Mini Review
Volume 4 Issue 1 - 2022
The Vesicular Residuum-Rathke Cleft Cyst
A.B. Diagnostics A-1, Rajouri Garden, New Delhi 110027 India
*Corresponding Author: Anubha Bajaj, A.B. Diagnostics A-1, Rajouri Garden, New Delhi 110027 India.
Received: March 28, 2022; Published: June 09, 2022
Preface Cystic lesions of the sellar region exhibit a heterogeneous countenance and a variable clinical representation. Cystic lesions can appear as benign, incidental lesions, may disrupt the hormonal axes or depict a cogent mass effect and may be associated with significant morbidity.
Segregation of cystic lesions of the sellar region can be challenging upon imaging. Nevertheless, pertinent clinical and imaging features can assist a preliminary disease discernment.
Differentiating features of cystic lesions are preponderantly comprised of heterogeneity of cyst contents, thickness of cyst wall, localization of cyst as anterior, posterior, midline or lateral, cyst configuration as globular or eccentric, cyst magnitude, proportionate invasion into circumscribing soft tissue along with occurrence of a solid component and an intra-cystic nodule. Nevertheless, the lesion can be appropriately discerned with evaluation of features such as heterogeneity of cyst wall, contents of cyst cavity and a discernible solid component upon preoperative magnetic resonance imaging (MRI).
Rathke cleft cyst is a common, benign, incidentally discovered, non neoplastic, epithelial cyst confined to the sellar or suprasellar region. Additionally designated as pars intermedia cyst, the lesion emerges from embryologic remnants of Rathke pouch of the pituitary gland.
Contrastingly, Rathke cleft cyst can be misinterpreted as cystic pituitary adenoma upon magnetic resonance imaging. Besides, Rathke cleft cyst and craniopharyngioma represent a histological continuum and cogent demarcation upon microscopic and molecular levels may be challenging. Disease CharacteristicsCystic lesions of the sellar region are classified according to features such as heterogeneity of cyst wall, contents of cystic cavity and a definitive solid component upon preoperative magnetic resonance imaging (MRI) into
- Type 1 cystic lesions display a well circumscribed, regular perimeter and homogeneous constituents within the cavity along with an absence of a solid component. Lesions are singularly composed of cyst contents and cyst wall [1,2].
- Type 2 cystic lesions are well circumscribed with an irregular outline, heterogeneous cyst cavity contents, septa traversing the cyst and a subtle solid, non-cystic component [1,2].
- Type 3 cystic lesions denominate a well circumscribed, regular perimeter and homogenous cyst contents along with a significant solid component.
- Type 4 cystic lesions describe an irregular perimeter, heterogeneous cyst cavity contents, septa traversing the cyst in addition to multiple poorly circumscribed, predominately solid cysts [1,2].
Type 1 lesions and Type 2 lesions can be associated with minimal elevation of serum prolactin levels, a feature which may be attributable to compression by pituitary gland stalk or the “stalk effect” [1,2].
Rathke cleft cyst originates from remnants of Rathke pouch and is predominantly located within midline pituitary gland, especially within pars intermedia. The lesion may enlarge continually as the cyst is posited to arise from a congenital substratum [1,2].
During embryogenesis, Rathke pouch is configured within fourth week and emerges as a rostral outpouching from the roof of primitive oral cavity. Anterior wall of the pouch engenders anterior lobe of pituitary gland or pars distalis whereas non proliferative posterior wall of the pouch persists as the intermediate lobe of pituitary gland or pars intermedia. Lumen of Rathke pouch narrows and configures Rathke cleft which may retrogress. However, persistence and expansion of Rathke cleft may engender a Rathke cleft cyst. Besides, posterior lesions arising from pars intermedia commonly engender anterior displacement of the infundibulum [1,2].
Rathke cleft cyst is commonly observed in up to ~ 22% of autopsies. The condition is exceptional in children and may be discerned within the adult population. A female preponderance is delineated with an estimated female to male proportion of 2:1[1,2].
Clinical Elucidation Type 1 lesions are miniature lesions preponderantly comprised of posteriorly situated Rathke cleft cyst wherein maximal magnitude of the lesion may extend beyond> one centimetre. Generally, young females are implicated. Headache is a common clinical symptom. Invasion of cavernous sinus is infrequent.
Majority of Rathke cleft cysts are asymptomatic and discovered incidentally. The cyst engendered from remnants of Rathke cleft can be impacted with mucus [3,4].
