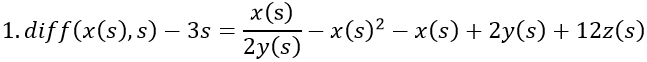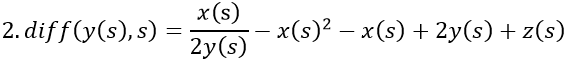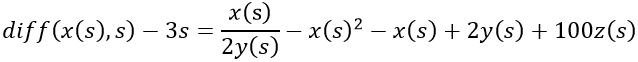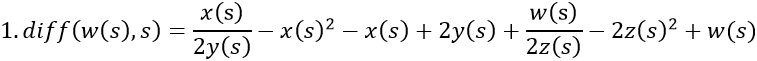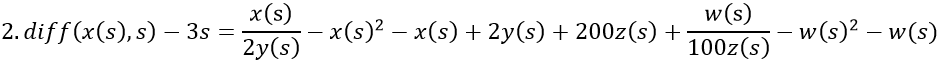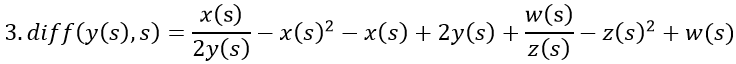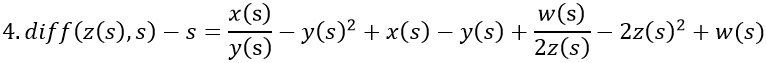Research Article
Volume 2 Issue 2 - 2020
Multiple sclerosis: Excess of Cytoplasmic Steroid Receptors to Corresponding Hormones in the Oligodendrocytes
M.D. Skolemarken 32, Broballe, 6430 Nordborg, Denmark
*Corresponding Author: Ebbe Lundsgaard, M.D. Skolemarken 32, Broballe, 6430 Nordborg, Denmark.
Email id: ebl@pc.dk
Email id: ebl@pc.dk
Received: July 15, 2020; Published: July 23, 2020
Abstract
An excess of zinc-enriched type 1 steroid receptors to matching steroid hormones may damage oligodendrocytes and cause central nervous system demyelination and multiple sclerosis. These receptors for vitamin D, estrogens, androgens, and other steroids can form cytoplasmic aggregates that occasionally release zinc in larger amounts, causing hyperphosphorylation of tau protein and degradation of the oligodendrocytes and the myelin sheath around the neurons.
Postmenopausal women and older men with multiple sclerosis and a hereditary low sex hormone level matched by excess receptors may develop symptoms of Alzheimer's.
Six facts about multiple sclerosis were inserted into innovative differential equations for the construction of the graphs, which show: A simultaneous increase in vitamin D receptors and multiple sclerosis. Vitamin D receptors are exposed to negative feedback from vitamin D followed by positive feedback from estrogen, which is initially repressed by vitamin D. Thus, growing levels of estrogen increase vitamin D receptors, while high levels in multiple sclerosis-pregnancies reduce the detrimental effect of vitamin D receptors by additional bonds to estrogen receptors, obviously augmented by a terminal drop in vitamin D receptors.
Keywords: Multiple-sclerosis; Oligodendrocytes; Steroid-receptors; Steroid-hormones; Vitamin-D; Estrogen.
Introduction
Two things separate this study from other studies dealing with multiple sclerosis (MS):
Firstly, I argue that MS originates in the myelin-forming oligodendrocytes and not, according to the well-established assumption, in an inflamed myelin sheath, which would require the presence of antigens in the myelin sheath and a concomitant defect in the blood-brain barrier allowing immune cells to enter the brain from the blood. Conditions that have not been verified despite decades of research.
Firstly, I argue that MS originates in the myelin-forming oligodendrocytes and not, according to the well-established assumption, in an inflamed myelin sheath, which would require the presence of antigens in the myelin sheath and a concomitant defect in the blood-brain barrier allowing immune cells to enter the brain from the blood. Conditions that have not been verified despite decades of research.
Secondly, the depicted dynamic figures are based on mathematical methods that are considered inferior to experiments, but the end justifies the means.
The aim is to show that damage to oligodendrocytes results from a cytoplasmic excess of type 1 steroid receptors relative to matching steroid hormones. This includes vitamin D receptors (VDR) generated by estrogens, especially estradiol, E2, [1] [2] [3], but apparently also by negative feedback from vitamin D.
