Research Article
Volume 6 Issue 1 - 2024
Zinc-Enriched Steroid Receptors May Cause Alzheimer’s
M.D. Skolemarken 32, Broballe, 6430 Nordborg, Denmark
*Corresponding Author: Ebbe Lundsgaard, M.D. Skolemarken 32, Broballe, 6430 Nordborg, Denmark. Email Id: ebl@pc.dk
Received: December 12, 2024; Published: December 21, 2024
Further Readings
Ebbe Lundsgaard. (2020). “Multiple sclerosis: Excess of Cytoplasmic Steroid Receptors to Corresponding Hormones in the Oligodendrocytes”. Journal of Medicine and Surgical Sciences 2.2. DOI: 10.5281/zenodo.3959103 https://escientificpublishers.com/multiple-sclerosis-excess-of-cytoplasmic-steroid-receptors-to-corresponding-hormones-in-the-oligodendrocytes-JMSS-02-0024
Ebbe Lundsgaard. (2020). The Autonomic Nervous System Fights Cancer in Mammals and Birds. Journal of Medicine and Surgical Sciences 2(3). DOI: 10.5281/zenodo.3993834 https://escientificpublishers.com/the-autonomic-nervous-system-fights-cancer-in-mammals-and-birds-JMSS-02-0025
Ebbe Lundsgaard. (2020). “Multiple sclerosis: Excess of Cytoplasmic Steroid Receptors to Corresponding Hormones in the Oligodendrocytes”. Journal of Medicine and Surgical Sciences 2.2. DOI: 10.5281/zenodo.3959103 https://escientificpublishers.com/multiple-sclerosis-excess-of-cytoplasmic-steroid-receptors-to-corresponding-hormones-in-the-oligodendrocytes-JMSS-02-0024
Ebbe Lundsgaard. (2020). The Autonomic Nervous System Fights Cancer in Mammals and Birds. Journal of Medicine and Surgical Sciences 2(3). DOI: 10.5281/zenodo.3993834 https://escientificpublishers.com/the-autonomic-nervous-system-fights-cancer-in-mammals-and-birds-JMSS-02-0025
Abstract
Decreasing steroid hormones, mostly estrogen, testosterone and vitamin-D - the latter predominantly associated with sparse sunlight - are accompanied by a reciprocal excess of their cytoplasmic type 1 receptors presumed to induce Alzheimer's Disease. Steroid receptors that escape binding to steroid hormones or other suitable ligands are not translocated to the cell-nucleus and may aggregate as unliganded zinc-enriched receptor-remnants in the cytoplasm, which by zinc-release promotes the formation of devastating neurofibrillary tangles. However, a part of these receptor-aggregates may become extruded via amyloid precursor protein (APP), contributing to Alzheimer's plaques outside the neurons. The evidence, as part of the research, is necessarily mathematical, which is, however, reasonably available.
Keywords: Alzheimer’s Disease; Steroid-Receptors; Steroid-Hormones; Vitamin-D; Zinc
Introduction
Increasing age and female gender are risk factors for developing Alzheimer's that coincides with the fall in sex hormone production at increasing age, starting in women after menopause and later as a gradual progression in men. Aging also reduces the formation of the steroid hormone, 1.25-dihydroxy vitamin-D, by 50% due to a decline in renal function and decreased synthesis of vitamin-D in the skin. However, the vitamin D- concentrations are significantly higher in males compared to females, and vitamin-D deficiency is substantially associated with Alzheimer's [1].
