Research Article
Volume 2 Issue 1 - 2020
Phytochemical, Antidiarrhoeal activity, Isolation and Characterisation of 11-Octadecenoic Acid, Methyl ester Isolated from the seeds of Acacia nilotica Linn.
1,2Department of Chemistry, Air force Institute of Technology, Nigerian Air force base, Kaduna, Nigeria
*Corresponding Author: Shoge M, Department of Chemistry, Air force Institute of Technology, Nigerian Air force base, Kaduna, Nigeria.
Received: January 22, 2020; Published: January 31, 2020
Abstrat
The antidiarrhoeal activity of 11-Octadecenoic acid, methyl ester isolated from the seeds of Acacia nilotica was determined using standard methods. The Phytochemical screening and the antidiarrhoeal screening of the crude extracts were determined and compared to the isolated fraction. The fraction was isolated by directing the fractionation of ethyl acetate extract of the air dried seeds with microbial sensitivity test. The result of thephytochemical screening showed that the seeds of Acacia nilotica Linn contains alkaloids, saponins, cardiac glycosides, anthraquinones, steroids, flavonoids,phenols, tannins, terpenoids and triterpenoids. The results of the antidiarrhoeal screening showed that the ethyl acetate extract of the seeds of Acacia nilotica Linn exhibited the highest activities against the test microbes with zones of inhibition diameter ranging from 27-32 mm against Salmonela typhi, Escherichia coli, Streptococcus feacalis, Staphylococcus aureus, Candida krusei and Shigella dysentriae and was subjected to activity guided isolation, leading to the isolation of fraction 21. The structure of the fraction was identified from 13CNMR, 1HNMR, IR and GC-MS spectral data. The isolation, structural elucidation, NMR spectral assignment and bioactivities were reported.
Keywords: Acacia nilotica; Structural elucidation; Antimicrobial activity; Isolation; Spectral data
Introduction
The use of and search for drugs and dietary supplements derived from plants have accelerated in recent years. Ethnopharmacologists, botanists, microbiologists, and natural-products chemists are combing the Earth for phytochemicals and “leads” which could be developed for treatment of infectious diseases. While 25 to 50% of current pharmaceuticals are derived from plants, none are used as antimicrobials. Traditional healers have long used plants to prevent or cure infectious conditions; Western medicine is trying to duplicate their successes. Plants are rich in a wide variety of secondary metabolites, such as tannins, terpenoids, alkaloids, and flavonoids, which have been found in vitro to have antimicrobial properties. This review attempts to summarize the current status of botanical screening efforts, as well as in vivo studies of their effectiveness and toxicity. The structure and antimicrobial properties of phytochemicals are also addressed. Since many of these compounds are currently available as unregulated botanical preparations and their use by the public is increasing rapidly, clinicians need to consider the consequences of patients self-medicating with these preparations [1].
It is against this background that Acacia nilotica extensively used as herbal preparation in some parts of Nigeria were investigated. It is commonly called Bagaruwa in Hausa and Booni by the Yorubas in Nigeria andis used in the treatments of intestinal pains, diarrhea, nerve stimulant, cold, congestion, coughs, dysentery, fever, hemorrhages, leucorrhea, ophthalmia and sclerosis The plants were found to be of medicinal importance among traditional medicine practitioners in the tropics, including West Africa. On a wider dimension, the disease causing organism commonly found in the affected sites of the patients is the target of this research. In this circumstance they are Salmonela typhi, Escherichia coli, Streptococcus feacalis, Staphylococcus aureus, Candida krusei and Shigella dysentriae. The aim of this research is to determine the antimicrobial potentialities of Acacia nilotica and isolate the active compound responsible for the activity.
Materials and Methods
Sample collection
The seeds and pods of Acacia nilotica were collected in Kaduna state. It was identified by Dr Ajibade, at the Herbarium of Biological Sciences, Faculty of Science, Nigerian Defence Academy, Kaduna and assigned a voucher number of 403.
The seeds and pods of Acacia nilotica were collected in Kaduna state. It was identified by Dr Ajibade, at the Herbarium of Biological Sciences, Faculty of Science, Nigerian Defence Academy, Kaduna and assigned a voucher number of 403.
Extraction
A portion (100 g) of the ground seeds was separately percolated in 300 cm3 each of methanol, ethyl acetate, chloroform and petroleum ether each for two weeks. The extracts were separately filtered and evaporated on rotary evaporator at 40°C. The marc was repercolated with the recovered solvents for an additional one week. The extracts were drained, filtered and combined with the previous extracts and evaporated on rotary evaporator. Each extract was cooled, weighed and stored in the refrigerator until needed [2].
A portion (100 g) of the ground seeds was separately percolated in 300 cm3 each of methanol, ethyl acetate, chloroform and petroleum ether each for two weeks. The extracts were separately filtered and evaporated on rotary evaporator at 40°C. The marc was repercolated with the recovered solvents for an additional one week. The extracts were drained, filtered and combined with the previous extracts and evaporated on rotary evaporator. Each extract was cooled, weighed and stored in the refrigerator until needed [2].
