Research Article
Volume 3 Issue 2 - 2021
Risk of Retinal Detachment Recurrence after Macular Surgery in eyes previously treated with 360° Encircling Band
1Department of Ophthalmology, P.O. Palagi, Azienda USL Toscana Centro, Florence, Italy
2Department of Neuroscience, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
3Ophthalmology Unit, Catholic University of the Sacred Heart, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy
2Department of Neuroscience, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
3Ophthalmology Unit, Catholic University of the Sacred Heart, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy
*Corresponding Author: Francesco Barca, Department of Ophthalmology, P.O. Palagi, Azienda USL Toscana Centro, Florence, Italy. Email address: francesco.barca@uslcentro.toscana.it
Received: July 25, 2021; Published: August 06 , 2021
Abstract
Aims: To evaluate functional outcomes and the safety profile of Pars Plana Vitrectomy (PPV) procedure for macular diseases in eyes already subjected to successful Scleral Buckle (SB) operation with encircling 360° band for Retinal Detachment (RD).
Methods: Observational, retrospective, consecutive case series on 55 patients affected by epiretinal membrane or macular hole, and previously subjected to encircling SB surgery for RD. All the patients underwent PPV procedure from May 2017 to September 2019 and they were followed up for at least 6 months.
Results: Three eyes out of 55 (5.45%) had a recurrence of RD within the first 30 days after PPV procedure. A retinal tear during a PPV for macular hole was found. The mean BCVA improved from 0.6 logMAR +/- 0.8 to 0.3 logMAR +/- 0.05.
Conclusion: The PPV procedure in patients who have already undergone SB operations with encircling 360° band exposes them to greater risks, and it is advisable to adopt safer and more effective approaches in order to avoid intra- and postoperative complications.
Keywords: Encircling scleral buckle; Novelty in the surgical approach; Pars plana vitrectomy; Safety profile
Introduction
Scleral Buckles (SB) play an important role in the treatment of Rhegmatogenous Retinal Detachment (RRD), by changing the geometry and physiology of the eye. SB helps in several ways to counteract the forces that tend to detach the retina. Indentation of the eye wall produced by the SB relieves vitreoretinal traction by decreasing the magnitude and possibly changing the direction of the vitreous traction on the retinal tear. Encircling scleral buckles help to decrease trans-retinal traction by decreasing the diameter and circumference of the vitreous base [1].
SB has a successful surgical repair rate of the RRD [2]. However, RRD may be associated with several post-operative complications that may require surgical treatment with Pars Plana Vitrectomy (PPV), such as the development of an Epiretinal Membrane (ERM) and Full Thickness Macular Hole (FTMH) [3,4,5].
We found no evidence in literature regarding possible implications of the encircling band on the risk of intra- and post-operative complication development during PPV procedure, as well as the recurrence of Retinal Detachment (RD). Nevertheless, this is an important issue when counseling patients with macular disease who have a previous history of retinal detachment, because it is important to assess the surgical risk.
The purpose of this study was therefore to evaluate the safety profile of PPV procedure in patients who had previously undergone an encircling 360° band for RD, by determining the rate over time of retinal re-detachments and of intra-operative complications, and comparing our results with those of the idiopathic forms reported in literature.
Patients and Methods
This was an observational, retrospective, consecutive case series of 55 patients affected by ERM or FTMH, who had previously undergone successful encircling SB surgery for RD. They were then subjected to PPV procedure from May 2017 to September 2019, and they were followed up for at least 6 months. Patients who underwent a combined phaco-vitrectomy procedure were also included. All operations were performed by 3 experienced vitreoretinal surgeons (FB, SR, TC).
This was an observational, retrospective, consecutive case series of 55 patients affected by ERM or FTMH, who had previously undergone successful encircling SB surgery for RD. They were then subjected to PPV procedure from May 2017 to September 2019, and they were followed up for at least 6 months. Patients who underwent a combined phaco-vitrectomy procedure were also included. All operations were performed by 3 experienced vitreoretinal surgeons (FB, SR, TC).
Written informed consent for participation was obtained from all the patients and the study is adherent with the Declaration of Helsinki.