Some contributing reasons for Alzheimer's with more or less penetrans should be briefly mentioned:
Apolipoprotein E (APOE4) poses a risk for Alzheimer's, but the frequencies vary by geographical region. Apolipoprotein E reduces for instance androgen binding to cognate receptors, but its role is obscure in severe vitamin-D deficiency leading to Alzheimer's. Estimates for APOE E4 from 142 samples showed an Alzheimer-prevalence of 48.7%. The rare allele combination 4/4 by APOE from 73 samples showed an Alzheimer-prevalence of 9.6% [2-3]
Apolipoprotein E (APOE4) poses a risk for Alzheimer's, but the frequencies vary by geographical region. Apolipoprotein E reduces for instance androgen binding to cognate receptors, but its role is obscure in severe vitamin-D deficiency leading to Alzheimer's. Estimates for APOE E4 from 142 samples showed an Alzheimer-prevalence of 48.7%. The rare allele combination 4/4 by APOE from 73 samples showed an Alzheimer-prevalence of 9.6% [2-3]
Virtually all individuals with Downs syndrome develop Alzheimer’s, and since the amyloid precursor protein APP-gene by trisomy 21 is found on three chromosomes it has been suggested that an overproduction of APP is a considerable cause of early-onset Alzheimer’s and even the sporadic type [4]. Nevertheless, some young individuals exhibit widespread extracellular amyloid plaques without any signs of Alzheimer's, which is possibly due to their high production of steroid hormones [5-6].
An alternative to APP might be the nuclear cofactor RIP 140 on chromosome 21 [7-8]. RIP 140 reduces the receptor-response to estrogens and perhaps to androgens too, at elevated ratio of RIP 140 to receptors, which retain the receptors unliganded.
There are some crucial factors missing in the above 'contributing reasons, hence my aim is to include cytoplasmic type 1 receptors in the pathogenesis of Alzheimer’s as they are donors of amyloid and zinc and can form aggregates [9] in cytoplasm by diminishing steroid hormones. They embrace the androgen receptors (X-chromosome) for testosterone and dihydrotestosterone, the estrogen receptors (chromosome 6 and 14) and the vitamin-D receptor [10]. The decisive trigger of Alzheimer’s may be zinc released from said receptor aggregates and capable of hyperphosphorylating tau protein in the neurons. As a result of the hyperphosphorylation neurofibrillary tangles are formed, causing degradation of microtubules with loss of the transport of essential substances in the neurons.
Binding of steroid hormones to cognate receptors releases the receptor-conformation from cytoplasm and allows nuclear translocation that progresses to a shuttle [11] between the cytoplasm and the nucleus, making the receptors recyclable. Regarding the estrogen receptors, this shuttle is selective [12] in the hippocampal CA subfields because ratio of estrogen receptors in the nucleus to their overall cellular level - a level, which is the same in both healthy and Alzheimer-patients - is approximately halved by Alzheimer’s compared to controls. This implies that the ratio of estrogen receptors or their remnants is increased in the cytoplasm by Alzheimer’s, which may accelerate the aggregation of unliganded receptors whether equipped with heat shock proteins or not [13].
A high number of steroid hormones desensitizes or down-regulates the number of receptors, which in turn is up-regulated by decreasing steroid hormones, figure 4. However, not everyone with a low level of sex hormones gets Alzheimer’s, which draws attention to the aforementioned change in the compartmentalization of steroid receptors showing an increment in the cytoplasm by Alzheimer’s.
The scope of this study assumes that Alzheimer's and Multiple Sclerosis are correlated diseases as they share several determinants [14].
Methods
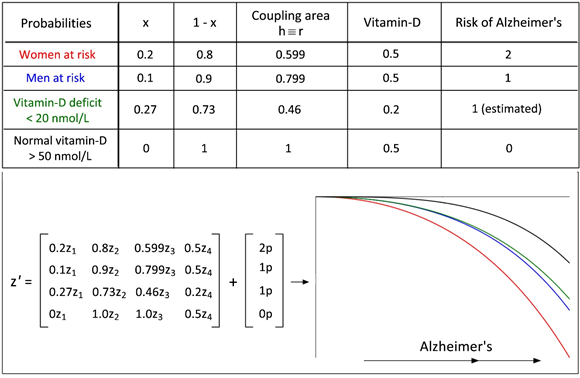
A woman's estimated lifetime risk of developing Alzheimer's at age 65 is 1 in 5 (0.2) and approximately the half (0.1) in men, because almost two-thirds of Americans with Alzheimer's are women [15].
