Review Article
Volume 2 Issue 3 - 2020
Physiological Relevance of the TLQP-21 and HSPA8 Interaction: A Review
Biotechnology and Genetic Engineering Discipline, Khulna University, Khulna-9208, Bangladesh.
*Corresponding Author: Md. Shamim Akhter, Biotechnology and Genetic Engineering Discipline, Khulna University, Khulna-9208, Bangladesh. Orcid id: 0000-0001-5630-3906
Received: May 11, 2020; Published: June 01, 2020
Abstract
By means of affinity chromatography and mass spectrometry-based protein identification, the heat shock cognate 71 kDa protein A8 (HSPA8) has been identified as a receptor of human TLQP-21. Cross-linking and FACS studies also established the binding of TLQP-21 to membrane associated HSPA8 in live SH-SY5Y cells. Additionally, TLQP-21 was found, as per in silico molecular modeling studies, to fit (docked) very well into the peptide binding pocket of HSPA8. So, it is expected that there would be physiological relevance between TLQP-21 and HSPA8 interaction. The aim of the review is to show the physiological relevance of the TLQP-21 and HSPA8 interaction. The physiological relevance of the TLQP-21 and HSPA8 interaction was evident in energy homeostasis, obesity, diabetes, reproduction, gastric acid secretion and hypertension. The major task moving forward is to elucidate the signaling pathways of the physiological relevance of the ligand TLQP-21 and its receptor HSPA8 to discover a new avenue for remedies where TLQP-21 has been found to have relation with human diseases.
Key words: TLQP-21; HSPA8; Physiological relevance; HSP family.
Abbreviations: CaM: calmodulin; FACS: Fluorescence-activated cell sorting; HSPA8: Heat shock cognate protein A8; HSPs: Heat shock proteins; HSP1A: Heat shock protein 1A; HSP70: Heat shock protein 70 (family); HPG: Hypothalamic-pituitary-gonadal; kDa: Kilo Dalton; Da: Dalton; MS: Mass spectrometry; SRY: Sex determining region on the Y chromosome; SH-SY5Y: A thrice cloned (SK-N-SH → SH-SY → SH-SY5 → SH-SY5Y) subline of the neuroblastoma cell line; TLQP-21: The first four amino acids, in short TLQP (Thr-Leu-Gln-Pro) generalizes the nomenclature of the peptide by its length; T2DM: Type 2 diabetes mellitus; VGF: Non acronymic, a neurotrophin; WAT: White adipose tissue
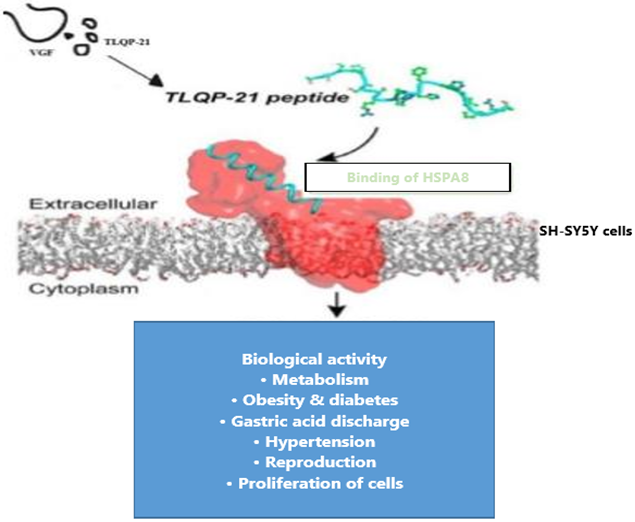
Graphical abstract: physiological relevance of the TLQP-21 and HSPA8 interaction (Modified from Cero et al., 2014).
Introduction
Out of several bioactive peptides derived from VGF, TLQP-21 is of great importance because of its many physiological roles. TLQP 21 plays a key role in energy expenditure (Possenti et al., 2012; Jethwa et al., 2007; Bartolomucci et al., 2006), metabolic functions (Bartolomucci et al., 2008), lipolysis (Possenti et al., 2012), glucose-stimulated insulin secretion (GSIS) ( Stephens et al., 2012), nociception (Fairbanks et al., 2014; Chen et al., 2013; Rizzi et al., 2008), blood pressure/hypertension regulation (Fargali et al., 2014), gastric contractility (Severini et al., 2009; Bartolomucci et al., 2008), regulation of gastric acid secretion ( Sibilia et al., 2012; Sibilia et al., 2010a; 2010b) reproduction (Aguilar et al., 2013; Pinilla et al., 2011), stress (Razzoli et al., 2012, Bartolomucci et al., 2011), neuroprotective agent (Severini et al., 2008), anorexia ( Bartolomucci et al., 2006; Jethwa et al., 2007).