Procedure for phytochemical screening
The presence of bioactive compounds in the chloroform, ethyl-acetate, methanol and petroleum ether extracts of the seeds Acacia nilotica were obtained using standard method [3].
The presence of bioactive compounds in the chloroform, ethyl-acetate, methanol and petroleum ether extracts of the seeds Acacia nilotica were obtained using standard method [3].
Column chromatography of theethyl acetate fraction
Ethyl acetate fraction that showed higher activity in most of the tested microbes was subjected to column chromatography. A portion (20g) of the fraction was dissolved in 80mls of ethylacetate and mixed with 15g of silica gel. It was evaporated to dryness in a water bath. The dried extract and silica gel were loaded on the column together with 10g of Celite. The column was first eluded with 6:4 Ethyl acetate: Petroleum ether. This was followed by 8:2 Ethyl acetate: Petroleum ether, 100% Ethyl acetate, 1:1 Ethyl acetate: Methanol and finally 100% Methanol. Each portion collected were evaporated using rotary evaporator [4]. A total of 83 fractions were collected and labeled from 1 to 83. These fractions were subjected to Thin Layer Chromatography and similar fractions were pooled together.
Ethyl acetate fraction that showed higher activity in most of the tested microbes was subjected to column chromatography. A portion (20g) of the fraction was dissolved in 80mls of ethylacetate and mixed with 15g of silica gel. It was evaporated to dryness in a water bath. The dried extract and silica gel were loaded on the column together with 10g of Celite. The column was first eluded with 6:4 Ethyl acetate: Petroleum ether. This was followed by 8:2 Ethyl acetate: Petroleum ether, 100% Ethyl acetate, 1:1 Ethyl acetate: Methanol and finally 100% Methanol. Each portion collected were evaporated using rotary evaporator [4]. A total of 83 fractions were collected and labeled from 1 to 83. These fractions were subjected to Thin Layer Chromatography and similar fractions were pooled together.
Thin Layer Chromatography (TLC)
Fraction 21 was one of the samples that gave a single spot on the TLC plates with an Rf value of 0.60cm using 8:2 Petroleum ether: Ethylacetate, Rf of 0.65cm using 7:3 Petroleum ether: Ethylacetate and 0.55cm using 9:1 Petroleum ether: Ethylacetate. It was therefore chosen for antidiarrhoeal activity, isolation and characterization.
Fraction 21 was one of the samples that gave a single spot on the TLC plates with an Rf value of 0.60cm using 8:2 Petroleum ether: Ethylacetate, Rf of 0.65cm using 7:3 Petroleum ether: Ethylacetate and 0.55cm using 9:1 Petroleum ether: Ethylacetate. It was therefore chosen for antidiarrhoeal activity, isolation and characterization.
Antidiarrhoeal activity of fraction 21.
The isolates of microbes were obtained from the Department of Medical Microbiology, Ahmadu Bello University Teaching Hospital Zaria from which the zone of inhibition, Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of fraction 21were determined against the collected isolates of Salmonela typhi, Escherichia coli, Streptococcus feacalis, Staphylococcus aureus, Candida krusei and Shigella dysentriaeThe antidiarrhoeal activities of fraction 21 was determined using agar well diffusion methods [5] and [6].
The isolates of microbes were obtained from the Department of Medical Microbiology, Ahmadu Bello University Teaching Hospital Zaria from which the zone of inhibition, Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of fraction 21were determined against the collected isolates of Salmonela typhi, Escherichia coli, Streptococcus feacalis, Staphylococcus aureus, Candida krusei and Shigella dysentriaeThe antidiarrhoeal activities of fraction 21 was determined using agar well diffusion methods [5] and [6].
Results and Discussion
Table 1 shows the result of phytochemical screening of the crude extracts of the seeds of Acacia nilotica Linn; Table 2 shows the results of Antidiarrhoeal sensitivity test of the raw extracts of the seeds Acacia nilotica Linn; Tables 3,4,5 and 6 show the results of the Minimum Inhibition Concentration (MIC) of the crude extracts of Acacia nilotica against the test microorganisms; Tables 7, 8, 9 and 10 show the results of the Minimum Bactericidal Concentration (MBC) of the crude extracts of Acacia nilotica against the test microorganisms; Table 11 shows the results of antidiarrhoeal assay of fraction 21; Table 12 shows the MIC of fraction 21 and the standard drug against the test microbes; Table 13 shows the MBC of fraction 21 and the standard drug against the test microbes.
| Solvents | Al. | Sa. | CG | Cd. G | F | Aq | St. | Ph | Tn | Tp | Ttp |
| Chloroform | + | + | _ | _ | + | _ | + | + | + | + | + |
| Ethylacetate | + | + | _ | _ | + | _ | + | + | + | + | + |
| Methanol | + | + | _ | _ | + | _ | + | + | + | + | + |
| Pet-ether | + | + | _ | _ | + | _ | + | + | + | + | + |
KEY; + - presence, _ - absence, Al - Alkaloids, Sa - Saponins, CG - Cyanogenic Glycosides, Cd. G - Cardiac glycosides, F - Flavonoids, Aq - Anthraquinones, St - Steroids, Ph - Phenols, Tn - Tannins, Tp – Terpenoids, Ttp – Triterpenoids
Table 1: Results of phytochemical screening of the crude extracts of the seeds of Acacia nilotica Linn.