However, incidence of Alzheimer's varies widely around the world. There are far more cases diagnosed in Europe and North America than in Africa, Asia or India [16] conceivably due to the intensity of sunlight. Severe deficiency of vitamin-D with a high risk of Alzheimer's was classified in 27% (0.27) [17].
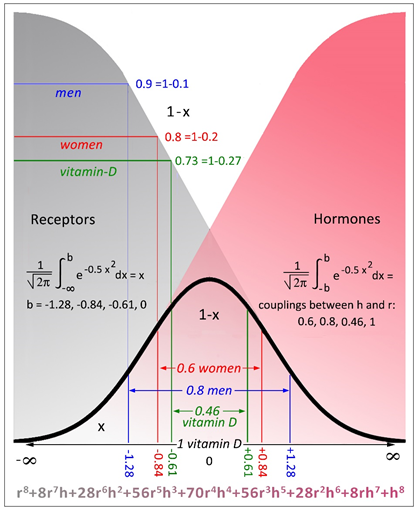
The binomial distribution (p+q)n, abbreviated to (h+r)8 at the bottom, displays a probability approaching 1 when the exponent, n, becomes infinitely large. The overall probability of the standard distribution - under the Gaussian curve - is 1. The zone of Alzheimer’s is displayed by x opposite to non-Alzheimer's, 1-x.
Women have a considerably lower level of steroid hormones than men, which is therefore not a satisfactory expression for the risk of Alzheimer's, but until menopause they are as well protected from Alzheimer's as men supposedly due to a higher receptor binding capacity.
Therefore, the probabilities p and q will be referred to as the logarithmic values of hormones (h) and receptors (r) because Alzheimer's predominantly occurs when the steroid production decreases.
Figure 2 is intended as a supplement to figure 1, and the differential equation system supports the assumption of the relationship between steroid hormones and Alzheimer’s. The colors of the table are reproduced in proper order in the graph and show the decline of the steroid hormones during increasing Alzheimer’s. (Maple: Runge-Kutta-Fehlberg 4-5th order).
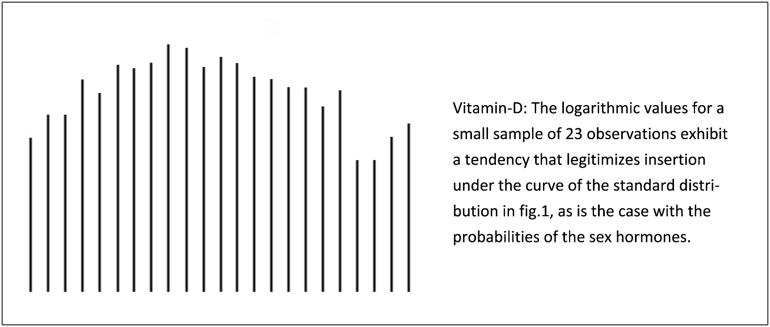
It is assumed that the logarithmic values of vitamin-D approach a standard distribution when the population is sufficiently large [18].
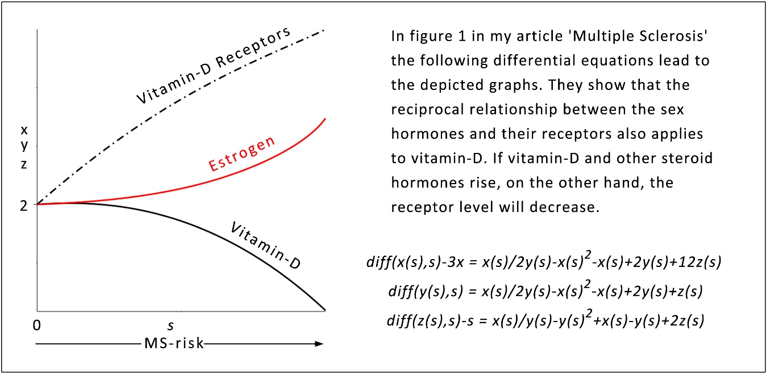
When s starts from zero, vitamin-D receptors is successively generated by negative feedback from vitamin-D (25-hydroxyvitamin D) and slightly by positive feedback from estrogen, which is initially inhibited by vitamin-D. As vitamin-D approaches the abscissa, the vitamin-D receptor continues its rise, now generated by the increasing estrogen concentration. (Maple: Runge-Kutta-Fehlberg 4-5th order).