TLQP-21 (human) is of molecular weight 2490.88 Da. The peptide sequence is –
TLQPPSALRRRHYHHALPPSR
Thr - Leu - Gln - Pro - Pro - Ser - Ala - Leu - Arg - Arg - Arg - His - Tyr His - His - Ala - Leu - Pro - Pro - Ser – Arg
TLQPPSALRRRHYHHALPPSR
Thr - Leu - Gln - Pro - Pro - Ser - Ala - Leu - Arg - Arg - Arg - His - Tyr His - His - Ala - Leu - Pro - Pro - Ser – Arg
HSPA8
HSPA8 (Heat shock cognate protein A8, 71 kDa), a constitutively expressed protein, is a fascinating member of the HSP70 (Heat shock protein) family. It is also referred to as stress inducible protein.
HSPA8 (Heat shock cognate protein A8, 71 kDa), a constitutively expressed protein, is a fascinating member of the HSP70 (Heat shock protein) family. It is also referred to as stress inducible protein.
While HSPA8 is usually localized within the cytoplasm and nucleus, where it carries out a variety of functions that are discussed below, it can also be found in a membrane-bound manner on cell surfaces, particularly in cancer cells, undifferentiated human embryonic stem cells, virus transformed B cells (Liao and Tang, 2014; Mambula et al., 2006; Kettner et al., 2007; Powers et al., 2008).
While HSPA8 is involved in housekeeping chaperoning functions (He M et al., 2010; Pfaffenbach et al., 2010; Daugaard et al., 2007; Powers et al., 2008), that is not its only role: additionally, it carries out biological functions relevant to immunity, antigenicity, hematopoiesis, autophagy (Stricher et al., 2013), vesicular trafficking, exocytosis, endocytosis and participates in multiple cellular signalling pathways (Meimaridou et al., 2009).
The HSP family of proteins
HSPs, the protein family to which HSPA8 belongs, are ubiquitously distributed and highly expressed in tissues (Hantschel et al., 2000). There are evidences of plasma membrane localization of cytoplasmin HSPs ranging from 70 to 90 KDa, though some of them term it as ‘unusual plasma membrane localization of cytoplasmin HSPs’(Altmeyer et al., 1996; Ferrarini et al., 1992; Piselli et al., 1995; Tamura , et al., 1993). In fact, HSPs are contained intracellularly but they are translocated into the plasma membrane and released into the extracellular environment especially after various pathological conditions (Campisi, 2003; Singh-Jasuja, 2001; Srivastava, 2002, Vega et al., 2008). It is now well established that the HSPs act in various intracellular compartments (Buchner, 1996; Frydman and Hohfeld, 1997; Hartl, 1996; Hightower, et al., 1994; Melnick and Argon, 1995), although there are evidences suggesting the function of certain HSPs after being expressed on the cell surface (Kaur, et al., 1993, Takashima, et al., 1996; Tamura, et al., 1993; Tsuboi, et al., 1994). HSPs interact with the proteins in unfolded, misfolded or aggregated states but do not react with the folded counterparts. However, they can interact with a limited set of native proteins (Mayer, 2013; Mayer and Bukau, 2005, Meimaridou, 2009). A list of wide variety of chemical scaffolds like polyamines, fatty acids, sulfoglycolipids, adenosines and peptideshave been identified which show remarkable affinity for HSPs (Evans et al., 2010).
HSPs, the protein family to which HSPA8 belongs, are ubiquitously distributed and highly expressed in tissues (Hantschel et al., 2000). There are evidences of plasma membrane localization of cytoplasmin HSPs ranging from 70 to 90 KDa, though some of them term it as ‘unusual plasma membrane localization of cytoplasmin HSPs’(Altmeyer et al., 1996; Ferrarini et al., 1992; Piselli et al., 1995; Tamura , et al., 1993). In fact, HSPs are contained intracellularly but they are translocated into the plasma membrane and released into the extracellular environment especially after various pathological conditions (Campisi, 2003; Singh-Jasuja, 2001; Srivastava, 2002, Vega et al., 2008). It is now well established that the HSPs act in various intracellular compartments (Buchner, 1996; Frydman and Hohfeld, 1997; Hartl, 1996; Hightower, et al., 1994; Melnick and Argon, 1995), although there are evidences suggesting the function of certain HSPs after being expressed on the cell surface (Kaur, et al., 1993, Takashima, et al., 1996; Tamura, et al., 1993; Tsuboi, et al., 1994). HSPs interact with the proteins in unfolded, misfolded or aggregated states but do not react with the folded counterparts. However, they can interact with a limited set of native proteins (Mayer, 2013; Mayer and Bukau, 2005, Meimaridou, 2009). A list of wide variety of chemical scaffolds like polyamines, fatty acids, sulfoglycolipids, adenosines and peptideshave been identified which show remarkable affinity for HSPs (Evans et al., 2010).
In stress conditions, HSPs are up-regulated and play an important role in cellular repair and protective mechanisms. For example, they bind as well as refold the denatured proteins due to be in stress, whereas in unstressed normal conditions, they perform their routine works like proper folding, assembly, and intercellular trafficking of newly synthesized proteins (Becker and Craig, 1994; Hartl, 1996, Lindquist and Craig, 1988).