Table 1: Results of phytochemical screening of the crude extracts of the seeds of Acacia nilotica Linn.
The results of the phytochemical screening of the chloroform, ethylacetate, methanol and petroleum ether extracts of Acacia nilotica Linn. showed that it contains saponins, alkaloids, steroids, flavonoids, phenols, tannins, terpenoids and triterpenoids (see Table 1). These chemical compounds occur naturally in plants, especially Tannins and Flavonoids are responsible for antidiarrhoeal activity by increasing colonic water and electrolyte reabsorption [9].
| Extracting solvents | C(µg/cm3(102) | Ec | Vc | Sf | Sd | St | Se | Cs | Ck |
| Chloroform | 4 | NI | NI | 8 | 9 | NI | 12 | NI | NI |
| 5 | 10 | 11 | 17 | 15 | NI | 8 | NI | 14 | |
| 6 | 15 | 17 | 22 | 19 | NI | 13 | NI | 18 | |
| 7 | 20 | 19 | 23 | 22 | NI | 15 | NI | 21 | |
| 8 | 25 | 20 | 27 | 24 | NI | 22 | NI | 24 | |
| C1 | 37 | NI | 41 | 42 | 47 | NI | 39 | NI | |
| C2 | NI | NI | NI | NI | NI | NI | 35 | NI | |
| Ethylacetate | 4 | NI | 8 | 13 | 9 | NI | 17 | NI | 8 |
| 5 | 10 | 13 | 17 | 12 | NI | 18 | NI | 11 | |
| 6 | 15 | 15 | 22 | 15 | NI | 20 | NI | 13 | |
| 7 | 25 | 20 | 25 | 27 | NI | 28 | NI | 26 | |
| 8 | 27 | 27 | 32 | 30 | NI | 30 | NI | 29 | |
| C1 | 37 | NI | 41 | 42 | 47 | NI | 39 | NI | |
| C2 | NI | NI | NI | NI | NI | NI | NI | 35 | |
| Methanol | 4 | NI | NI | 5 | 11 | NI | 11 | NI | 12 |
| 5 | NI | NI | 12 | 13 | NI | 15 | NI | 17 | |
| 6 | NI | 7 | 15 | 17 | NI | 18 | NI | 18 | |
| 7 | NI | 11 | 20 | 22 | NI | 19 | NI | 19 | |
| 8 | 22 | 21 | 24 | 21 | NI | 20 | NI | 21 | |
| C1 | 37 | NI | 41 | 42 | 47 | NI | 39 | NI | |
| C2 | NI | NI | NI | NI | NI | NI | NI | 35 | |
| Pet-ether | 4 | NI | NI | NI | NI | NI | 5 | NI | 7 |
| 5 | NI | NI | NI | 5 | NI | 9 | NI | 11 | |
| 6 | NI | NI | 7 | 7 | NI | 6 | NI | 13 | |
| 7 | NI | 11 | 13 | 15 | NI | 5 | NI | 19 | |
| 8 | 19 | 18 | 18 | 17 | NI | 18 | NI | 18 | |
| C1 | 37 | NI | 41 | 42 | 47 | NI | 39 | NI | |
| C2 | NI | NI | NI | NI | NI | NI | NI | 35 | |
Zone of inhibition diameter (mm)
KEY; NI-No inhibition, C1- Reference standard 1 (Ciprofloxacin=50µg/cm3), C2- Reference standard 2 (Fluconazole=50µg/cm3), Ec-Escherichia coli, Vc-Vibro cholera, Sf- Streptococcus feacalis, Sd-Shigella dysentriae, St-Salmonella typhi, Se-Salmonella enteritidis, Cs-Campylobacter sp., Ck-Candida krusei.
Table 2: Results of Antidiarrhoeal sensitivity test of the crude extracts of the seeds of Acacia nilotica Linn.
KEY; NI-No inhibition, C1- Reference standard 1 (Ciprofloxacin=50µg/cm3), C2- Reference standard 2 (Fluconazole=50µg/cm3), Ec-Escherichia coli, Vc-Vibro cholera, Sf- Streptococcus feacalis, Sd-Shigella dysentriae, St-Salmonella typhi, Se-Salmonella enteritidis, Cs-Campylobacter sp., Ck-Candida krusei.