TABLE 1 summarizes patient demographics and baseline clinical data. The exclusion criteria were previous traumatic RD, segmental SB surgery, previous operations for diabetic retinopathy, previous PPV procedure, patients who underwent scleral buckle revision or combined PPV procedure with encircling SB procedure.
| Variables | |
| Mean age (SD), years | 59 ± 16 |
| Male-Female, No. | 23-32 |
| Pre-operative BCVA, mean (SD), logMAR | 0.6 logMAR ± 0.8 |
| Phakic, No. (%) | 4 (7.27) |
Table 1: Patient demographics and baseline clinical data.
The main outcomes were the recurrence of RD and BCVA improvement at 6 months. Secondary endpoints were the development of intraoperative retinal breaks during PPV procedure.
Before surgery each patient underwent a complete ophthalmologic examination including assessment of Best-Corrected Visual Acuity (BCVA), slit-lamp biomicroscopy, Intraocular Pressure (IOP) evaluation with Goldmann applanation tonometry, axial length assessment with IOL Master (Carl Zeiss Meditec, Dublin, CA, USA), spectral-domain Optical Coherence Tomography (OCT) (AngioVue Optovue, Fremont CA USA) analysis and ultra-wide retinography (Daytona, Optos Inc. Marlborough USA).
At the 6-month follow up, patients underwent a complete ophthalmologic examination including BCVA assessment, slit-lamp biomicroscopy, IOP measurement, spectral-domain OCT (AngioVue Optovue, Fremont CA USA) and ultra-wide retinography (Daytona, Optos Inc. Marlborough USA).
Surgical Technique
All patients underwent a standard 3-port 23/25-gauge complete PPV (Alcon Laboratories, Fort Worth, TX) with basal vitreous shaving and 360-degree peripheral photocoagulation anterior and posterior to back edge of the scleral indentation of the buckle. The Alcon NGENUITY 3D viewing-system was used for all operations. The display was positioned next to the operating table (right side), and 120 to 180 cm away from the surgeon as recommended. Passive circularly polarized glasses were needed to visualize the display in 3D. Before starting surgery, plugging and setting the Image Capture Module (ICM) on the OPMI Lumera 700 (Carl Zeiss Meditec, Inc, Jena, Germany) surgical microscope, with Resight instrument for panoramic vision, was performed.
All patients underwent a standard 3-port 23/25-gauge complete PPV (Alcon Laboratories, Fort Worth, TX) with basal vitreous shaving and 360-degree peripheral photocoagulation anterior and posterior to back edge of the scleral indentation of the buckle. The Alcon NGENUITY 3D viewing-system was used for all operations. The display was positioned next to the operating table (right side), and 120 to 180 cm away from the surgeon as recommended. Passive circularly polarized glasses were needed to visualize the display in 3D. Before starting surgery, plugging and setting the Image Capture Module (ICM) on the OPMI Lumera 700 (Carl Zeiss Meditec, Inc, Jena, Germany) surgical microscope, with Resight instrument for panoramic vision, was performed.
In 4 patients the cataract-extraction procedure was performed before vitrectomy. In all the patients after core vitrectomy a mixture of Vital Dyes (Twins, AL.CHI.MI.A, ITALY) was used to highlight both ERM and the Internal Limiting Membrane (ILM) within the posterior pole before the peeling maneuver. Air was chosen as endo-tamponade in the ERM cases, while Sulfur Hexafluoride (SF6) 20% endo-tamponade in the FTMH cases (See supplemental digital content 1). All patients were asked to maintain the face-down position for 3 post-operative days.
Results
Fifty-five eyes of 55 patients affected by ERM or FTMH, who had already undergone successful SB procedure for RD, were included in this study. All the patients underwent PPV or combined phaco-PPV procedure with the 3D-viewing system, from May 2017 to September 2019, and they were followed up for at least 6 months. Twenty-five patients out of 55 were affected by FTMH and 30 by ERM. Three eyes out of 55 (5.45%) had a recurrence of RD within the first 30 days after PPV procedure, 1 after ERM removal and 2 after surgical operations for MHs. In one case, a retinal tear during PPV for FTMH was found and endolaser-barrage was performed. No other recurrences of RD were reported. Mean 6 months post-operative BCVA was 0.3 logMAR +/- 0.05.