Physiological relevance of the TLQP-21 and HSPA8 interaction
Although it was unanticipated at first sight that HSPA8 be a receptor for TLQP-21, this was consistent with several earlier and very recent findings.
Obesity and diabetes
First, expression of HSPA8 has been found to increase in association with obesity and diabetes (Tiss et al., 2014). Increased expression of HSPs is required in persons with obesity to minimize inflammatory and metabolic stresses imposed by obesity. HSPs are believed to play crucial role in renovating cellular and metabolic homeostasis (McArdle et al., 2002; Morton et al., 2006) by decreasing inflammation, uprising skeletal muscle oxidation (Morino et al., 2008; Gupte et al., 2009; Liu et al., 2012; Henstridge et al., 2014).
First, expression of HSPA8 has been found to increase in association with obesity and diabetes (Tiss et al., 2014). Increased expression of HSPs is required in persons with obesity to minimize inflammatory and metabolic stresses imposed by obesity. HSPs are believed to play crucial role in renovating cellular and metabolic homeostasis (McArdle et al., 2002; Morton et al., 2006) by decreasing inflammation, uprising skeletal muscle oxidation (Morino et al., 2008; Gupte et al., 2009; Liu et al., 2012; Henstridge et al., 2014).
In consistent with the finding of less expression of HSPs in T2DM (Kurucz et al., 2002; Bruce et al., 2003); HSPs have been considered with importance for the treatment of insulin resistance and obesity related T2DM (Chung et al., 2008; Literati-Nagy et al., 2009). In fact, the role of HSPs was under-recognized previously in the maintenance of homeostasis in the physiology, with reference to the obesity-driven inflammation that promotes insulin resistance (Hooper and Hooper, 2009). All these lend support to the argument in favour of a TLQP21-HSPA8 connection in modulating obesity. In this respect, it should be remembered that TLQP-21 is the first identified VGF-derived peptide involved in energy homeostasis (Bartolomucci et al., 2007) and considered as putative target to treat obesity related disorders (Cero et al., 2014) as its role in diabetes (Zhang et al., 2013; Stephens et al., 2012), hypertension (Fargali et al., 2014) and lypolysis (Possenti et al., 2012) has already been confirmed.
Metabolism
TLQP-21 exerts important effects on energy metabolism, its administration decreased food intake and increased energy expenditure in Siberian hamsters (Jethwa et al., 2007). It also decreased body weight and WAT in mice fed high fat diet for 14 days, blocking hormonal changes associated with this diet and provoking the autonomic activation of the adrenal medulla and adipose tissue. Furthermore, intracerebroventricular administration of TLQP-21 reduced early phase diet induced obesity (Bartolomucci et al., 2006). It is therefore noteworthy that expression of HSPA8 has been shown to be modulated by diet. Thus, significant downregulation was seen in liver from rats fed high-fat sucrose diet versus starch-rich control diet (Bondia-Pons et al., 2011), and in response to increased bean consumption ( Daniell et al., 2012).
TLQP-21 exerts important effects on energy metabolism, its administration decreased food intake and increased energy expenditure in Siberian hamsters (Jethwa et al., 2007). It also decreased body weight and WAT in mice fed high fat diet for 14 days, blocking hormonal changes associated with this diet and provoking the autonomic activation of the adrenal medulla and adipose tissue. Furthermore, intracerebroventricular administration of TLQP-21 reduced early phase diet induced obesity (Bartolomucci et al., 2006). It is therefore noteworthy that expression of HSPA8 has been shown to be modulated by diet. Thus, significant downregulation was seen in liver from rats fed high-fat sucrose diet versus starch-rich control diet (Bondia-Pons et al., 2011), and in response to increased bean consumption ( Daniell et al., 2012).
Reproduction
Another argument favoring the physiological relevance of this newly discovered TLQP 21/HSPA8, ligand-receptor relationship comes from the study of reproduction. HSPA8 contributes to survival of sperm in the oviduct (Elliott et al., 2009). And enhances the ability of spermatozoa to bind oviductal epithelial cells, boosting up in-vitro fertilization performance (Moein-Vaziri et al., 2014). Furthermore, HSPA8 has crucial protective functions in the process of spermatogenesis and epididymal maturation of male germ cells under stress conditions (Zaprjanova et al., 2013). SRY (sex determining region on the Y chromosome) plays a key role in mammalian sex determination within the nucleus. HSPA8 plays a key role in calcium-binding protein, calmodulin (CaM) dependent nuclear import of SRY (Kaur et al., 2013). On the other hand, after administration of TLQP-21 on adolescent males with chronic food deprivation, the gonadotrophin response of hyphothalamic-pituitary-gonadal (HPG) axis was found to be reduced and in fed males it caused puberty delay (Pinilla et al., 2011), consistent with the infertility found in male VGF knockout mice (Hahm et al., 1999; 2002).