Table 2: Results of Antidiarrhoeal sensitivity test of the crude extracts of the seeds of Acacia nilotica Linn.
| Organisms | Conc (x102) µg/cm3 |
Colour change | MIC (x102) µg/cm3 |
MIC of Ciprofloxacin (x 102 µg/cm3) |
| E. coli | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| V. cholerae | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| S. feacalis | 8 | None | ||
| 7 | None | |||
| 6 | None | |||
| 5 | None | 5 | 6 | |
| 4 | Light pink | |||
| S. dysentriae | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| S. enteritidis | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| C. krusei | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink |
Table 3: Results of the Minimum Inhibition Concentration (MIC) of the crude chloroform extract of Acacia nilotica against the test microorganisms.
| Organisms | Conc (x102) µg/cm3 |
Colour change | MIC (x102) µg/cm3 |
MIC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | ||
| 7 | None | |||
| 6 | None | |||
| 5 | None | 5 | 6 | |
| 4 | Light pink | |||
| V. cholerae | 8 | None | ||
| 7 | None | |||
| 6 | None | |||
| 5 | None | 5 | 6 | |
| 4 | Light pink | |||
| S. feacalis | 8 | None | ||
| 7 | None | |||
| 6 | None | |||
| 5 | None | 5 | 6 | |
| 4 | Light pink | |||
| S. dysentrae | 8 | None | ||
| 7 | None | |||
| 6 | None | |||
| 5 | None | 5 | 6 | |
| 4 | Light pink | |||
| S. enteritidis | 8 | None | ||
| 7 | None | |||
| 6 | None | |||
| 5 | None | 5 | 6 | |
| 4 | Light pink | |||
| 4 | Light pink | |||
| C. krusei | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink |
Table 4: Results of the Minimum Inhibition Concentration (MIC) of the crude ethylacetate extract of Acacia nilotica against the test microorganisms.
| Organisms | Conc (x102) µg/cm3 | Colour change | MIC (x102) µg/cm3 | MIC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| V. cholerae | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| S. feacalis | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| S. dysentriae | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| S. enteritidis | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink | |||
| C. krusei | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 6 | |
| 5 | Light pink | |||
| 4 | Moderate pink |
Table 5: Results of the Minimum Inhibition Concentration (MIC) of the crude methanol extract of Acacia nilotica against the test microorganisms.
| Organisms | Conc (x102) µg/cm3 | Colour change | MIC (102) µg/cm3 | MIC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | ||
| 7 | None | 7 | 6 | |
| 6 | Light pink | |||
| 5 | Moderate pink | |||
| 4 | Deep pink | |||
| V. cholerae | 8 | None | ||
| 7 | None | 7 | 6 | |
| 6 | Light pink | |||
| 5 | Moderate pink | |||
| 4 | Deep pink | |||
| S. feacalis | 8 | None | ||
| 7 | None | 7 | 6 | |
| 6 | Light pink | |||
| 5 | Moderate pink | |||
| 4 | Deep pink | |||
| S. dysentriae | 8 | None | ||
| 7 | None | 7 | 6 | |
| 6 | Light pink | |||
| 5 | Moderate pink | |||
| 4 | Deep pink | |||
| S. enteritidis | 8 | None | ||
| 7 | None | 7 | 6 | |
| 6 | Light pink | |||
| 5 | Moderate pink | |||
| 4 | Deep pink | |||
| C. krusei | 8 | None | ||
| 7 | None | 7 | 6 | |
| 6 | Light pink | |||
| 5 | Moderate pink | |||
| 4 | Deep pink |
Table 6: Results of the Minimum Inhibition Concentration (MIC) of the crude petroleum ether extract of Acacia nilotica against the test microorganisms.
The results of the MIC of the crude chloroform extracts of Acacia nilotica (see Table 3) shows MIC value of 500 µg/cm3 against Streptococcus feacalis and MIC value of 600 µg/cm3 against Escherichia coli, Vibro cholera, Shigella dysentriae, Salmonella enteritidis and Candida krusei. The ethylacetate extract of Acacia nilotica (Table 4) shows MIC value of 600 µg/cm3 against Candida krusei and MIC value of 500 µg/cm3 against Escherichia coli, Vibro cholera, Shigella dysentriae, Salmonella enteritidis and Streptococcus feacalis. The reference standard (Ampicloxacillin) shows MIC value of 600 µg/cm3. The crude methanol extract of Acacia nilotica (Table 5) shows MIC value of 600 µg/cm3 against the test microbes while the crude petroleum ether extract of Acacia nilotica (see Table 6) shows MIC value of 700 µg/cm3 against the test microbes. The reference standard (Ciprofloxacin) shows MIC value of 600 µg/cm3 for all the extracts, indicating that the extracts of Acacia nilotica shows moderate to high activity against the test microbes with the ethylacetate extract being the most active, with MIC value of 500 µg/cm3 against all the test microbes except Candida.
| Organisms | Conc (x102) µg/cm3 | Colony growth | MBC (x102) µg/cm3 | MBC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| V. cholerae | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| S. feacalis | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| S. dysentriae | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| S. enteritidis | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| C. krusei | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy |
Table 7: Results of the Minimum Bactericidal Concentration (MBC) of the crude chloroform extract of Acacia nilotica against the test microorganisms.
| Organisms | Conc (x102) µg/cm3 | Colony growth | MBC (x102) µg/cm3 | MBC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| V. cholerae | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| S. feacalis | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 8 | |
| 5 | Scanty | |||
| 4 | Moderate | |||
| S. dysentriae | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 8 | |
| 5 | Scanty | |||
| 4 | Moderate | |||
| S. enteritidis | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 8 | |
| 5 | Scanty | |||
| 4 | Moderate | |||
| C. krusei | 8 | None | ||
| 7 | None | |||
| 6 | None | 6 | 8 | |
| 5 | Scanty | |||
| 4 | Moderate |
Table 8: Results of the Minimum Bactericidal Concentration (MBC) of the crude ethylacetate extract of Acacia nilotica against the test microorganisms.
