Research Article
Volume 2 Issue 2 - 2020
The Best Method for Estimating the Density of Malaria plasmodium Infection
National Centre for Malaria Control and Vectors, UAE
*Corresponding Author: Atef Ali Kloub, National Centre for Malaria Control and Vectors, UAE.
Received: May 16, 2020; Published: May 25, 2020
Introduction
Vector-borne diseases is a group of diseases which spread by insects and other vectors and cause more than one million deaths annually, Malaria is a disease belongs to Vector-borne diseases and it is the most dangerous one of this group causing about 45% of the death cases by the Vector-borne diseases and most malaria related deaths occur in children under five years of age and in pregnant women.
Malaria also, belongs for that diseases which are linked with water (the vector of the disease spends a lot of its lifespan in water). Malaria is caused by protozoan parasites belonging to the genus Plasmodium and about 150 species which have been identified to date, infecting mammals, birds, and reptiles, whereas only 5 species out of the 150 species cause malaria for human, they are:
- Plasmodium falciparum
- Plasmodium vivax
- Plasmodium ovale
- Plasmodium malariae
- Plasmodium knowlesi
Plasmodium falciparumis the most dangerous of the malaria plasmodium parasites because:
- It causes ‘malignant’ or cerebral malaria that can quickly progress to unconsciousness and death.
- Untreated or poorly treated infections can cause recurring fevers and are communicable from several months to two years (P. falciparum).
3.4 billion people in 91 countries are living in areas where malaria is present (45% of the world’s population) are under risk of infection with malaria. In Sub Saharan Africa one in ten deaths of children under 12 months of age and one in four deaths of children between (1) and (4) years of age are caused by malaria. Some parts of the world are also experiencing a resurgence of malaria and malaria has even been recorded in areas where it was previously unknown.
Infection with malaria parasites may result in a wide variety of symptoms, ranging from absent or very mild symptoms to severe disease and even death, but in general, malaria is a curable disease if diagnosed and treated promptly and correctly. so malaria disease can be categorized as uncomplicated or severe (complicated) and all the clinical symptoms associated with malaria are caused by the asexual erythrocytic or blood stage parasites.
Methods of Malaria Transmission
Female Anopheles mosquitoes: by the biting of infected female Anopheles mosquitoes for some species of Anopheles mosquitoes which able to transmit the infection.
Blood transfusion: Infection can also occur by blood transfusion of infected donor.
Contaminated tools: injection by needles and syringes contaminated with infected blood.
Congenital malaria: from a mother to her fetus before or during delivery.
The Danger Level of Malaria Plasmodium Species
Life cycle for the pathogenic malaria plasmodium stages in erythrocytic (schizogony stage) extending from 1 to 3 days according to plasmodium species which needs for one day in case of plasmodium knowlesi infection but 2 days for Plasmodium falciparum, Plasmodium vivax and Plasmodium ovale infections, while needs for 3 days in case of plasmodium malariae.
Life cycle for the pathogenic malaria plasmodium stages in erythrocytic (schizogony stage) extending from 1 to 3 days according to plasmodium species which needs for one day in case of plasmodium knowlesi infection but 2 days for Plasmodium falciparum, Plasmodium vivax and Plasmodium ovale infections, while needs for 3 days in case of plasmodium malariae.
Merozoite is the first parasitic stage of malaria plasmodium enters the red blood cell whereas mature Schizont is the last product stage in schizogony cycle and the mature schizont is composed of numbers of merozoites from 6 - 32 merozoites regarding to the specie of malaria plasmodium (the biggest in falciparum up to 32 merozoites while the smallest in knowlesi, ovale and malariae start from 6 up to 12 merozoites and for vivax 12 - 16 merozoites and sometimes extending to 24).
Schizogony cycle is an important indicator to determine and distinguish the level of danger between the five species of malaria plasmodium which infect human, so regarding for that; malaria plasmodium falciparum is the most dangerous specie followed by malaria plasmodium knowlesi and vivax.