Another argument favoring the physiological relevance of this newly discovered TLQP 21/HSPA8, ligand-receptor relationship comes from the study of reproduction. HSPA8 contributes to survival of sperm in the oviduct (Elliott et al., 2009). And enhances the ability of spermatozoa to bind oviductal epithelial cells, boosting up in-vitro fertilization performance (Moein-Vaziri et al., 2014). Furthermore, HSPA8 has crucial protective functions in the process of spermatogenesis and epididymal maturation of male germ cells under stress conditions (Zaprjanova et al., 2013). SRY (sex determining region on the Y chromosome) plays a key role in mammalian sex determination within the nucleus. HSPA8 plays a key role in calcium-binding protein, calmodulin (CaM) dependent nuclear import of SRY (Kaur et al., 2013). On the other hand, after administration of TLQP-21 on adolescent males with chronic food deprivation, the gonadotrophin response of hyphothalamic-pituitary-gonadal (HPG) axis was found to be reduced and in fed males it caused puberty delay (Pinilla et al., 2011), consistent with the infertility found in male VGF knockout mice (Hahm et al., 1999; 2002).
Gastric acid discharge
HSPA8 was reported to be involved in the healing of acetic acid-induced gastric ulcers in rats. The protein was expressed highly in the normal mucosa and ulcerated tissue, though the level of protein expression was not changed during the ulceration and ulcer healing process (Tsukimi et al., 2001). Remarkably, the central inhibitory effect of TLQP-21 on gastric acid secretion was proved, confirming that it is mediated by endogenous somatostatin and prostaglandins and requires the integrity of sensory nerve fibres (Sibilia et al., 2012).
HSPA8 was reported to be involved in the healing of acetic acid-induced gastric ulcers in rats. The protein was expressed highly in the normal mucosa and ulcerated tissue, though the level of protein expression was not changed during the ulceration and ulcer healing process (Tsukimi et al., 2001). Remarkably, the central inhibitory effect of TLQP-21 on gastric acid secretion was proved, confirming that it is mediated by endogenous somatostatin and prostaglandins and requires the integrity of sensory nerve fibres (Sibilia et al., 2012).
Hypertension
Altered gene expression of HSPA8 was also found to be involved in hypertension in patients with blood-stasis syndrome (Lian et al., 2014), in Japaneese individuals with chronic kidney disease (Oguri et al., 2009), patients with thoracic aortic aneurysm (Kato et al, 2008), patients with arterial hypertension (Timofeeva et al., 2006). The findings that acute and chronic administration of the VGF-derived peptide TLQP-21 to rodents improved hypertension (Fargali et al., 2014) indicates that the action of TLQP 21 may be mediated by the receptor HSPA8.
Altered gene expression of HSPA8 was also found to be involved in hypertension in patients with blood-stasis syndrome (Lian et al., 2014), in Japaneese individuals with chronic kidney disease (Oguri et al., 2009), patients with thoracic aortic aneurysm (Kato et al, 2008), patients with arterial hypertension (Timofeeva et al., 2006). The findings that acute and chronic administration of the VGF-derived peptide TLQP-21 to rodents improved hypertension (Fargali et al., 2014) indicates that the action of TLQP 21 may be mediated by the receptor HSPA8.
Survival and proliferation of cells
At this stage, it is difficult and premature to conclude whether binding of TLQP-21 to cell surface HSPA8 has a positive or negative effect for the survival and proliferation of SH-SY5Y cells. On the one hand, it might result to activation of signals leading to proliferation, perhaps by activating some secondary membrane partner bound to HSPA8. This would be consistent with the general neurotrophic character of VGF and its derived peptides. Simultaneous targeting of HSPA8 and HSP1A induces tumor-specific apoptosis (Powers et al., 2008), which highlights the important role of HSPA8 in viability of tumor cells.
At this stage, it is difficult and premature to conclude whether binding of TLQP-21 to cell surface HSPA8 has a positive or negative effect for the survival and proliferation of SH-SY5Y cells. On the one hand, it might result to activation of signals leading to proliferation, perhaps by activating some secondary membrane partner bound to HSPA8. This would be consistent with the general neurotrophic character of VGF and its derived peptides. Simultaneous targeting of HSPA8 and HSP1A induces tumor-specific apoptosis (Powers et al., 2008), which highlights the important role of HSPA8 in viability of tumor cells.
Concluding remarks and future directions
The findings highlighting the physiological relevance of the HSPA8 and TLQP-21 connection give more credence to the interaction of HSPA8 and TLQP-21 or at least the probable involvement of HSPA8 in TLQP-21 induced biological actions. In fact, due to all these physiological relevancies of HSPA8 and TLQP-21, it is highly likely that the HSPA8-TLQP-21 interaction prompts various cellular responses, though the consequences of HSPA8-TLQP-21 are still elusive and need more clarification in signaling pathways.
What is now left and of upmost important in the field of HSPA8-TLQP-21 interaction is complete elucidation of physiologically important downstream signaling pathways with further characterization. The further read out of TLQP-21 signaling can be exploited to explore new horizon in diagnosis and therapies for TLQP-21 related human diseases, especially in which it has been shown to have effect and related with thereof, notably metabolism, gastric acid secretion, reproduction, hypertension, lipolysis, Obesity, diabetes (as mentioned above).