| Organisms | Conc (x102) µg/cm3 | Colony growth | MBC (x102) µg/cm3 | MBC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Very Heavy | |||
| V. cholerae | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| S. feacalis | 8 | None | ||
| 7 | None | 7 | 8 | |
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| S. dysentriae | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| S. enteritidis | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| C. krusei | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy |
Table 9: Results of the Minimum Bactericidal Concentration (MBC) of the crude methanol extract of Acacia nilotica against the test microorganisms.
| Organisms | Conc (µg/cm3) (x102) | Colony growth | MBC (x102) µg/cm3 | MBC of Ciprofloxacin (x102 µg/cm3) |
| E. coli | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| V. cholerae | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| S. feacalis | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| S. dysentriae | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Moderate | |||
| 4 | Heavy | |||
| S. enteritidis | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Moderate | |||
| 5 | Heavy | |||
| 4 | Heavy | |||
| C. krusei | 8 | None | 8 | 8 |
| 7 | Scanty | |||
| 6 | Scanty | |||
| 5 | Moderate | |||
| 4 | Heavy |
Table 10: Results of the Minimum Bactericidal Concentration (MBC) of the crude petroleum ether extract of Acacia nilotica against the test microorganisms.
The results of the MBC of the crude chloroform extracts of Acacia nilotica (see Table 7) shows MBC value of 700 µg/cm3 against Streptococcus feacalis, Escherichia coli, Shigella dysentrae and Candida krusei and MBC value of 800 µg/cm3 against Vibro cholera and Salnonella enteritidis. The crude ethylacetate extract of Acacia nilotica (Table 8) shows MBC value of 700 µg/cm3 against Escherichia coli and Vibro cholerae and MBC value of 600 µg/cm3 against Salmonella feacalis, Shigella dysentriae, Salmonella enteritidis and Candida krusei. The crude methanol extract(Table 9) shows MBC value of 700 µg/cm3 against Streptococcus feacalis and MBC value of 800 µg/cm3 against Escherichia coli, Vibro cholera, Shigella dysentriae, Salmonella enteritidis and Candida krusei. The crude petroleum ether extractof Acacia nilotica (Table 10) shows MBC value of 800 µg/cm3 against the test microbes. The reference standard (Ciprofloxacin) shows MBC value of 800 µg/cm3 for all the extracts, indicating that the extracts of Acacia nilotica shows moderate to high activity against the test microbes.
| Organisms | Concentration (µg/cm3) | Zone of inhibition diameter (mm) |
| Shigella dysentriae | 1000 | 15 |
| 500 | No inhibition | |
| 250 | No inhibition | |
| Escherichia coli | 1000 | 16 |
| 500 | 9 | |
| 250 | 5 | |
| Staphylococcus aureus | 1000 | 25 |
| 500 | 20 | |
| 250 | 17 | |
| Salmonella typhi | 1000 | 20 |
| 500 | 18 | |
| 250 | 12 | |
| Streptococcus feacalis | 1000 | 30 |
| 500 | 24 | |
| 250 | 20 | |
| Candida krusei | 1000 | No inhibition |
| 500 | No inhibition | |
| 250 | No inhibition |
Table 11: Results of antidiarrhoeal assay of fraction 21.
At 1000 µg/cm3, fraction 21 exhibited zone of inhibition diameter of 15, 16, 25, 20 and 30 mm against Shigella dysentriae, Escherichia coli, Staphylococcus aureus, Salmonella typhi and Streptococcus feacalis respectively while it shows no activity against Candida krusei at this concentration. At 500 µg/cm3, fraction 21 exhibited moderate activities (zone of inhibition diameter of 9, 20, 18 and 24 mm respectively) against Escherichia coli, Staphylococcus aureus, Salmonella typhi and Streptococcus feacalis while it is inactive against Shigella dysentriae and Candida krusei. At 250 µg/cm3, fraction 21 generally exhibited moderate activities (zone of inhibition diameters of 5, 17, 12 and 20 mm respectively) against Escherichia coli, Staphylococcus aureus, Salmonella typhi and Streptococcus feacalis while it is inactive against Shigella dysentriae and Candida krusei.
| Organisms | Concentrations (x102 µg/cm3) | Colour change | MIC (x102 µg/cm3) | MIC of Ampicloxacillin (x102 µg/cm3) |
| S. dysentriae | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Light pink | |||
| 2 | Light pink | |||
| 1 | Light pink | |||
| 0.5 | Pink | |||
| E. coli | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Light pink | |||
| 2 | Light pink | |||
| 1 | Light pink | |||
| 0.5 | Pink | |||
| S. aureus | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Light pink | |||
| 2 | Light pink | |||
| 1 | Light pink | |||
| 0.5 | Pink | |||
| S. typhi | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Light pink | |||
| 2 | Light pink | |||
| 1 | Light pink | |||
| 0.5 | Pink | |||
| S. feacalis | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Light pink | |||
| 2 | Light pink | |||
| 1 | Light pink | |||
| 0.5 | Pink | |||
| C. krusei | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Light pink | |||
| 2 | Light pink | |||
| 1 | Light pink | |||
| 0.5 | Pink |
Table 12: The MIC of fraction 21 and the standard drug against the test microbes.
