Review Article
Volume 3 Issue 2 - 2021
Standard Operating Procedures for the collection of information Remotely via Phone Calls in healthcare organizations
1Gentium Healthcare, Cairo, Egypt
2University of South Wales, School of Law, Accounting and Finance, Pontypridd, Wales
3McMaster University, Hamilton, Canada
2University of South Wales, School of Law, Accounting and Finance, Pontypridd, Wales
3McMaster University, Hamilton, Canada
*Corresponding Author: Mohamed Refaat, Gentium Healthcare, Cairo, Egypt.
Received: July 02, 2021; Published: October 08, 2021
Abstract
The purpose of this paper is to describe procedures for how members of a healthcare organizations should handle phone calls to ensure, enhance, and implement quality measures and to reduce errors in the collection of information [1].
This Standard Operating Procedure (SOP) applies to all phone calls regarding Patient Support and/or Patient Oriented Programs conducted by an organization and all employees who receive phone calls [2]. It is the responsibility of employee manager’s and the Quality Assurance manager to ensure all employees adhere to the defined procedures [2].
Keywords: SOPs; Collection; Information; Remote; Phone Calls; Healthcare organizations
Introduction
Employee Training
- Organizations should create a defined procedure for handling phone calls using defined procedures for creating Standard Operating Procedures. This phone script should then be approved by the Marketing Authorization Holder before being communicated to agents [3].
- The organization is responsible for conducting and documenting phone training for each agent before they begin work to ensure they understand accepted procedures.
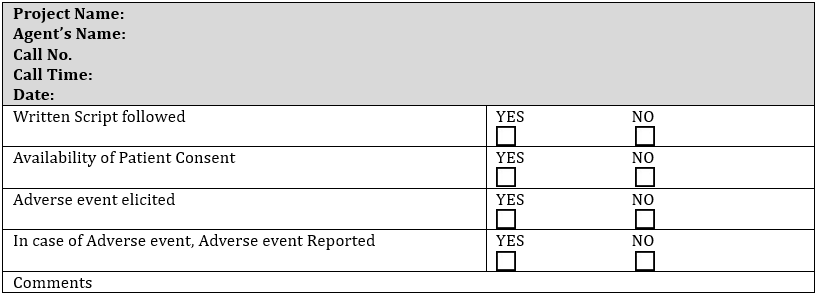
- Each agent will be trained to spot, record, report, and archive Adverse Events as per the organization’s defined procedures [4].
- Each agent will be trained to secure patient’s consent for phone call recording at the start of each call. If consent is not given, the call will be terminated [5].
- Simulated calls will be carried out before employees experience actual patient interactions to ensure good communication and adherence to phone procedures. These simulations will also test the employee’s awareness and application of Adverse Event (AE) reporting procedures [6].
- The organization will confirm that each agent follows the approved script. A printed copy will be present in front of each agent’s desk for easy availability [7].
- All out-bound and In-bound calls will be recorded, it is the responsibility of the agent and their direct manager to confirm that the recording is active before each call
- Complete and signed induction plan for Marketing Authorization Holder call center educators will be provided upon hiring
- At the start of the project, each agent’s first 5 patient interactions will be supervised by the direct manager for quality control purposes [8].
- Each agent will be required to fill out a phone call handling form after each call for quality control purposes [9].
Procedures
Call Quality Assurance Procedures
Call Quality Assurance Procedures
- It is the responsibility of the Agents’ Manager or Quality Assurance manager to perform Test Calls on a daily basis before the start of taking calls to validate the recording/archiving system and to test sound clarity for call participants. This Test Call will be remain on the system records for proof of testing [10]
- It is the responsibility of the Agent’s manager or Quality Assurance manager to perform random daily audits of 5% of calls per agent per project ensure compliance with appropriate procedures, including and not limited to post-vaccination adverse events, Health Professional Communication, Business Continuity Plan and fulfillment of requested Marketing Authorization Holder task orders, including and not limited to the approved script, transcript, patient consent, etc.