Acknowledgments
The authors expresses thanks to his respective institute: Khulna University, Bangladesh.
The authors expresses thanks to his respective institute: Khulna University, Bangladesh.
Copyright
Submission of a manuscript implies that the work described has not been published before (except in the form of an abstract or as part of a published lecture, or thesis) and that it is not under consideration for publication elsewhere.
Submission of a manuscript implies that the work described has not been published before (except in the form of an abstract or as part of a published lecture, or thesis) and that it is not under consideration for publication elsewhere.
References
- Aguilar, E., Pineda, R., Gayta´ n, F., Sa´nchez-Garrido, M. A., Romero, M., Romero-Ruiz, A., Ruiz-Pino, F., Tena-Sempere, M., and Pinilla, L. (2013). Characterization of the reproductive effects of the Vgf-derived peptide TLQP-21 in female rats: in vivo and in vitro studies. Neuroendocrinology 98: 38–50
- Altmeyer, A., Maki, R. G., Feldweg, A. M., Heike, M., Protopopov, V. P., Masur, S. K., Srivastava, P. K. (1996). Tumor-specific cell surface expression of the KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 69: 340–349
- Bartolomucci, A., Corte, G. L., Possenti, R., Locatelli, V., Rigamonti, A. E., Torsello, A., Bresciani, E., Bulgarelli, I., Rizzi, R., Pavone, F., D'Amato, F. R., Severini, C., Mignogna, G., Giorgi, A., Schinina, M. E., Elia, G., Brancia, C., Ferri, G. L., Conti, R., Ciani, B., Pascucci, T., Dell'Omo, G., Muller, E. E., Levi, A., Moles, A. (2006). TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity, Proc. Natl. Acad. Sci. U. S. A. 103 : 14584–14589
- Bartolomucci, A., Possenti, R., Levi, A., Pavone, F., Moles, A. (2007) The role of the vgf gene and VGF-derived peptides in nutrition and metabolism. Genes Nutr. 2 (2): 169-80
- Bartolomucci, A., Moles, A., Levi, A., Possenti, R., (2008). Pathophysiological role of TLQP-21: gastrointestinal and metabolic functions. Eat Weight Disord. 13(3): e49-54. PMID: 19011364
- Bartolomucci, A., Possenti, R., Mahata, S. K., Fischer-Colbrie, R., Loh, Y. P., Salton, S. R. (2011) The extended granin family: structure, function, and biomedical implications. Endocr Rev. 32 (6): 755-97
- Buchner, J. (1996) Supervising the fold: functional principlesof molecular chaperones. FASEB. 10: 10-19
- Becker, F., Craig, E. (1994). Heat shock proteins as molecular chaperones. Eur J Biochem. 219: 11–23
- Bruce, C. R., Carey, A. L., Hawley, J. A., Febbraio, M. A. ( 2003). Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 52: 2338-2345
- Bondia-Pons, I., Boqué, N., Paternain, L., Santamaría, E., Fernández, J., Campión, J., Milagro, F., Corrales, F., Martínez, J. A. (2011). Liver proteome changes induced by a short-term high-fat sucrose diet in wistar rats. J Nutrigenet Nutrigenomics. 4 (6): 344-53
- Chen, Y. C., Pristerá, A., Ayub, M., Swanwick, R. S., Karu, K., Hamada, Y., Rice, A. S., Okuse, K. (2013). Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J BiolChem. 288 (48): 34638-46.
- Campisi, J., Leem, T. H., Fleshner, M. (2003). Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 8: 272-286
- Chung, J., Nguyen, A. K., Henstridge, D. C., Holmes, A. G., Chan, M. H., Mesa, J. L., et al. (2008) HSP72 protects against obesity-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America 105: 1739-1744
- Cero, C., Vostrikov, V. V., Verardi, R., Severini, C., Gopinath, T., Braun, P. D., Sassano, M. F., Gurney, A., , Roth, B. L., Vulchanova, L., Possenti, R., Veglia, G., Bartolomucci, A. (2014). The TLQP-21 peptide activates the G-proteincoupled receptor C3aR1 via a folding-upon-binding mechanism. Structure 22: 1744–1753.
- Daugaard, M., Rohde, M., Jäättelä, M., (2007). The heat shock protein 70family: Highly homologous proteins with overlapping and distinctfunctions. FEBS Lett 581: 3702-10.
- Daniell, E. L., Ryan, E. P., Brick, M. A., Thompson, H. J. (2012). Dietary dry bean effects on hepatic expression of stress and toxicity-related genes in rats. Br J Nutr. 108 Suppl 1: S37-45
- Evans, C. G., Chang, L., Gatwick, J. E. (2010) Heat shock protein 70 (Hsp70) as an emerging drug target. J Med Chem. 24: 53 (12): 4585–4602
- Elliott, R. M. A., Lloyd, R. E., Fazeli, A., Sostaric, E., Georgiou, A. S. Satake1, N., Watson, P. F., Holt, W. V. (2009) Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 137: 191–203
- Fairbanks, C. A., Peterson, C. D., Speltz, R. H., Riedl, M. S., Kitto, K. F., Dykstra,J. A., Braun, P. D., Sadahiro, M., Salton, S. R., Vulchanova, L. (2014). TheVGF-derived peptide TLQP-21 contributes to inflammatory and nerve injuryinducedhypersensitivity. Pain 155: 1229–1237.