For computational reasons, the receptors are limited to estrogen receptors (ER) and VDR, whose hormones are strongly associated with the onset and frequency of MS.
Each receptor is enriched with four zinc atoms [4] and can, regardless of the presence of heat shock proteins, form aggregates [5] in cytoplasm unless it binds to corresponding hormones, after which the complex is translocated to DNA in the cell nucleus without contributing to MS.
The decisive trigger of MS may be zinc released from said receptor aggregates and capable of hyperphosphorylating tau protein in oligodendrocytes and neurons [6] [7] [8] [9], which, by the way, is also a hallmark of Alzheimer's disease (AD) [10]. The activity of zinc is, however, not embraced by the subsequent mathematics.
As a result of the hyperphosphorylation, glial fibrillary tangles [11] and neurofibrillary tangles [12] are formed, causing degradation of microtubules with loss of the transport of essential substances in the cell and finally decay of the myelin sheath covering the neurons [13].
Accordingly, steroid hormones are restricted to the following hormones: Estrogen is included as women are three times more likely to develop MS than men [14], but after menopause and in elderly men, the drop in testosterone may become important. However, vitamin D plays a role from puberty to senescence and appears to be a key steroid in MS although external factors such as the geographical location are involved in the synthesis of this steroid, with sunlight being the most potent factor [15] [16].
Steroid hormones generally auto-regulate their receptor levels [17]. An excess of sex hormones desensitizes or down-regulates the number of ER (chromosome 6 alpha and 14 beta) and androgen receptors (AR) (X chromosome), which in turn are upregulated by a decreasing level of sex hormones, a regulation that also appears to occur between vitamin D and VDR.
The scope of this study sporadically includes neurodegenerative diseases, which in my opinion share determinants with MS.
Materials
The six fundamentals below are used in the following equations that determine the course of the graphs:
- Within certain limits, elevated estrogen levels generate VDR [1] [2] [3].
- Ratio of men to women with MS approximates 1/3 [14].
- Vitamin D deficiency poses a risk for MS [15] [16].
- Ratio of estrogen concentrations, postmenopausal- to fertile women, approximates 1/12 [18] [19] [20].
- Ratio of estrogen concentrations, men to fertile women, approximates 1/4 [18] [19] [20].
- Free estrogen increases by a factor exceeding twenty late in pregnancy [21].
However, an elaboration of these fundamentals seems opportune:
MS is pronounced in fertile women [22], possibly because VDR is generated by estrogen. In addition, VDR is significantly more prevalent in MS compared to healthy individuals [23] [24]. Nevertheless, the overall pool of unliganded steroid receptors in oligodendrocytes and neurons plays an essential role, especially at aging in those who are hereditarily predisposed for low levels of sex hormones against high levels of their receptors. This group can exhibit progression of MS resembling the pathology of AD [25].
MS is pronounced in fertile women [22], possibly because VDR is generated by estrogen. In addition, VDR is significantly more prevalent in MS compared to healthy individuals [23] [24]. Nevertheless, the overall pool of unliganded steroid receptors in oligodendrocytes and neurons plays an essential role, especially at aging in those who are hereditarily predisposed for low levels of sex hormones against high levels of their receptors. This group can exhibit progression of MS resembling the pathology of AD [25].
Pregnant women with MS often experience improvement in late pregnancy, characterized by a very high estrogen level. The reason may be the reciprocal relationship between hormones and receptors as well as the high receptor binding capacity in women [26], both of which reducing the number of unliganded ER.
Women with MS also have a subnormal testosterone concentration [26], which causes an increase in AR, creating a cumulative effect with VDR.
Men with MS and a high estrogen level exhibit a greater degree of brain injury [27] [28], while testosterone supplementation causes cognitive enhancement [29], possibly because the unliganded AR are reduced, whereby the effect of estrogen-generated VDR attenuates.
Methods
The models depend on the fractions VDR/vitamin-D (x/y) and ER/estrogen (w/z) and estrogen, z. The unknown concentration of receptors (x, w) is placed in the numerator, whereas the concentration of hormones (y, z) in the denominator is defined. The same rules apply to x/y and w/z, why x/y is chosen.