According to schizogony cycle we will know that parasites density will multiply 32 times in case of plasmodium falciparum infections every 48 hours while it could multiply 8-12 times in plasmodium knowlesi infection daily and 12 - 16 times in case of plasmodium vivax infection each 48 hours and rarely 24 times and 6 - 12 times in case of plasmodium ovale infection each 48 hours and 8-12 times in plasmodium malariae infection every 72 hours.
Main reasons for complications and mortality:
- Late lab diagnosis
- Error lab diagnosis
- Inaccurate lab diagnosis
- Not received recommended treatment
How to decrease numbers of mortality?
- Treat reasons which lead for complication by:
- Treat reasons which lead for complication by:
- Early case detection
- Correct & accurate lab diagnosis
- Give professional lab report (include parasite stages, specie and density)
- Use recommended treatment and doses
- Follow correct and accurate algorithm for management of malaria
- Comprehensive (for lab technologists, pharmacists, nurses and clinicians)
- Prepared by experts from various malaria field specialties
- Prepare effective malaria policy drug
- Continuous health education and training programs for all medical staffs
- Qualified lab technologists for correct microscopy diagnosis.
- Promote knowledge and awareness about malaria topic
- Know all lab diagnostic methods
- Know the mechanism of malaria transmission fluently
- Health education programs for different community groups
- Continuous programs
- For different age groups
- Using different methods of health education
- Continue vector controlling programs
- For both larvae and adult mosquitoes
- Put in account environment safety
Discussion
Malaria patient will deal with three medical departments the clinic, the lab and the pharmacy, it is known functions of these three departments is complement each to other always but sometimes one of them become basic for others, therefore next procedures will be done according to the result of that department so in malaria case the lab is the basic agent by which both the clinician and the pharmacist will choose and continue treatment methods as quality and doses, therefore when everything in lab go ahead correctly, it will also become correct in other departments which will reflect positivity for the patient case.
In malaria lab, it is common to receive requests for malaria test as the following form:
Blood film for malaria, without asking for counting parasites or estimating parasite density, therefore some laboratories only gives reports positive or negative for malaria while many other laboratories determine the specie of the plasmodium when the test is positive; like positive for p.falciparum or positive for P.vivax for examples.
Blood film for malaria, without asking for counting parasites or estimating parasite density, therefore some laboratories only gives reports positive or negative for malaria while many other laboratories determine the specie of the plasmodium when the test is positive; like positive for p.falciparum or positive for P.vivax for examples.
This is not sufficient and incomplete report, whereas it is essential that the malaria lab report must declare the species of malaria plasmodium which were seen also mention to the parasite stages which are seen and density of each stage and must determine parasitaemia density, all of these data must be included in malaria lab report even the clinician requested that or not.
So, the typical malaria lab report for example must be like:
P. vivax ring form +++ trophozoite ++ gametocyte + schizont +
Parasitaemia density: 100000 parasites/mL
Infected RBCs 2%
Parasitaemia density: 100000 parasites/mL
Infected RBCs 2%
Preparing comprehensive malaria lab report needs qualified lab technologist has technical skills for microscopy diagnosis and background about methods of counting malaria parasites and estimated parasitaemia density.
Methods of Counting Malaria Plasmodium Parasites
- Parasite / μL.
- Determining the percentage of parasitemia.
- The ‘plus system’
1. Parasite / μL (Thick Film):
- Parasites are counted in relation to a predetermined number of WBCs and an average of 8000/µl is taken as standard.
- 200 leucocytes are counted.
- All parasite species and forms including both sexual and asexual forms are counted together.
(No. of Parasites/ No. of WBCs counted) x 8000 = No. of parasites/µl
2. Determining the percentage of parasitemia (Thin Blood Film):
(The percentage of infected Red Blood Cells)
(The percentage of infected Red Blood Cells)
- The number of infected red cells (and not number of parasites) in 1000 RBCs is converted to percentage.
- If 1000 red cells are counted, then divide the number of parasitized red cells by 10 to get the percentage (i.e. if 30 out of 1000 cells are parasitized, then the parasitized red cell count is 3%).