Fraction 21 showed MIC value of 600 µg/cm3 against Shigella dysentriae, Escherichia coli, Staphylococcus aureus, Salmonella typhi, Streptococcus feacalis and Candida krusei when compared to ampicloxacillin (reference standard) with MIC value of 800 µg/cm3, which shows that fraction 21 is more active than the reference standard.
| Organisms | Concentrations (x102 µg/cm3) | Colony growth | MBC (x102 µg/cm3) | MBC of Ampicloxacillin (x102 µg/cm3) |
| S. dysentriae | 10 | None | ||
| 8 | None | 8 | 8 | |
| 6 | Scanty | |||
| 4 | Moderate | |||
| 2 | Moderate | |||
| 1 | High | |||
| 0.5 | High | |||
| E. coli | 10 | None | ||
| 8 | None | 8 | 8 | |
| 6 | Scanty | |||
| 4 | Moderate | |||
| 2 | Moderate | |||
| 1 | High | |||
| 0.5 | High | |||
| S. aureus | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Scanty | |||
| 2 | Scanty | |||
| 1 | Moderate | |||
| 0.5 | High | |||
| S. typhi | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Scanty | |||
| 2 | Scanty | |||
| 1 | High | |||
| 0.5 | High | |||
| S. feacalis | 10 | None | ||
| 8 | None | |||
| 6 | None | 6 | 8 | |
| 4 | Scanty | |||
| 2 | Scanty | |||
| 1 | High | |||
| 0.5 | High | |||
| C. krusei | 10 | None | ||
| 8 | None | 8 | 8 | |
| 6 | Scanty | |||
| 4 | Scanty | |||
| 2 | Moderate | |||
| 1 | High | |||
| 0.5 | High |
Table 13: The MBC of compound F21 and the standard drug against the test microbes.
The results of the Minimum bactericidal Concentration (MBC) tests of fraction 21 (Table 4.53 to 4.58) showed that it exhibited MBC values of 800 µg/cm3 against Shigella dysentriae, Escherichia coli, Candida krusei and MBC value of 600 µg/cm3 against Staphylococcus aureus, Salmonella typhi and Streptococcus feacalis while the reference standard (ampicloxacillin) showed MBC value at 800 µg/cm3 against Shigella dysentriae, Escherichia coli, Staphylococcus aureus, Salmonella typhi, Streptococcus feacalis and Candida krusei.
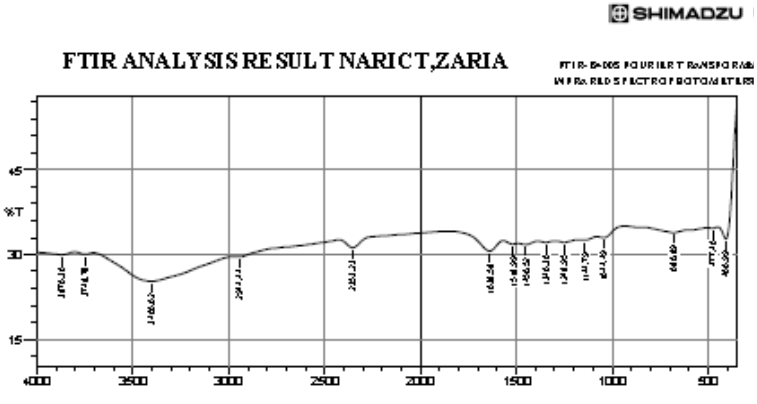
Infra-red analysis
The IR spectrum showed absorption at 1638.58 cm-1 which is due to the carbonyl stretching of an ester. 680.89 cm-1 which is due to C=H of alkenes. 2,944.44 cm-1 which is due to C-H of alkanes. Absorption at 2,353.23 cm-1 is due to C=C of alkenes. Absorption at 1,144.79 cm-1 is due to the presence of C-O-C stretch of an ester [7].
The IR spectrum showed absorption at 1638.58 cm-1 which is due to the carbonyl stretching of an ester. 680.89 cm-1 which is due to C=H of alkenes. 2,944.44 cm-1 which is due to C-H of alkanes. Absorption at 2,353.23 cm-1 is due to C=C of alkenes. Absorption at 1,144.79 cm-1 is due to the presence of C-O-C stretch of an ester [7].