- Fargali, S., Garcia, A. L., Sadahiro, M., Jiang, C., Janssen, W. G., Lin, W. J., Cogliani, V., Elste, A., Mortillo, S., Cero, C., et al. (2014) The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J. 28: 2120–2133
- Ferrarini, M., Heltai, S., Zocchi, M. R., Rugarli, C. (1992). Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 51: 613– 619.
- Frydman, J.,Hohfeld, J. (1997) Chaperones get in touch: the Hip-Hop connection. Trends Biochem.Set 22 : 87
- Gupte, A. A., Bomhoff, G. L., Swerdlow, R. H., Geiger, P. C. ( 2009) Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567-578
- He, M., Guo, H., Yang, X., et al. (2010) Genetic variations in HSPA8 gene associated with coronary heart disease risk in a Chinese population. PLoS One 5: 9684
- Hantschel, M., Pfister, K., Jordan, A., Scholz, R., Andreesen, R., Schmitz, G., Schmetzer, H., Hiddemann, W., Multhoff, G. (2000). Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 5(5): 438-42.
- Hartl, F. U. (1996) Molecular chaperones in cellular protein folding. Nature 381: 571–580
- Hightower, L. E., Sadis, S. E., and Takenaka, I. M. (1994) Interactionsof vertebrate hsc70 and hsp70 with unfold proteinsand peptides. In "The Biology of Heat Shock Proteins and Molecular Chaperones."(R.I. Morimoto, A. Tissieres and C.Georgopoulos, Eds.), Cold Spring Harbor Press, Cold Spring
- Henstridge, D. C., Bruce, C. R., Drew, B. G., Tory, K., Kolonics, A., Estevez, E., et al., ( 2014). Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63: 1881-1894
- Hooper, P. L., Hooper, P. L. (2009) Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress and Chaperones. 14: 113–115
- Hahm, S., Mizuno, T. M., Wu, T. J., Wisor, J. P., Priest, C. A., Kozak, C. A., Boozer, C. N., Peng, B., McEvoy, R. C., Good, P., et al. (1999) Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron 23: 537-548
- Hahm, S., Fekete, C., Mizuno, T. M., Windsor, J., Yan, H., Boozer, C. N., Lee, C., Elmquist, J. K., Lechan, R. M., Mobbs, C. V., et al. (2002). VGF is required for obesity induced by diet, gold thioglucose treatment, and agouti and is differentially regulated in pro-opiomelanocortin- and neuropeptide Y-containing arcuate neurons in response to fasting. J Neurosci 22: 6929-6938.
- Jethwa, P. H., Warner, A., Nilaweera, K. N., Brameld, J. M., Keyte, J. W., Carter, W. G., Bolton, N., Bruggraber, M., Morgan, P. J., Barrett, P., et al. (2007) VGFderived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology 148: 4044-4055
- Kettner, S., Kalthoff, F., Graf, P., Priller, E., Kricek, F., Lindley, I., Schweighoffer, T. (2007). EWI-2/CD316 is an induciblereceptor of HSPA8 on Human Dendritic Cells. Mol Cell Biol 27: 7718-26.
- Kaur, I., Voss, S. D., Gupta, R. S., Schell, K., Fisch, P., Sondel, P. M. (1993) Human Peripheral yd T cells recognize hsp60 molecules on Daudi Burkitt's ymphoma cells. J. Immunol 150: 2046-2055
- Kurucz, I., Morva, A., Vaag, A., Eriksson, K. F., Huang, X., Groop, L., et al. (2002). Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 51: 11021109.
- Kato, K., Oguri, M., Kato, N., Hibino, T., Yajima, K., Yoshida, T., Metoki, N., Yoshida, H., Satoh, K., Watanabe, S., Yokoi, K., Murohara, T., Yamada, Y. (2008) Assessment of genetic risk factors for thoracic aortic aneurysm in hypertensive patients. Am J Hypertens. 21(9): 1023-7
- Liao, Y., Tang, L., (2014). The Critical Roles of HSC70 in Physiological and Pathological Processes. Current Pharmaceutical Design 20: 101-107
- Lindquist, S., Craig, E. A. (1988) The heat-shock proteins. Annu Rev Genet 22: 631–677
- Liu, T., Daniels, C. K., Cao, S. (2012). Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 136 (3): 354-74
- Literati-Nagy, B., Kulcsar, E., Literati-Nagy, Z., Buday, B., Peterfai, E., Horvath, T., et al. (2009) Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized doubleblind clinical trial. Hormone and Metabolic Research. 41: 374-380
- Lian, Y. H., Fang, M. X., Chen, L. G. (2014). Constructing protein-protein interaction network of hypertension with blood stasis syndrome via digital gene expression sequencing and database mining. J Integr Med. 12(6): 476-82.