If MS is due to receptor dominance, x is greater than y in x/y (but less than 2y), or z is elevated. In the function, F(x/y) → x/y + αxy + (x – αx), is αx the subset of x that binds to y in an equivalent quantity. Therefore, αx, when mul-tiplied by y, forms the bond, αxy. Since αx equals y, follows, αxy = yy = y2, which is negative, – y2, as it is translocated to the cell nucleus. There remains an unused part of x, x – αx, identical to x – y.
By adding z, the final model now equals the disease risk: x/y – y2 + x – y + z = s.
At vitamin D dominance, associated with the absence of MS, y is greater than x in x/y, and the level of z is normal.
In the function, G(x/y) → x/y + βyx + (y – βy), is βy the subset of y that binds to x in an equivalent quantity. Therefore, βy, when multiplied by x, forms the bond, βyx. Since βy equals x, follows, βyx = xx = x2, which is negative, – x2, as it is translocated to the cell nucleus. There remains an unused part of y, y – βy, identical to y – x.
By adding z, the final model now equals the disease risk: x/y - x2 - x + y + z = s.
An unknown quantity of hormones and receptors is unliganded at any given time, but this does not significantly affect the method.
Results
Figures 1, 2 and 3 show the concentration of unliganded VDR, estrogen, vitamin D and ER versus the risk of MS and are based on systems of nonhomogeneous differential equations. The computations can only be performed numerically in MAPLE with the 'boundary values' set to 2 on the ordinate for the dependent variables, hormones and receptors, at the instant the independent variable, s, the risk of MS, equals 0 on the abscissa.
Moreover, the independent variable must be consistent with an approximately straight section of the line formed by age versus disease frequency, what is met here [22].
MS progresses from zero to the numbers, 0.19, 0.085, and 0.067, representing the respective upper limits of MAPLE's performance.
- Fertile woman with MS. The level of vitamin D, 2y(s), is normal, but the level of estrogen is increased from the average of 8z(s) to 12z(s). The disease rate is 3s, i.e. 3 times that of men.
- Postmenopausal woman without MS and with normal levels of vitamin D, 2y(s), and estrogen, z(s).
- Man with MS. There is a deficiency of vitamin D, y(s), but the level of estrogen, 2z(s) - a quarter of fertile women - is normal. The disease rate is s.
When s starts from zero, VDR is successively generated by negative feedback from vitamin D and slightly by positive feedback from estrogen, which is initially inhibited by vitamin D. As vitamin D approaches the abscissa, VDR continues its rise, now generated by the increasing estrogen concentration. (Runge-Kutta-Fehlberg 4-5th order).
The estrogen concentration in the first equation is now raised to a level of 100z(s) that may occur late in pregnancy. The other two equations remain unchanged.
VDR is crossed by a pregnancy-equivalent estrogen concentration. This alleviates MS symptoms, among others due to increasing number of couplings between estrogen and ER, which is partly attributed to the high receptor binding capacity in women. The small arrow shows the coincidence between exhausted vitamin D and markedly increasing estrogen. (Runge-Kutta-Fehlberg 4-5th order).
In the desire to visualize the course of ER after consecutive couplings with estrogen, the above equation system is expanded by w/z, identical to ER/estrogen.
- Man without MS. The levels of vitamin D, 2y(s), and estrogen, 2z(s), are normal. It is assumed that the diminutive 2z(s) is smaller than w(s) due to the reciprocal relationship. 2z(s) and -2z(s) cancel each other out.
- Pregnant woman with MS. Vitamin D, 2y(s), is normal, but the level of estrogen increases to 100z(s), which surpasses w(s) and occurs twice, yielding 200z(s). The disease rate is 3s.
- Postmenopausal woman without MS and with normal levels of vitamin D, 2y(s), and estrogen, z(s). It is assumed that z(s) is smaller than w(s) due to the reciprocal relationship. z(s) and -z(s) cancel each other out.
- Man with MS. There is a deficiency of vitamin D, y(s), but the level of estrogen, 2z(s), is normal. It is assumed that 2z(s) is smaller than w(s) due to the reciprocal relationship. The disease rate is s. 2z(s) and -2z(s) cancel each other out.