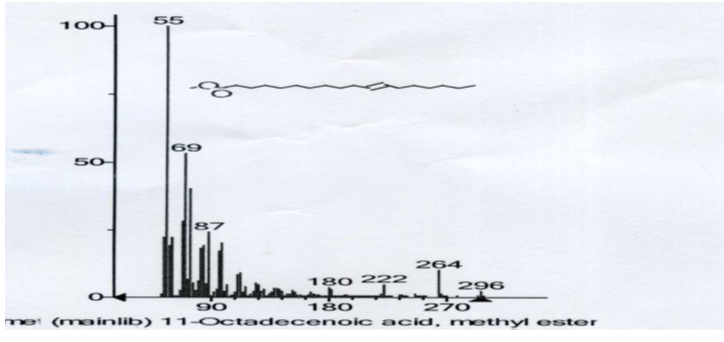
GC-MS Analysis
The GC/MS gave the molecular weight of the molecule as 296. The signal at m/z 265 correspond to the loss of CH3O+, the signal at 222, correspond to the loss of C3H6+, the signal at 180 also correspond to the loss of C3H6+, the signal at 87, correspond to the loss of C7H9+, at 69, the signal correspond to the loss of H2O molecule and at 55, which is the base peak, correspond to the loss of CH2+.
The GC/MS gave the molecular weight of the molecule as 296. The signal at m/z 265 correspond to the loss of CH3O+, the signal at 222, correspond to the loss of C3H6+, the signal at 180 also correspond to the loss of C3H6+, the signal at 87, correspond to the loss of C7H9+, at 69, the signal correspond to the loss of H2O molecule and at 55, which is the base peak, correspond to the loss of CH2+.
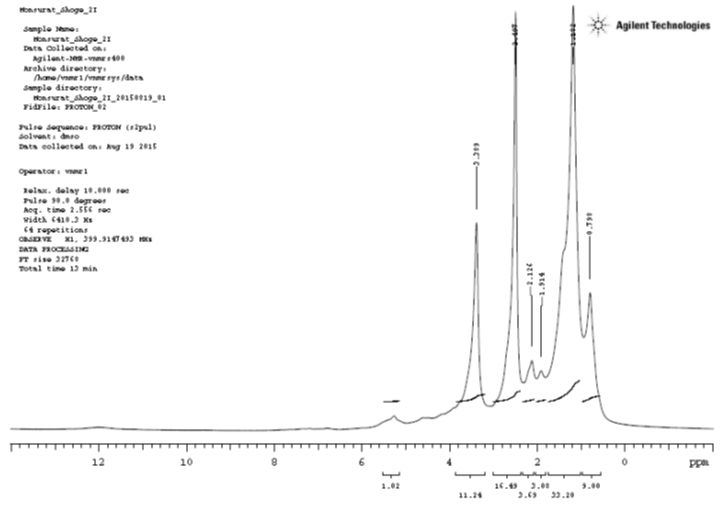
1HNMR Analysis
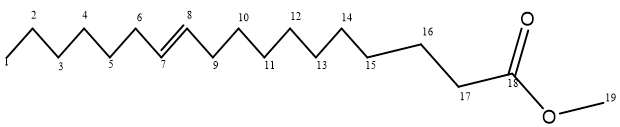
1HNMRrecorded signals at δ0.789 which is a methyl proton (C1); signal at δ1.182 correspond to the methylene protons (C2, C3, C4, C5, C11, C12, C13 and C14); the signal at δ1.914 correspond to the methylene protons (C15 and C16); the signal at δ2.126 correspond to the methylene protons (C6 and C9); the signal at δ2.497 correspond to the methylene proton (C17); the signal at δ3.389 correspond to the methyl proton (C20); the signal at δ5.4 correspond to the methine proton (C7 and 8).
1HNMRrecorded signals at δ0.789 which is a methyl proton (C1); signal at δ1.182 correspond to the methylene protons (C2, C3, C4, C5, C11, C12, C13 and C14); the signal at δ1.914 correspond to the methylene protons (C15 and C16); the signal at δ2.126 correspond to the methylene protons (C6 and C9); the signal at δ2.497 correspond to the methylene proton (C17); the signal at δ3.389 correspond to the methyl proton (C20); the signal at δ5.4 correspond to the methine proton (C7 and 8).
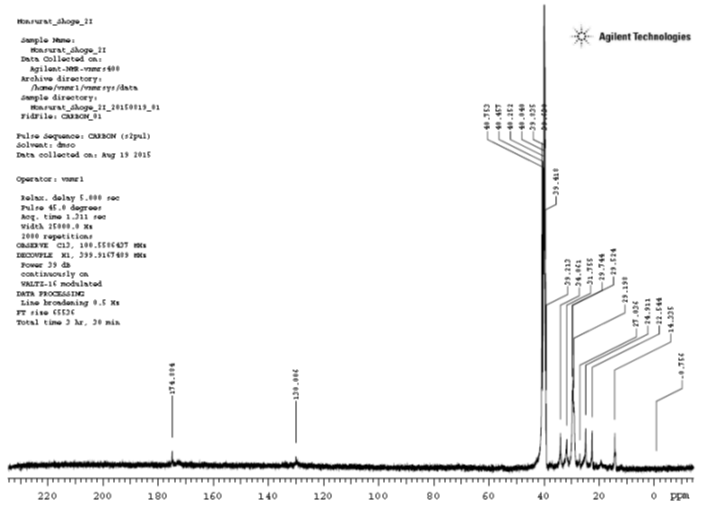
13CNMR Analysis
The 13CNMR spectrum had signals recorded at δ174.884 which is due to the carbonyl carbon (C18); the signal at δ130.006 is due to the methine carbon (C7 and C8); the signal at δ40.753 is due to the methyl carbon (C19); the signal at δ40.457 correspond to the carbon bonded to the –COO group (C17); the signal at δ40.252 correspond to the carbon bonded to the double bond (C6); the signal at δ40.04 correspond to the methylene carbon (C16); the signal at δ39.835 correspond to the methylene carbon (C5 and C6); the signal at δ39.63 correspond to the methylene carbon (C15); the signal at δ39.418 is due to the methylene group (C9 and C10); the signal at δ34.061 is due to the methylene group (C14); the signal at δ31.755 is due to the methylene group (C14); the signal at δ29.744 is due to the methylene group (C13); the signal at δ29.524 is due to the methylene group (C12); the signal at δ29.198 is due to the methylene group (C11); the signal at δ27.036 is due to the methylene group (C4); the signal at δ24.911 is due to the methylene group (C3); the signal at δ22.544 is due to the methylene group (C2); the signal at δ14.355 is due to the methyl group (C1).