Discussion
The SB is universally recognized as a successful procedure for RD; however, RD may be associated with complications that require PPV, such as ERM or FTMH. In this study, vitrectomy for ERM or FTMH after a SB-RRD successful repair resulted in significant visual and anatomical improvement.
ERM development is a known complication of RD surgery, with an extremely variable incidence after SB procedure, ranging from 3% to 51% among different reports [6,7]. Secondary MHs is a rare condition first described by Brown in patients who had undergone SB surgery for RD repair; the incidence of MH following RD repair with SB was reported to be between 0.32% to 0.96% [8]. The presence of vitreomacular traction during or after SB has been advocated as the main pathological mechanism that may cause the development of MH; recently other patho-genetical factors including Posterior Vitreous Detachment (PVD), degeneration of the outer retina due to neurosensory RD, and phagocytosis of damaged photoreceptors seem to be involved in MH formation [8].
Performing the PPV for ERM and MH on our patients with a 360° encircling band, we faced greater technical difficulty in removing the vitreous base. Other than the changes in the geometry of the scleral wall altered by the buckle, the presence of abnormal vitreous adhesions complicated the procedure to a great extent. This could be explained by the fact that during a SB procedure an evacuative puncture and an injection of air as endotamponade is usually performed: these maneuvers generally alter the basal vitreous, inducing greater vitreal adhesions and making its subsequent removal more challenging due to the increased risk of intraoperative retinal breaks. Moreover, the retinal area on which cryotherapy is performed becomes atrophic; its thinning, combined with the presence of vitreous adherences, could perhaps predispose the retina to the development of intraoperative breaks.
Furthermore, an encircling scleral buckle increases the axial length and decreases the volume and internal surface area of the eye, especially when the band is too tight, making the retina redundant and therefore difficult to lay on a narrow scleral bed. The redundant retina creates radial folds, at which level the adhesion between neuroretina and underlying RPE is less tight (Figure 1). This causes greater intraoperative mobility of the retina, leading to greater technical difficulty in removing the vitreous base at this level. In the presence of over-tightened encircling SBs, the removal of the vitreous is also made more challenging by the physical presence of the indentation of the scleral wall, which creates a shadow area behind it, making it more technically difficult to perform vitrectomy at this level (See supplemental digital content 1) (Figure 2).
An intra-operative retinal tear during the PPV procedure for MH was found in 1 patient. The retinal tear was positioned at the edge of the 7.5 mm encircling band; the scleral-wall indentation was found excessive and the buckle was found over-tightened, inducing the formation of radial retinal folds with greater intra-operative mobility. Fluid-air exchange and endolaser-barrage were performed. This patient did not experience RD occurrences in the 6 months of follow-up.
In our series we had a total RD recurrence rate of 5.45% (3 out of 55) after PPV procedure performed to treat ERM and FTMH after successful SB surgery.
The occurrence of RD after PPV for idiopathic ERM is reported to be lower than 1% [10] in literature. The reported recurrence of RD after ERM removal ranges from 1% to 7%, nevertheless these percentages refer to patients treated with both SB and PPV [10].
In our series 1 eye out of 30 (3,33%) had a recurrence of RD after ERM removal within the first 30 days after PPV procedure, in accordance with data reported in literature.
There are no data in literature on the recurrence of retinal detachment after MH surgery, while the RD rates following primary MH surgery range from 0% to 16% [11].
In our series, 2 eyes out of 25 (8%) had a recurrence of RD after PPV procedure for MH, which is a quite high percentage. However, our sample is small, and in many vitrectomies in our study we found vitreomacular attachment, and we had to induce surgical detachment of the posterior hyaloid membrane. The intra-operative induction of PVD has been correlated with a significant increase of the incidence of intraoperative and postoperative retinal breaks [12,13] and this finding could therefore motivate some of the recurrences of RD.
Our percentage of recurrent RD (5.45%, 3 out of 55) is higher than the incidence of RD after vitrectomy for idiopathic macular pathologies [14]. Analyzing this incongruence, we found no evidence in literature regarding possible implications of the encircling band on the recurrence of RD after PPV. As mentioned before, some maneuvers performed at the time of the SB surgery could have altered the basal vitreous, making it difficult to remove it during the vitrectomy procedure, and predisposing its remnants to contraction with a higher risk of RD recurrence. However, we noticed that all our 3 cases of recurrent RD after PPV had an over-tightened encircling SB and PVR in the area in front of the buckle. The formation of anterior PVR may have been exacerbated by the seeding of pigment epithelial cells onto the retinal surface at the time of drainage of subretinal fluid during the SB procedure. Cryotherapy has also been shown to have an exacerbating role in causing PVR [15] by enhancing intravitreal dispersion of RPE cells.