Estrogen exhibits negative feedback on ER, but this is less pronounced where the inclination of estrogen (small arrow) is located along with the decline in VDR, which continues as estrogen resumes its increase. This coinciding loss of free estrogen and VDR suggests that bonds are established between them. (Rosenbrock stiff 3-4th order).
The assumed reciprocal relationship between ER [w(s)] and the low estrogen levels in men and postmenopausal women can only be depicted by non-feasible symbolically computations in MAPLE.
Discussion and Conclusion
Although the six fundamentals in ‘Materials’ set rules for the construction of the equations, the concentration of the receptors (VDR, ER) is unknown, and their tendency in the graphs is unpredictable, as is convincingly seen in in figure 3.
As figure 1 shows, vitamin D exerts negative feedback on VDR and inhibits the estrogen synthesis [30] until vitamin D approaches the abscissa and continues at a zero slope without further participating in the increase of VDR, which now occurs by increased estrogen activity. This results in a decrease in ER in accordance with figure 3, which confirms the reciprocal relationship.
The sudden drop in VDR at high estrogen concentrations is not described in the literature but is perhaps the primary cause of the relief of MS symptoms in pregnant women. The dosage of estrogen in a course of treatment should, therefore, match the values of late pregnancy as smaller doses according to figure 2 can generate an excess of VDR.
Certainly, vitamin D supplementation, preferably sunlight, is an alternative, but in all likelihood vitamin D does not cross-react with estrogen- and androgen receptors, which would restrain their formation of lumps in the oligodendrocytes. However, a comprehensive effect occurs when vitamin D supplementation is succeeded by tamoxifen, which competitively binds to cytoplasmic estrogen- and androgen receptors and has a beneficial effect on MS [31]. Binding of tamoxifen to these receptors may inhibit receptor aggregation and reduce the release of zinc, a process that takes place in the cytoplasm and is independent of transcription.
Incidentally, tamoxifen counteracts AD and amyotrophic lateral sclerosis, presumably due to the same mechanisms [32] [33].
In a genetic perspective, MS seems to be related to polygenes responsible for the variation in the reciprocal relationship between steroids and their receptors. For example, when the vitamin D level decreases, the increase in VDR seems beneficial because it improves the utilization, but this benefit is temporary as aggregates can be formed. Such aggregates conceal zinc for an extended period of time and may occasionally release it in larger quantities, which would likely overload the zinc transport mechanisms as the concentration of cytosolic zinc is usually negligible [34]. Does that constitute the cause of 'relapsing remitting' MS?
Treatment with zinc transporters has been suggested as a temporary improvement occurs in Lewis rats [6]. However, the source of zinc, the zinc-enriched aggregates, is unlikely to be affected.
References
- F.E. Nashold, K.M. Spach, J.A. Spanier, C.E. (2009). Hayes Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression(Abstract and Discussion). J Immunol.183 (6) 3672-3681.
- L.A. Gilad, T. Bresler, J. Gnainsky, P. Smirnoff, B. Schwartz. (2005). Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK ½ signaling pathway in colon and breast cancer cells J Endocrinol. Jun; 185(3): 577-92.
- Y. Liel, S. Shany, P. Smirnoff, B. Schwartz (1999). Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa Endocrinology. Jan; 140(1):280-5.
- Steroid hormone receptors and their response elements
- AB Vector: Role of Hsp90 machinery in native folding and activity of steroid/nuclear receptors (Hsp90 implications for receptor stability in HTS assays).
- M. Penkowa, J. Hidalgo (2003). Treatment with metallothionein prevents demyelination and axonal damage and increases oligodendrocyte precursors and tissue repair during experimental autoimmune encephalomyelitisJournal of Neuroscience Research, Vol 72, Issue 5, p. 574-86
- S. Mato, M.V. Sánchez-Gómez, A. Bernal-Chico, C. Matute (2013). Cytosolic zinc accumulation contributes to excitotoxic oligodendroglial death Glia. May; 61(5):750-64.
- P. LoPresti. (2018). Tau in oligodendrocytes takes neurons in sickness and in health Int J Mol Sci. Aug; 19(8): 2408.
- X.Y. Sun, Y.P. Wei, Y. Xiong, X.C. Wang, et al. (2012). Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). J Biol Chem. Mar 30; 287(14): 11174-82.