The 13CNMR spectrum had signals recorded at δ174.884 which is due to the carbonyl carbon (C18); the signal at δ130.006 is due to the methine carbon (C7 and C8); the signal at δ40.753 is due to the methyl carbon (C19); the signal at δ40.457 correspond to the carbon bonded to the –COO group (C17); the signal at δ40.252 correspond to the carbon bonded to the double bond (C6); the signal at δ40.04 correspond to the methylene carbon (C16); the signal at δ39.835 correspond to the methylene carbon (C5 and C6); the signal at δ39.63 correspond to the methylene carbon (C15); the signal at δ39.418 is due to the methylene group (C9 and C10); the signal at δ34.061 is due to the methylene group (C14); the signal at δ31.755 is due to the methylene group (C14); the signal at δ29.744 is due to the methylene group (C13); the signal at δ29.524 is due to the methylene group (C12); the signal at δ29.198 is due to the methylene group (C11); the signal at δ27.036 is due to the methylene group (C4); the signal at δ24.911 is due to the methylene group (C3); the signal at δ22.544 is due to the methylene group (C2); the signal at δ14.355 is due to the methyl group (C1).
Conclusion
The ethyl acetate extract of the seeds of Acacia nilotica Linn was found to have higher activities against the test microbes. Chromatographic separation and thin layer chromatography carried out on it led to the isolation of a compound with a melting point of 80°C. Structural elucidation using 1HNMR, 13CNMR, IR and GC-MS showed that the compound is 11-Octadecenoic acid, methyl ester.
Acknowledgement
Authors acknowledge the contribution of Alhaji Muhammad Munir, Mr Amusan, all the laboratory technologist and staffs of Chemistry Department, Nigerian Defence Academy, Kaduna and Chemistry department, Air force Institute of Technology Kaduna, for their contributions towards the success of this research work.
Authors acknowledge the contribution of Alhaji Muhammad Munir, Mr Amusan, all the laboratory technologist and staffs of Chemistry Department, Nigerian Defence Academy, Kaduna and Chemistry department, Air force Institute of Technology Kaduna, for their contributions towards the success of this research work.
References
- Kathe W. (2005). The revision of the “WHO/IUCN/WWF guidelines on the conservation of medicinal plants”: a step forward in medicinal plant conservation and sustainable use. Herbal Gram. 66: 60-61.
- Anees T.P. (2010). International market scenario of traditional Indian herbal drugs: India declining. Int. J. Green. Pharm., 122: 184-190.
- Harborne, J. B. (1984). “Phytochemical methods, a guide to modern techniques in plants analysis”, 2nd edition, Chapman and Hall, London; pp. 1-10, 100-117.
- Garba S, Okeniyi SO, 2012. Antimicrobial activities of total alkaloids extracted from some Nigerian medicinal plants. J Microbiology and Antimicrob, 4(3): 60-63.
- Cannell RJP, 1998. Natural Products Isolation. GlaxoWellcome Research & Development, Stevenage, Herts, UK.
- Navorro V, Villaeal ML, Rojas G, Lozoya X, (1996). Antimicrobial evaluation of some medicinal plants used in Mexican traditional medicine for the treatment of infectious diseases. J Ethnopharmacol, 53: 143-147.
- Okeke MI, Iroegbu CU, Eze EN, Okoli AS, Esimone CO, (2001). Evaluation of extracts of the root of Landolphia owerrience for antimicrobial activity. J Ethnopharmacol, 78(2): 119-127.
- Williams and Fleming, (2008). Spectroscopic methods in organic chemistry. (Mc-Graw Hill), 6th edition, p27.
- Palombo, E. A. (2006). Phytochemicals from traditional medicinal plants used in the treatments of diarrhoea: modes of action and effects on intestinal function. Phytother Res; 20(9): pp717-724.
Citation: Shoge M and Amusan T. (2020). Phytochemical, Antidiarrhoeal activity, Isolation and Characterisation of 11-Octadecenoic Acid, Methyl ester Isolated from the seeds of Acacia nilotica Linn. Journal of Biotechnology and Immunology 2(1). DOI: 10.5281/zenodo.3669434
Copyright: © 2020 Shoge M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.