The intra-sellar or supra-sellar Rathke cleft cyst may be discovered as an incidental finding on autopsy. Enlarged cysts appearing in adults may be symptomatic and associated with headache or visual disturbances occurring due to compression of optic chiasm [3,4].
An estimated three fourths of symptomatic cysts demonstrate pituitary dysfunction arising due to compression of adjoining pituitary tissue and distortion of pituitary stalk. Preoperative endocrine dysfunction with hormonal symptoms is commonly observed with Rathke cleft cyst [3,4].
Histological Elucidation Upon macroscopy, an intraluminal, white nodule appears adherent to the cyst wall. Alternatively, the nodule may be detached and is composed of solid tissue arising from desquamated cellular debris. Macroscopically, cyst wall is attenuated and cyst cavity is imbued with mucinous contents [5,6].
Characteristically,Rathke cyst wall is coated with singular layer of frequently ciliated, columnar epithelium which may incorporate goblet cells. Foci of squamous metaplasia are observed along with focal xanthogranulomatous reaction comprised of foamy macrophages. Fragments of pituitary tissue circumscribe the cystic cavity [5,6].
Layering cyst epithelium is immune reactive to cytokeratin (CK) and epithelial membrane antigen (EMA) [5,6].
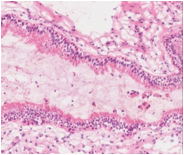
Figure 2: Rathke cleft cyst demonstrating a cystic cavity lined by columnar epithelium with sprinkled goblet cells and imbued cellular debris with circumscribing fibrous tissue (12).
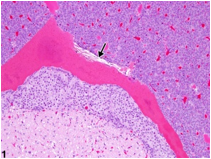
Figure 3: Rathke cleft cyst delineating a cavity lined by columnar epithelium with focal haemorrhage and red cell extravasation (13).
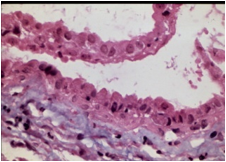
Figure 4: Rathke cleft cyst exhibiting a cystic cavity coated with ciliated columnar epithelium and impacted cellular debris (14).
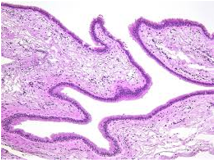
Figure 5: Rathke cleft cyst exemplifying a cyst cavity with layering ciliated columnar epithelium (15).
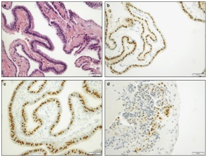
Figure 6: Rathke cleft cyst enunciating a cystic cavity with lining ciliated columnar epithelium and immune reactivity to FOXJ1 (16).
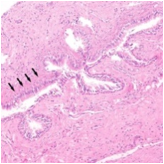
Figure 7: Rathke cleft cyst depicting a cystic cavity layered with ciliated columnar epithelium with impacted cellular debris and surrounding fibrotic tissue (17).
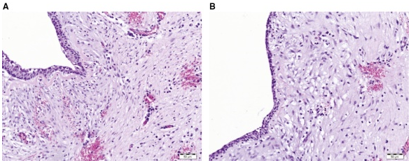
Figure 8: Rathke cleft cyst displaying a cystic cavity layered with ciliated columnar epithelium and enveloping fibro-connective tissue (18).
Differential Diagnosis Rathke cleft cyst requires a segregation from pathological conditions of the sella such as cystic pituitary adenoma, craniopharyngioma, arachnoid cyst, epidermoid cyst, dermoid cyst or xanthogranuloma. Especially, a cystic mass devoid of solid enhancement upon magnetic resonance imaging (MRI) within the sella requires segregation whereas surgical intervention is indicated for symptomatic lesions [7,8].
Demarcation is required from conditions such as •cystic pituitary adenoma wherein diverse pituitary adenomas are composed of cells imbued with moderately abundant cytoplasm, uniform nuclei, stippled chromatin and inconspicuous nucleoli. Pertaining to variant staining hues, adenoma cells are categorized as acidophilic, basophilic or chromophobic.
Cytoplasmic appearance is usually concordant with hormonal content of densely granulated or sparsely granulated secretory cells. Crooke’s hyaline change exhibits enlarged chromophobic or eosinophilic cells with glassy, hyaline countenance on account of accumulated keratin filaments and appears as a component of neoplastic or non-neoplastic corticotroph adenomas. Upon MRI, the neoplasm may depict fluid-fluid level or haemorrhagic debris [7,8].