Moreover, the presence of an encircling SB changes the geometry of the scleral wall. In particular, when tightening the buckle, the cross-section of the eyeball is deformed to an ellipsoidal shape [16,17]. If the buckle is over-tightened, the embedding of the band could determine a configuration that is no longer elliptical, but an hourglass. This can even lead to a shortening of the eye [18]. Tight buckles cause a pronounced indentation of the eye wall, making the curvature radius of the wall smaller at its location. The radial inward force from the epiretinal membrane is however inversely proportional to the radius of curvature of the eye wall [1]. Thus, tighter buckles induce a stronger radial inward force applied by the PVR that is found in this area on the retina, and which may facilitate the formation of retinal breaks, playing a role in RD recurrence (Figure 1).
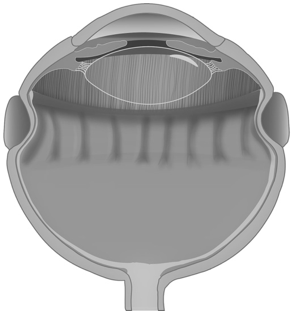
Figure 1: Over-tightened encircling scleral buckle, making the retina redundant. The redundant retina creates radial folds, at which level the adhesion between neuroretina and underlying RPE is less tight.
We wish to report that these patients underwent subsequent multiple surgical interventions to manage recurrences of retinal detachment.
In addition to the technical difficulties already mentioned in performing vitrectomy procedure in patients with encircling SBs, we must remember that the area in front of the encircling buckle, as well as the lower sectors, is where the action of a tamponade agent is most reduced, because of its geometry. This also predisposes the retina to the formation of PVR, which is technically difficult to remove in subsequent surgery due to its position, and this could explain why multiple interventions are necessary in this kind of patient (Figure 2).
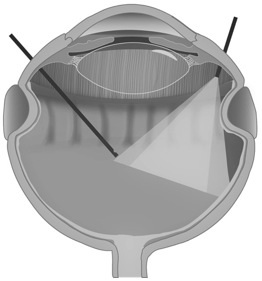
Figure 2: In presence of over-tightened encircling scleral buckle, the removal of the vitreous is made more challenging by the physical presence of the indentation of the scleral wall, which creates a shadow area behind it, making more technically difficult to perform vitrectomy at this level.
Usually we approach “standard” macular surgery by performing an extended vitrectomy without the shaving of the basal vitreous. In light of the results obtained from this study, the latter approach does not seem extensive enough in case of eyes with SB. Instead, we found it safer to perform an accurate vitreous base shaving, in order to avoid vitreous remnants that may induce new breaks and recurrences of retinal detachment. Similarly, in “standard” macular surgery we usually perform laser photocoagulation just on eventual retinal degeneration areas or intraoperative retinal breaks; based on this study, our impression is that in SB patients it is safer to perform 360° peripheral photocoagulation anterior and posterior to back edge of the scleral indentation of the buckle, to reduce the risk of RD recurrence. We have indeed experienced RD recurrences when we performed PPV with a “standard” approach, without basal vitreous shaving nor 360° peripheral photocoagulation. Examining the surgical register, we retrospectively understood that we had to apply an “extended” technique; once we applied it, we had no RD recurrences.
In conclusion, PPV with membrane peeling may restore macular anatomy and improve visual acuity even in secondary cases after RD treated with SB, but performing a PPV on patients with a 360° encircling SB increases the risk of intra-and postoperative complications. We believe the findings presented in this manuscript can open the way to safer and more effective alternatives to the current approaches to PPV surgery after SB procedure.
Each author warrants that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) That might pose a conflict of interest in connection with the submitted article
LIST OF SUPPLEMENTAL DIGITAL CONTENT:
Supplemental Digital Content 1 (PPV in in eyes with 360° encircling band)
References
- Schachat A. P., Wilkinson, C. P., Hinton, D. R. et al. (2017). Ryan's retina e-book. Elsevier Health Sciences,
- Wang, Aijing, and Martin P. Snead. (2019). "Scleral buckling—a brief historical overview and current indications." Graefe's Archive for Clinical and Experimental Ophthalmology 1-12.