Results
Deviation Management
- When deviations from phone handling protocol are identified by any means, (e.g. during random sample checks by the organizations managers, during source data verification processes (SDV)) deviation reporting procedures will be followed to resolve the issue(refer to deviation Standard Operating Procedures) [11].
Discussion
- Deviation reporting should occur within 24 hours of the incident, at which time the Marketing Authorization Holder will be notified and the agreed upon actions carried out to resolve the deviation.
- Upon review of the deviation, the related agent may be suspended for re-training at which time the agent will sign a consent for re-training. If the deviation does not warrant full re-training, the agent will not take any new calls until the deviation has been discussed and corrected by a senior officer.
- When the call agent resumes duties after retraining their first 5 calls will be accompanied and supervised by the direct manager.
- Monthly meetings will be held to share deviation incidents for continuous learning and improvement.
Conclusion
- In case of Adverse Events reporting during a call, the agent will not start a new call before reporting the event through the Adverse Events reporting procedure (refer to Pharmacovigilance and Adverse Event Standard Operating Procedure).
Phone Call Handling Checklist Form [12]
Daily Random Sample Audit Form [13]
| Project Name: Agent Name: Call Date & Time: Auditor Signature: |
|
| • If there is a Written Transcript, state your comments mentioning the integrity with the voice call. | Comments |
| • Was the approved script followed? | |
| • Is there an Adverse event elicited & was it reported? | |
| Is there an Adverse event elicited & was it reported? | |
| • Was the patient consent secured? | |
References
- Laboratory Quality Management System Handbook Laboratory Quality Management System; (2011).
- Martínez-Mesa, J.; González-Chica, D. A.; Duquia, R. P.; Bonamigo, R. R.; Bastos, J. L. (2016). Sampling: How to Select Participants in My Research Study? An. Bras. Dermatol. 91 (3), 326–330.
- Meischke, H.; Painter, I.; Turner, A. M.; Weaver, M. R.; Fahrenbruch, C. E.; Ike, B. R.; Stangenes, S. (2016). Protocol: Simulation Training to Improve 9-1-1 Dispatcher Identification of Cardiac Arrest. BMC Emerg. Med. 16 (1).
- Munroe, B.; Buckley, T.; Curtis, K.; Morris, R. (2016). Designing and Implementing Full Immersion Simulation as a Research Tool. Australas. Emerg. Nurs. J., 19, 90–105.
- Kahn, K.; Collinson, M. A.; Xavier Gómez-olivé, F.; Mokoena, O.; Twine, R.; Mee, P.; Afolabi, S. A.; Clark, B. D.; Kabudula, C. W.; Khosa, A.; Khoza, S.; Shabangu, M. G.; Silaule, B.; Tibane, J. B.; Wagner, R. G.; Garenne, M. L.; Clark, S. J.; Tollman, S. M. (2012). Profile: Agincourt Health and Socio-Demographic Surveillance System. Int. J. Epidemiol. 41 (4), 988–1001.
- Alinier, G. 61_f_scenario-Healthcare A Guide for Developing High-Fidelity Simulation Scenarios in Healthcare Education and Continuing Professional Development.
- Alinier, G. (2011). Developing High-Fidelity Health Care Simulation Scenarios: A Guide for Educators and Professionals. Simul. Gaming, 42 (1): 9–26.
- https://www.laerdal.com/uk/UK-NewsletterIssue15.pdf (accessed May 14, 2020).
- Devices and Techniques for Low-Engagement Interaction.
- https://nlp.cs.princeton.edu/CRW/WWC_vocab.txt (accessed May 14, 2020).
- David Brown, M. (1999). The Construction of Muslim Identities in the United Kingdom and France: A Contribution to the Critique of Orientalism.
- Management of Safety Information from Clinical Trials; (2007).
- Frequently Asked Questions - Office of the Registrar - Santa Clara University https://www.scu.edu/registrar/frequently-asked-questions/ (accessed May 14, 2020).
Citation: Mohamed Refaat. (2021). Standard Operating Procedures for Change Control in Healthcare Organizations. Journal of Biotechnology and Immunology 3(2).
Copyright: © 2021 Mohamed Refaat. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.