- Craniopharyngioma occurs in adults and is devoid of gender predilection. Tumefaction may appear entirely within the suprasellar region or a suprasellar component is delineated. Tumefaction is comprised of solid sheets of well differentiated, non-keratinizing squamous epithelial cells. Poorly configured papillae display fibro-vascular cores with cellular invagination. Miniature, collagenous whorls and focal calcification may ensue. Upon low power examination, papillary variant with papillary configuration demonstrates cauliflower-like excrescences [7,8].
- Arachnoid cyst emerges in elderly individuals and is devoid of a specific gender preponderance. Cyst wall is composed of delicate, fibro- connective tissue and a focal or diffuse layer of meningothelial cells. The meningothelial cells are immune reactive to epithelial membrane antigen (EMA) and immune non reactive to cytokeratin(CK), glial fibrillary acidic protein (GFAP), transthyretin or synaptophysin [7,8].
- Epidermoid cyst arising within the central nervous system is usually suprasellar in location. The unilocular epidermal inclusion cyst is layered with stratified squamous epithelium with prominent granular cells. The cystic cavity is imbued with acellular, lamellar keratin exhibiting a “basket weave” pattern. Upon diffusion weighted imaging (DWI), image enhancement may be restricted [7,8].
- Teratoma confined to the central nervous system is usually composed of solid components. Pineal teratoma is commonly discerned in males. Comprised of tissues derived from ectoderm, endoderm and mesoderm, configuration of teratoma requires a minimal of two of three germinal cell layers. Central nervous system teratomas are usually well differentiated neoplasms of grade I category. Mature teratoma is constituted of well differentiated tissues derived from three germinal layers along with neuroectoderm along with epithelial component represented as solid, cystic, glandular or tubular, cartilaginous tissue or diverse mesenchymal elements, glial and neuronal tissue. Imaging procedures frequently depict a signal intensity concordant with mature adipose tissue. Immature teratoma exhibits minimally differentiated tissue arising from singular or three germinal layers [7,8].
- Normal cysts of the intermediate lobe may manifest as miniature, asymptomatic lesions wherein layering epithelium is non ciliated and devoid of goblet cells [7,8].
Investigative Assay Upon imaging, Rathke cleft cyst characteristically depicts a non enhancing, non calcified cystic lesion confined to intrasellar or suprasellar region. Additionally, an intra-cystic nodule is significantly associated with Rathke cleft cyst [9,10].
Rathke cleft cyst appears as a well defined, non-enhancing, midline cyst confined to the sella between anterior and intermediate lobes of pituitary gland. Nearly 40% cysts are intra-sellar whereas roughly 60% lesions depict a suprasellar extension[9,10].
Rathke cleft cyst singularly confined to suprasellar region is exceptional. Lateral radiographs of the skull adopted for enlarged cysts may infrequently demonstrate expansion of the sella [9,10].
Non contrast computerized tomography (CT) typically exhibits a non calcified, homogeneous lesion with minimal attenuation. Exceptionally, the lesion may enunciate a combination of iso-attenuation and minimal attenuation. Alternatively, cyst wall can exemplify miniature, curvilinear calcifications [9,10].
Post contrast computerized tomography delineates a non enhancing lesion although few cystic lesions may demonstrate enhancement of the cyst wall [9,10]. Enhancement of cyst wall is critical in differentiating neoplastic from non-neoplastic cysts. However, Rathke cleft cyst is frequently circumscribed along with enhancement of normal pituitary gland. The cyst can display patchy or ring enhancement on account of inflammation or squamous metaplasia of the cyst wall [9,10].
Upon T1 weighted imaging and T2 weighted imaging, signal intensities may vary as hypo-intense to hyper-intense. Variability of magnetic resonance signal intensities is contingent to cyst composition as predominantly protein, muco-polysaccharides or cholesterol and is denominated as mucoid or serous [9,10].
Contingent to cystic contents and occurrence of an intra-cystic nodule, Rathke cleft cyst may depict diverse signal intensities upon T1 weighted and T2 weighted imaging. Hyper-intense signals upon T1 weighted imaging and hypo-intense signals upon T2 weighted imaging are associated with elevated intra-cystic protein within a Rathke cleft cyst. Upon T1 weighted imaging, certain protein-imbued cysts are hyper-intense while remaining cysts emerge as hypo-intense. Upon T2 weighted imaging, majority of cysts are hyper-intense whereas remaining cysts appear iso-intense or hypo-intense [9,10]. Upon diffusion weighted magnetic resonance imaging (DWI), Rathke cleft cyst demonstrates a significantly elevated apparent diffusion coefficient (ADC) values [9,10].