Discussion
It should be noted that Apolipoprotein E and RIP140 reduce the bindings of androgens and estrogens to their receptors and at the same time pose a risk of Alzheimer's because more unbound zinc-enriched receptors accumulate in the cytoplasm. This is also seen by Downs Syndrome with the difference that, as mentioned in the introduction, there is an increased production of receptors.
The matter of ligand function is exemplified by the action of tamoxifen [19], partly an inhibitor who competitively binds to cytoplasmic estrogen and androgen receptors and appears to have a curative effect in Alzheimer’s. With some reservations it is substantiated that the conformation tamoxifen-receptor neither aggregates nor becomes transcripted. This implies that a transcription mediated by the conformation hormone-receptor is not required in order to impede the development of amyloid plaques because the mere conformation ligand-receptor seems sufficient for that purpose.
According to the binomial distribution in figure 1: The later in life Alzheimer’s occurs, the less the fraction of unliganded receptors on the left tail of the Gaussian curve. In other words, the amount of unliganded receptors determines the speed of aggregation that accumulates gradually. On the right tail of the curve the steroid hormones dominate successively with decreasing bindings to the dwindling receptors and do not participate in Alzheimer's.
The half-life of sex hormone receptors amounts to a few hours. It is anticipated that they during this time will adhere to each other and to the amorphous clusters of protein they have gradually formed unless they become liganded. The aggregates are largely resistant to lysosomal degradation due to the zinc-content, which progressively might be released and demonstrably plays a role in the hyperphosphorylation of tau protein. Such aggregates may occasionally release zinc in larger quantities, which would likely overload the zinc transport mechanisms as the concentration of cytosolic zinc is usually negligible [20].
Unliganded steroid receptors tend to clump together, and it is logical to assume that the more there are, the more rapidly it proceeds. From this it can be inferred that the early onset of Alzheimer’s may be caused by a high ratio of receptors to hormones, while the late occurrence can be attributed to a small ratio with slow aggregative receptors. The lag time seems thus variable and abide by age; therefore, the dosage of therapeutic steroid hormones is not straightforward as it must be initiated early before the irreversible damage to the brain reaches a certain level.
References
- Thomas J. Littlejohns, MSc, William E. Henley, PhD, Iain A. Lang, PhD, Cedric Annweiler, MD, PhD, Olivier Beauchet, MD, PhD, Paulo H.M. Chaves, MD, PhD, Linda Fried, MD, MPH, and David J. Llewellyn, PhD (2014). Vitamin D and the risk of dementia and Alzheimer disease. (Full Text). Neurology Journals. September 2. 83 (10) 920-928.
- Alex Ward; Sheila Crean; Catherine J. Mercaldi; Jenna M. Collins; Dylan Boyd; Michael N. Cook; H. Michael Arrighi (2012). Prevalence of Apolipoprotein E4 Genotype and Homozygotes (APOE e4/4) among Patients Diagnosed with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. (Abstract). Neuroepidemiology. Dec 17. 38 (1): 1–17.
- Jacob Raber (2009). AR, apoE, and cognitive function. (Full Text). National Library of Medicine. Feb 26;53(5): 706–715.
- Chia-Chen Liu, Chia-Chan Liu, Takahisa Kanekiyo, Huaxi Xu, Guojun Bu (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. (Abstract). Nat Rev Neurol. Feb;9(2): 106-18.