- Weng C. Y., Gregori N. Z., Moysidis S. N. et al. (2015). “Visual and anatomical outcomes of macular epiretinal membrane peeling after previous rhegmatogenous retinal detachment repair.” Retina 35.1: 125-135.
- Benzerroug M., Genevois O., Siahmed K. et al. (2008). "Results of surgery on macular holes that develop after rhegmatogenous retinal detachment." British Journal of Ophthalmology 92.2: 217-219
- Shah, R., Byanju, R., Pradhan, S., & Ranabhat, S. (2018). Factors affecting the outcome of scleral buckling surgery for primary rhegmatogenous retinal detachment. Journal of Ophthalmology, 2018.
- Hagler WS, Aturaliya U. (1971). Macular puckers after retinal detachment surgery. Br J Ophthalmol 55: 451–457;
- Cacioppo V., Govetto A., Radice P., Virgili G et al. (2019). "Premacular membrane formation after scleral buckling for primary rhegmatogenous retinal detachment: prospective study and pathophysiological insights." British Journal of Ophthalmology 103.4: 481-487.
- Byon, I. S., Kwon, H. J., Park, G. H., Park, S. W. et al. (2014). Macular hole formation in rhegmatogenous retinal detachment after scleral buckling. Korean Journal of Ophthalmology. 28(5): 364-372.
- Shah, R., Byanju, R., Pradhan, S., & Ranabhat, S. (2018). Factors affecting the outcome of scleral buckling surgery for primary rhegmatogenous retinal detachment. Journal of Ophthalmology, 2018.
- Council, M. D., Shah, G. K., Lee, H. C., & Sharma, S. (2005). Visual outcomes and complications of epiretinal membrane removal secondary to rhegmatogenous retinal detachment. Ophthalmology, 112(7): 1218-1221.
- Vaziri, K., Schwartz, S. G., Kishor, K. S., Fortun, J. A. et al. (2016). Rates of reoperation and retinal detachment after macular hole surgery. Ophthalmology, 123(1), 26-31.
- Chung, Song Ee, Kuk-Hyoe Kim, and Se Woong Kang. (2009). "Retinal breaks associated with the induction of posterior vitreous detachment." American journal of ophthalmology 147.6: 1012-1016.
- Hikichi, T., Kosaka, S., Takami, K., Ariga, H. et al. (2012). Incidence of retinal breaks in eyes undergoing 23-gauge or 20-gauge vitrectomy with induction of posterior vitreous detachment. Retina, 32(6): 1100-1105.
- Guillaubey, A., Malvitte, L., Lafontaine, P. O., Hubert, I. et al. Incidence of retinal detachment after macular surgery: a retrospective study of 634 cases. British journal of ophthalmology, (2007) 91(10), 1327-1330.
- Campochiaro, P. A., Kaden, I. H., Vidaurri-Leal, J., & Glaser, B. M. (1985). Cryotherapy enhances intravitreal dispersion of viable retinal pigment epithelial cells. Archives of Ophthalmology, 103(3), 434-436.
- Lanchares, E., Buey, M. A. D., Cristobal, J. A., Calvo, B. et al. (2016). Computational simulation of scleral buckling surgery for rhegmatogenous retinal detachment: On the effect of the band size on the myopization. Journal of ophthalmology. 2016.
- Heimann, Heinrich, and Silvia Bopp. (2011). "Retinal folds following retinal detachment surgery." Ophthalmologica 226(Suppl. 1): 18-26.
- Vukojevi?, N., Šiki?, J., ?urkovi?, T., Juratovac, Z. et al (2005). Axial eye length after retinal detachment surgery. Collegium antropologicum, 29(1): 25-27.
Citation: Francesco Barca., et al. (2021). Risk of Retinal Detachment Recurrence after Macular Surgery in eyes previously treated with 360° Encircling Band. Journal of Ophthalmology and Vision Research 3(2). DOI: 10.5281/zenodo.5494450
Copyright: © 2021 Francesco Barca. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