Upon gadolinium contrast, the cyst is devoid of contrast enhancement. However, an attenuated rim of circumscribing, compressed pituitary tissue may be enhanced [9,10].
Majority (~75%) of instances exhibit a miniature, non enhancing, intra-cystic nodule which is characteristic of Rathke cleft cyst. Occasionally, a fluid-fluid level is observed which is concurrent with occurrence of haemorrhage although haemorrhage is exceptional within Rathke cleft cysts. Nevertheless, imaging features such as an off-midline location, fluid-fluid level or hypo-intense lesion perimeter upon T2 weighted magnetic resonance imaging, septa traversing the cyst cavity and an intra-cystic nodule are pathognomonic of Rathke cleft cyst [9,10].
Therapeutic Options Incidentally discovered lesions may be managed conservatively with regular monitoring. Rathke cleft cyst may decimate in magnitude and resolve spontaneously in the absence of cogent therapy. Symptomatic Rathke cleft cyst can be appropriately treated with pertinent surgical resection [9,10].
Surgical intervention is necessitated on account of hormonal symptoms, headache or localized mass effect. Partial resection of cyst wall and comprehensive evacuation of cyst contents are an optimal treatment strategy for managing Rathke cleft cyst [9,10].
Unnecessary or excessive surgical eradication of Rathke cleft cyst can be associated with infection, effusion of cerebrospinal fluid or injury to the hypothalamus. Lesion reoccurrence may ensue following surgical extermination [9,10].
References
- Tavakol S, Catalino MP et al. (2021). “Cyst Type Differentiates Rathke Cleft Cysts From Cystic Pituitary Adenomas” Front Oncol 11:778824.
- Park M, Lee SK et al. (2015). “Differentiation between Cystic Pituitary Adenomas and Rathke Cleft Cysts: A Diagnostic Model Using MRI” AJNR Am J Neuroradiol 36(10): 1866-1873.
- Cote DJ, Smith TR et al. (2021). Body Habitus Across the Lifespan and Risk of Pituitary Adenoma” J Clin Endocrinol Metab 106(4): e1591–602.
- Burke WT, Penn DL et al. (2019). “Prolactinomas and Nonfunctioning Adenomas: Preoperative Diagnosis of Tumour Type Using Serum Prolactin and Tumour Size” J Neurosurg 133: 321–8.
- Chabot JD, Chakraborty S et al. (2015). “Evaluation of Outcomes After Endoscopic Endonasal Surgery for Large and Giant Pituitary Macro-adenoma : A Retrospective Review of 39 Consecutive Patients” World Neurosurg 84(4): 978–88.
- Zamora C, Castillo M. (2017). “Sellar and Parasellar Imaging”. Neurosurg 80(1) :17-38.
- Shatri J, Ahmetgjekaj I. (2018). “Rathke’s Cleft Cyst or Pituitary Apoplexy: A Case Report and Literature Review” Open Access Maced J Med Sci 6(3): 544–7.
- Gittleman H, Ostrom QT et al. (2014). “Descriptive Epidemiology of Pituitary Tumours in the United States, 2004-2009”. J Neuro-surg 121(3): 527–35.
- Xiao D, Wang S et al. (2017). “Fluid-Fluid Level on Magnetic Resonance Images may Predict the Occurrence of Pituitary Adenomas in Cystic Sellar-Suprasellar Masses” Exp Ther Med 13(6): 3123–9.
- Zada G. (2011). “Rathke Cleft Cysts: A Review of Clinical and Surgical Management”. Neurosurg Focus 31(1): E1.
- Image 1 Courtesy: Science direct
- Image 2 Courtesy: Radiopedia.com
- Image 3 Courtesy: National Toxicology Programme
- Image 4 Courtesy: Anocef.com
- Image 5 Courtesy: Twitter.com
- Image 6 Courtesy: Nature.com
- Image 7 Courtesy: Research gate
- Image 8 Courtesy: De gruyter.com
Citation: Anubha Bajaj. (2022). The Vesicular Residuum-Rathke Cleft Cyst. Journal of Biotechnology and Immunology 4(1).
Copyright: © 2022 Anubha Bajaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