- Mambula, S. S., Calderwood, S. K. (2006). Heat Shock Protein 70 is secretedfrom tumor cells by a nonclassical pathway involving lysosomalendosomes. J Immunol 177: 7849-57
- Meimaridou, E., Gooljar, S. B., Chapple, J. B. (2009) From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J MolEndocrinol. 42: 1-9
- Melnick, J., Argon, Y. (1995). Molecular chaperones andthe biosynthesis of antigen receptors. Immunol. Today. 16: 243-250
- Mayer, M. P. (2013). Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 38(10): 507-14
- Mayer, M. P., Bukau, B. (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 62: 670–684
- McArdle, A., Jackson, M. (2002) Stress proteins and exercise-induced muscle damage. In exercise and stress response. The role of stress proteins. Edited by Locke FM, Noble EG, Boca R. London: CRC; 137–150
- Morton, J. P., MacLaren, D. P., Cable, N. T., Bongers, T., Griffiths, R. D., Campbell, I. T., Evans, L., Kayani, A., McArdle, A., Drust, B. (2006). Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J Appl Physiol. 101: 176–182
- Morino, S., Kondo, T., Sasaki, K., Adachi, H., Suico, M.A., Sekimoto, E., et al. (2008). Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS ONE 3: 4068
- Moein-Vaziri, N., Phillips, I., Smith, S., Almi?ana, C., Maside, C., Gil, M. A., Roca, J., Martinez, E. A., Holt, W. V., Pockley, A. G., Fazeli, A. (2014) Heatshock protein A8 restores sperm membrane integrity by increasing plasma membrane fluidity. Reproduction 147(5): 719-32
- Oguri, M., Kato, K., Yokoi, K., Watanabe, S., Metoki, N., Yoshida, H., Satoh, K., Aoyagi, Y., Nishigaki, Y., Yoshida, H., Nozawa, Y., Yamada, Y. (2009). Association of polymorphisms of THBS2 and HSPA8 with hypertension in Japanese individuals with chronic kidney disease. Mol Med Rep. 2(2): 205-11
- Possenti, R., Muccioli, G., Petrocchi, P., Cero, C., Cabassi, A., Vulchanova, L., Riedl, M. S., Manieri, M., Frontini, A., Giordano, A., Cinti, S., Govoni, P., Graiani, G., Quaini, F., Ghè, C., Bresciani, E., Bulgarelli, I., Torsello, A., Locatelli, V., Sanghez, V., Larsen, B. D., Petersen, J. S., Palanza, P., Parmigiani, S., Moles, A., Levi, A., Bartolomucci, A. (2012). Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP21. Biochem J. 441 (1): 511-22
- Pinilla, L., Pineda, R., Gaytan, F., Romero, M., Garcia-Galiano, D., SanchezGarrido, M.A., Ruiz-Pino, F., Tena-Sempere, M., and Aguilar, E. (2011) Characterization of the reproductive effects of the anorexigenic VGF-derived peptide TLQP-21: in vivo and in vitro studies in male rats. American journal of physiology Endocrinology and metabolism 300: 837-847
- Powers, M. V., Clarke, P. A., Workman, P. (2008). Dual targeting of HSC70 and HSP72 inhibits HSP90 function and Induces tumor-specific apoptosis. Cancer Cell 14: 250-62
- Pfaffenbach, K. T., Lee, A. S. (2010). The critical role of GRP78 in physiologicand pathologic stress. Curr Opin Cell Biol 23: 150-6
- Piselli, P., Vendetti, S., Poccia, F., Cicconi, R., Mattei, M., Bolognesi, A., Stripe, F., Colizzi, V., (1995). In vitro and in vivo efficacy of heat shock protein specific immunotoxins on human tumor cells. J Biol Regul Homeost Agents. 9: 55–62
- Rizzi, R., Bartolomucci, A., Moles, A., D’Amato, F., Sacerdote, P., Levi, A., La Corte, G., Ciotti, M. T., Possenti, R., Pavone, F. (2008) The VGF-derived peptide TLQP-21: a new modulatorypeptide for inflammatory pain. Neurosci Lett 441: 129–133
- Razzoli, M., Bo, E., Pascucci, T., Pavone, F., D’Amato, F.R., Cero, C.,Sanghez, V., Dadomo, H., Palanza, P., Parmigiani, S., et al. (2012) Implication of the VGFderived peptide TLQP-21 in mouse acute and chronicstress responses. Behav. Brain Res. 229: 333–339
- Stephens, S. B., Schisler, J. C., Hohmeier, H. E., An, J., Sun, A. Y., Pitt, G. S., Newgard, C. B. (2012). A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab. 16 (1): 33-43.