- If occasional parasites are seen when scanning the smear, but none are identified during the process of counting 300-500 red blood cells, a percentage value of less than 1% of red blood cells parasitized is assigned.
3. The ‘plus system’
- “The ‘plus system’ is an old method, which is simple but far less accurate for establishing parasite density in thick blood films” (1).
- “-Because of its unreliability, it has been replaced by the method described above and is no longer recommended”. (2).
• In this system:
+ = 1–10 parasites per 100 oil-immersion thick film fields
++ = 11–100 parasites per 100 oil-immersion thick film fields
+++ = 1–10 parasites per single oil-immersion thick film field
++++ = more than 10 parasites per single oil-immersion thick film field
+ = 1–10 parasites per 100 oil-immersion thick film fields
++ = 11–100 parasites per 100 oil-immersion thick film fields
+++ = 1–10 parasites per single oil-immersion thick film field
++++ = more than 10 parasites per single oil-immersion thick film field
(1), (2)A quote words from WHO publication titled “Basic Malaria Microscopy” Part I Learner’s guide – second edition Page No.75 ISBN: 978 92 4 154782 6 (Part I)
What is the accuracy of this? Let’s see the following slides, then decide:
Example one
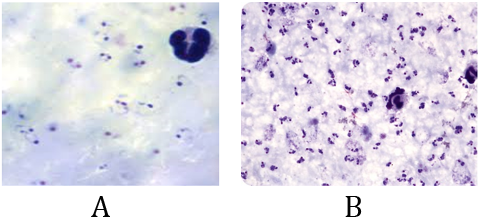
Both slides A & B are positive slides for plasmodium falciparum contain many rings form stage (thick film).
Regarding to the ‘plus system method the lab report result will be written for both A & B slides as: P.f r ++++
This didn't represent the fact, which appear that the severity of infection in slide B is too much more than it in slide A. (Lab result for both slides A & B will be reported as: P.f r ++++)
So, status like this, surely, using "Parasite/µl method is the best choice.
Example two
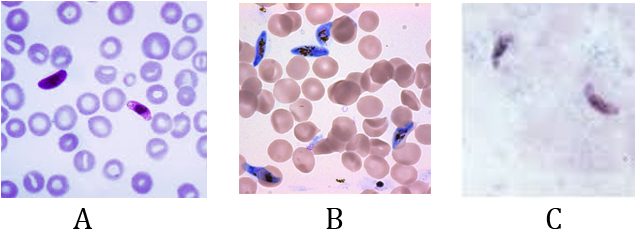
Slides A, B and C appear the presence of gametocyte form stage only, the nonpathogenic stage. (It is common to see this when following up the falciparum cases after finishing the treatment doses).
Using the percentage method for both slides A & B will show that the density of parasitemia in slid A is less than it in slide B.
For the slides A,B: Using “the percentage method and parasite/µl method "for the slide C will appear the amount of parasitemia density but unable to indicate for the presence of the gametocyte stage only.
So, status like this, surely, using “Plus system method” is the best additional choice. Report result (slide C): P.f g +++
Example three
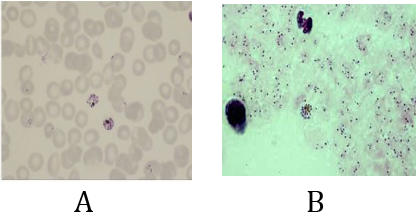
Both slides A & B are positive for a plasmodium falciparum infection contain early trophozoite forms & schizont form stage.
Slide A (thin film): using “percentage method for estimating parasite load” will develop the severity level of infection but unable to indicate for the presence of the schizont form of stage.
Slide B (thick film): using” Parasite/µl method” for estimating parasite load will represent about the heavy severity of infection but unable to indicate for the presence of the schizont form stage.
So, status like this, surely, using “Plus system method” is the best additional choice. Report result: P.f r++++ sch +
Note: appearance of schizont stage in blood smears in falciparum infection is an indicator for high risk case because it is common to appear in advanced cases and in cerebral malaria cases.