- Huang, Han-Changa, Jiang, Zhao-Fenga (2009). Accumulated Amyloid-β Peptide and Hyperphosphorylated Tau Protein: Relationship and Links in Alzheimer's Disease Journal of Alzheimer's Disease. vol. 16, no. 1, pp. 15-27.
- C. Richter-Landsberg (2016) Protein aggregate formation in oligodendrocytes: tau and the cytoskeleton at the intersection of neuroprotection and neurodegeneration Biol Chem. Mar; 397(3):185-94.
- C. Björkdahl, M.J. Sjögren, B. Winblad, J.J. Pei (2005). Zinc induces neurofilament phosphorylation independent of p70 S6 kinase in N2a cells Neuroreport. Apr 25;16(6):591-5.
- H. Lassmann (2010). What drives disease in multiple sclerosis: Inflammation or neurodegeneration? Wiley Online Library.
- L. Airas. (2015). Hormonal and gender-related immune changes in multiple sclerosis Wiley Online Library. Acta Neurologica Scandinavica.
- K.L. Munger, L.I. Levin, B.W. Hollis, N.S. Howard, A. (2006). Ascherio Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis JAMA. Dec 20; 296(23):2832-8.
- M.B. Sintzel, M. Rametta, A.T. Reder (2018). Vitamin D and multiple sclerosis: A comprehensive review Neurol Ther. Jun; 7(1): 59–85.
- C. Klinge, C. Rao (2008). The Steroid Hormone Receptors Glob. libr. women's med. (ISSN: 1756-2228)
- Mayo Clinic, Laboratories Test ID, EEST. Estradiol free, serum (includes estradiol and SHBG)
- Lab Dept, Chemistry Testosterone, total and free
- Laboratory reference ranges
- Perinatology.com (2007) Reference values during pregnancy
- Prevalence of multiple sclerosis, by sex and age group, British Columbia (Figure 3-3). 2009/2010, BC Administrative Data Project [1].
- Multiple Sclerosis Society of Canada (2015). Early stage study shows that vitamin D can promote myelin repair (Results). December 18.
- J. Smolders, K.G. Schuurman, M.E. van Strien, J. Melief, et al. (2013). Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue J Neuropathol Exp Neurol. Feb;72(2): 91-105.
- A. Dal Bianco, M. Bradl, J. Frischer, A. (2008). Kutzelnigg Multiple sclerosis and Alzheimer's disease Ann Neurol. Feb; 63(2):174-83.
- V. Tomassini, E. Onesti, C. Mainero, E. Giugni, et al. (2005). Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. (Abstract and Discussion). BMJ Journals.
- A.B. Nicot. (2009). Gender and sex hormones in multiple sclerosis pathology and therapy.(4). Front Biosci (Landmark Ed). 14: 4477–4515.
- S.M. Gold, R.R. Voskuhl (2009). Estrogen treatment in multiple sclerosis J Neurol Sci. Nov 15; 286(1-2): 99–103.
- S.M. Gold, R.R. Voskuhl (2009). Estrogen and testosterone therapies in multiple sclerosis. (3.1 and 4) Prog Brain Res. 175: 239–251.
- A.V. Krishnan, S. Swami, D. Feldman (2012). The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer Steroids. Sep; 77(11): 1107–1112.
- G.A. Gonzalez, et al. (2016). Tamoxifen accelerates the repair of demyelinated lesions in the central nervous system Sci. Rep. 6, 31599.
- L.M. Sun, H.J. Chen, J.A. Liang, C.H. Kao (2016). Long-term use of tamoxifen reduces the risk of dementia: a nationwide population-based cohort study QJM: An International Journal of Medicine. Volume 109, Issue 2, Pages 103–109.
- M. Orsini, et al. (2015). Amyotrophic lateral sclerosis: New perspectives and update. (Study medications for patients with amyotrophic lateral sclerosis: tamoxifen). Neurol Int. Sep 24; 7(2): 5885.
- W. Maret (2017) Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. (1). Int J Mol Sci. Nov; 18(11): 2285.
Citation: Ebbe Lundsgaard. (2020). “Multiple sclerosis: Excess of Cytoplasmic Steroid Receptors to Corresponding Hormones in the Oligodendrocytes”. Journal of Medicine and Surgical Sciences 2.2. DOI: 10.5281/zenodo.3959103
Copyright: © 2020 Ebbe Lundsgaard. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.