- Severini, C., Ciotti, M. T., Biondini, L., Quaresima, S., Rinaldi, A. M., Levi, A., Frank, C., Possenti, R. (2008). TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem 104: 534-544
- Severini, C., La Corte, G., Improta, G., Broccardo, M., Agostini, S., Petrella, C., Sibilia, V., Pagani, F., Guidobono, F., Bulgarelli, I., et al. (2009) In vitro and in vivo pharmacological role of TLQP-21, a VGF-derived peptide, in the regulation of rat gastric motor functions. British journal of pharmacology 157: 984-993
- Sibilia, V., Pagani, F., Bulgarelli, I., Mrak, E., Broccardo, M., Improta, G., Severini, C., Possenti, R., Guidobono, F. (2010a). TLQP-21, a VGF-derived peptide, prevents ethanol-induced gastric lesions: insights into its mode of action. Neuroendocrinology. 92 (3): 189-97.
- Sibilia, V., Pagani, F., Bulgarelli, I., Tulipano, G., Possenti, R., Guidobono, F. (2010b). Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a vgf-derived peptide, in rats. Amino Acids 10.1007/s00726010-0818-6
- Sibilia, V., Pagani, F., Bulgarelli, I., Tulipano, G., Possenti, R., Guidobono, F., (2012) Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a vgf-derived peptide, in rats. Amino Acids. 42 (4): 1261-8. doi: 10.1007/s00726-010-0818-6. Epub 2010 Dec 4.
- Stricher, F., Macri, C., Ruff, M., Muller, S. (2013) HSPA8/HSC70 chaperone protein Structure, function, and chemical targeting. Autophagy 9: (12), 1937–1954
- Singh-Jasuja, H., Hilf, N., Arnold-Schild, D., Schild. H. (2001). The role of heat shock proteins and their receptors in the activation of the immune system. Biol. Chem. 382: 629-636.
- Tamura, Y., Tsuboi, N., Sato, N., Kikuchi, K. (1993). 70 kDa heat shock cognate protein is a transformation-associated antigen and a possible target for the host's anti-tumor immunity. J Immunol. 151: 5516–5524
- Takashima, S., Sato, N., Kishi, A., Tamura, Y., Hirai, I., Torigoe, T., Yagihashi, A., Takahashi, S., Sagae, S., Kudo,R., Kikuchi, K. (1996). Involvement of peptide antigens inthe cytotoxicity between 70-kDa heat shock cognate proteinlikemolecule and CD3+, CD4~, CD8", TCRa/3- killer T cells. J. Immunol. 157: 3391-3395
- Tsuboi, N., Ishikawa, M., Tamura, Y., Takayama, S., Tobioka, H., Matsuura, A., Hirayoshi, K., Nagata, K., Sato, N., Kikuchi, K. (1994). Monoclonal antibody specifically reacting against 73-kilodalton heat shock cognate protein: possible expression on mammalian cell surface. Hybridoma 13: 373-81
- Tiss A., Khadir, A., Abubaker, J., Abu-Farha, M., Al-Khairi, I., Cherian, P., John, J., Kavalakatt, S., Warsame, S., Al-Ghimlas, F., Elkum, N., Behbehani, K., Dermime, S., Dehbi, M. (2014). Immunohistochemical profiling of the heat shock response in obese non-diabetic subjects revealed impaired expression of heat shock proteins in the adipose tissue. Lipids in Health and Disease 13: 106
- Tsukimi, Y., Nakai, H., Itoh, S., Amagase, K., Okabe, S. (2001). Involvement of heat shock proteins in the healing of acetic acid-induced gastric ulcers in rats. J Physiol Pharmacol. 52(3): 391-406
- Timofeeva, A. V., Goryunova, L. E., Khaspekov, G. L., Kovalevskii, D. A., Scamrov, A. V., Bulkina, O. S., Karpov, Y. A., Talitskii, K. A., Buza, V.V., Britareva, V. V., Beabealashvilli, R. S. (2006). Altered gene expression pattern in peripheral blood leukocytes from patients with arterial hypertension. Ann N Y Acad Sci. 1091: 319-35
- Vega, V. L., Rodríguez-Silva, M., Frey, T., Gehrmann, M., Diaz, J. C., Steinem, C., Multhoff, G., Arispe, N., De Maio, A. (2008). Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. The Journal of Immunology 180: 6, 4299-4307.
- Zhang, W., Ni, C., Sheng, J., Hua, Y., Ma, J., et al. (2013). TLQP-21 protects human umbilical vein endothelial cells against high-glucose-induced apoptosis by increasing G6PD expression. PLoS ONE 8(11): 79760
- Zaprjanova, S., Rashev, P., Zasheva, D., Martinova, Y., Mollova, M. (2013). Electrophoretic and immunocytochemical analysis of Hsp72 and Hsp73 expression in heat-stressed mouse testis and epididymis. Eur J Obstet Gynecol Reprod
Citation: Md. Shamim Akhter. (2020). Physiological Relevance of the TLQP-21 and HSPA8 Interaction: A Review. Journal of Biotechnology and Immunology 2(3).
Copyright: © 2020 Md. Shamim Akhter. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.