Example four
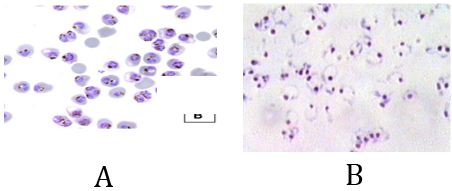
Both slides A & B are positive for a heavy infection of plasmodium falciparum contain early & late trophozoite form stage.
Slide A (thin film): using “percentage method for estimating parasite load” will represent about the heavy severity of infection but unable to indicate for the presence of the late trophozoite form stage.
Slide B (thick film): using” Parasite/µl method” for estimating parasite load will represent about the heavy severity of infection but unable to indicate for the presence of the late trophozoite form stage.
So, status like this, surely, using “Plus system method” is the best additional choice. Report result: P.f r++++ tr +
Note: appearance of late trophozoite stage in blood smears in falciparum infection is an indicator for high risk case because it is common to appear in advanced cases and in cerebral malaria cases.
Conclusion
Disagree what has been published by WHO publication titled “Basic Malaria Microscopy”
Part I Learner’s guide – second edition Page No.75 ISBN: 978 92 4 154782 6 (Part I) about “The ‘plus system’ that:
- “The ‘plus system’ is an old method, which is simple but far less accurate for establishing parasite density in thick blood films”.
- “Because of its unreliability, it has been replaced by the method described above and is no longer recommended”.
Because above examples number two, three and four show the important of appearance of some developing malaria plasmodium stages in stained blood smears, whereas this feature is exclusive for plus system method only.
Mentioning these stages in malaria report will indicate for the level risk of the malaria case which will assist the physician how to deal with the case by using the suitable choices on the time, therefore the plus system is the only counting method which able doing this.
So, absolutely, I disagree describing this method that it is unreliable and but far less accurate for establishing parasite density in thick blood films “because this isn’tadapt with logic scientific nor with practical experience in malaria lab.
References
- WHO. Bench aids for malaria diagnosis. Geneva; (2009). WHO. QA manual for malaria microscopy. 2nd Edition. Geneva; 2015. WHO.
- Basic malaria microscopy. Part I. Learner’s guide. Second edition. Geneva; (2010). WHO. National malaria slide bank standard operating procedures. Geneva; 2015 (in preparation).
- Jan (2016) 1 Reviewed and finalized by experts, edited and formatted Glenda Gonzales, Technical Officer, WPRO
- Basic Malaria Microscopy, part II Tutor’s guide, second edition – WHO
- Manual for Malaria Control in the UAE
- Andrej Trampuz, Matjaz Jereb, Igor Muzlovic, Rajesh M Prabhu. Clinical review: Severe malaria.
- World Health Organization. (2006). Guidelines for the Treatment of Malaria. Geneva: WHO.
- Global technical strategy for malaria 2016–2030/WHO
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. (2004). The global distribution and population at risk of malaria: past, present, and future. Lancet Infectious Diseases. 4: 327-336.
- Breman JG, Alilio MS, Mills A. (2004). Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. American Journal of Tropical Medicine and Hygiene. 71 (suppl 2): 1-15.
- Variability in determining malaria parasite density by microscopy. American Journal of Tropical Medicine and Hygiene. (2005).
Both slides A & B are positive for a heavy infection of plasmodium falciparum contain early & late trophozoite form stage.
Slide A (thin film): using “percentage method for estimating parasite load” will represent about the heavy severity of infection but unable to indicate for the presence of the late trophozoite form stage.
Slide B (thick film): using” Parasite/µl method” for estimating parasite load will represent about the heavy severity of infection but unable to indicate for the presence of the late trophozoite form stage.
So, status like this, surely, using “Plus system method” is the best additional choice. Report result: P.f r++++ tr +
Citation: Atef Ali Kloub. (2020). The Best Method for Estimating the Density of Malaria plasmodium Infection. Journal of Biotechnology and Immunology 2(2).
Copyright: © 2020 Atef Ali Kloub. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.