- Alzheimer’s Disease Amyloid Plaques Build Over A Lifetime; Found In Brains As Young As 20 (2015). The Grapevine. Mar 02.
- Alaina Baker-Nigh, Shahrooz Vahedi, Elena Goetz Davis, Sandra Weintraub, Eileen H. Bigio, William L. Klein, Changiz Geula (2015). Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer’s disease (Full Text). Brain. Volume 138, Issue 6, June, Pages 1722–1737.
- V Cavaillès, S Dauvois, F L'Horset, G Lopez, S Hoare, P J Kushner, M G Parker (1995). Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. The Embo Journal. Aug 1;14(15): 3741–3751.
- Nicholas Katsanis, Jane H. Ives, Jeurgen Groet, Dean Nizetic & E. M. C. Fisher (1998). Localisation of receptor interacting protein140 (RIP140) within 100kb of D21S13 on 21q11, a gene-poor region of the human genome. (Abstract). Springer Nature. February. Volume 102, pages 221–223.
- Huang, Han-Chang, | Jiang, Zhao-Feng (2009). Accumulated Amyloid-β Peptide and Hyperphosphorylated Tau Protein: Relationship and Links in Alzheimer's Disease. (Abstract). Journal of Alzheimer's Disease. 4 January, vol. 16, no. 1, pp. 15-27, 2009.
- Thomas J. Littlejohns, MSc, William E. Henley, PhD, Iain A. Lang, PhD, Cedric Annweiler, MD, PhD, Olivier Beauchet, MD, PhD, Paulo H.M. Chaves, MD, PhD, Linda Fried, MD, MPH, and David J. Llewellyn, PhD (2014). Vitamin D and the risk of dementia and Alzheimer disease. (Full Text). Neurology Journals. September 2. 83 (10): 920-928
- Carolyn M. Klinge, PhD, C. V. Rao, PhD (2008). The Steroid Hormone Receptors. (Full Text). Glowm. December. 1756-2228.
- Ya-Ping Lu, Mei Zeng, Dick F Swaab, Rivka Ravid, Jiang-Ning Zhou (2004). Colocalization and alteration of estrogen receptor-alpha and -beta in the hippocampus in Alzheimer's disease. (Abstract). Hum Pathol. Mar;35(3): 275-80.
- Role of Hsp90 machinery in native folding and activity of steroid/nuclear receptors. AB Vector.
- Ebbe Lundsgaard M.D. (2020) Multiple sclerosis: Excess of Cytoplasmic Steroid Receptors to Corresponding Hormones in the Oligodendrocytes. (Full Text). EScientific Publishers. July 23. Vol. 2 Issue 2.
- Alzheimer’s Association. Women and Alzheimer's.
- McGill, Office for Science and Society. Diet, Hygiene and Alzheimer’s Disease. 20. mar. 2017.
- David A. Hanley, K. Shawn Davison (February 2005). Vitamin D Insufficiency in North America. (Full Text). The Journal of Nutrition. Volume 135, Issue 2. Pages 332-337.
- Geoffrey Amuka Omuse PhD. (2018). Vitamin D status in healthy black African adults at a tertiary hospital in Nairobi, Kenya: A cross-sectional study. Aga Khan University Hospital, Nairobi.
- B Breuer, R Anderson (2000). The relationship of tamoxifen with dementia, depression, and dependence in activities of daily living in elderly nursing home residents. (Abstract). National Library of Medicine. 31(1): 71-85.
- W. Maret (2017). Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. (1). Int J Mol Sci. Sep24; 7(2): 5885.
Citation: Ebbe Lundsgaard. (2024). “Zinc-Enriched Steroid Receptors May Cause Alzheimer’s”. Journal of Medicine and Surgical Sciences 6.1. DOI: 10.5281/zenodo.14540378
Copyright: © 2024 Ebbe Lundsgaard. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.