Research Article
Volume 1 Issue 2 - 2019
Iron Deficiency Anemia in Adolescent School Children: Effects on Biochemical and Immune Functions
Department of Biochemistry and Molecular Biology, University of Dhaka, Dhaka-1000, Bangladesh
*Corresponding Author: Laila N. Islam, Department of Biochemistry and Molecular Biology, University of Dhaka, Dhaka-1000, Bangladesh.
Received: November 19, 2019; Published: December 03, 2019
Abstract
The prevalence of iron deficiency anemia (IDA) and its effects on the biochemical and immune functions were compared in 135 adolescents, comprising of 70 boys and 65 girls. Their monthly family income (MFI), nutritional and demographic data were recorded. The results showed 26.7% of the adolescents were suffering from anemia and the percentages of anemic boys and girls were 10.0% and 44.6%, respectively. The mean hemoglobin, hematocrit, RBC counts, and serum proteins of the girls were significantly lower. Their mean serum iron level (128.71±26.22 µg/dL) and total iron binding capacity 386.13±78.65 µg/dL were significantly lower than the boys, 153.84±48.51 µg/dL and 461.50±145.53 µg/dL, respectively. The girls had lower MFI than the boys and lower intake of protein- and mineral- rich food items, which did not correlate with MFI. About 17% of the girls had menorrhagia and all of them were anemic. The levels of IgG, complement C3, myeloperoxidase activity and bactericidal function were lower in the girls. These results showed nutritional deficiency and menorrhagia might be associated with higher prevalence of IDA and lower humoral immune functions in adolescent girls.
Key words: IDA; Serum iron; TIBC; Myeloperoxidase; Immune functions
Abbreviations: IDA: Iron deficiency anemia; MFI: Monthly family income; TIBC: Total iron binding capacity; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MBL: Menstrual blood loss; IgG: Immunoglobulin G; MPO: Myeloperoxidase
Introduction
Anemia, a disorder with a manifestation of several diseases and pathological conditions, is one of the most common public health problem worldwide and especially in developing countries. Based on the criteria of the World Health Organization, more than two billion people globally and 149 million people in the Eastern Mediterranean Region are estimated to be anemic [1]. Among adolescents the worldwide prevalence of anemia is 15%, 27% in developing countries and 6% in developed countries [2].More than 400 types of anemia have been identified so far. Among them the irondeficiency anemia (IDA) tops the list [3].
Iron plays an important role in body´s energy metabolism, gene regulation, cell growth and differentiation, oxygen binding and transport, muscle oxygen use and storage, enzyme reactions, neurotransmitter synthesis and protein synthesis [4]. In IDA, lack of iron leads to reduce production of hemoglobin in the red blood cells. As a result, blood cannot carry oxygen effectively. Without enough iron people may feel tired, breathless, dizzy, weak and irritable [5]. IDA can be defined as the hemoglobin concentration lower than 11 to 12 g/dL in children younger than 12 years, 12 g/dL in adolescent and women, and 13 g/dL in men [6].
Inadequate iron intake, poor absorption and increased iron demand or blood loss are the major causes of IDA [7]. In developing countries, low standards of living, low socioeconomic conditions, restricted access to food and a lack of knowledge for good dietary practices and personal hygiene contribute even more than nutritional deficit to a high occurrence of iron deficiency and hence anemia. Moreover, intestinal parasitic infection due to poor hygienic conditions interferes with iron absorption by reducing it, thus expanding the prevalence of IDA in the developing world [8].
A nutritional surveillance project of Helen Keller International showed that 68% of under-five children, 40% of adolescent girls and 31% adolescent boys as well as 46% of non-pregnant and 39% of pregnant women were anemic in Bangladesh [9]. Adolescence is the only time following infancy when the rate of physical growth actually increases. This sudden growth spurt is associated with hormonal, cognitive, and emotional changes that make adolescence an especially vulnerable period of life. As a result, besides other vulnerable age groups, adolescence is placed at a high risk level for developing iron deficiency, due to a combination of menstrual iron losses in girls and a rapid physical growth, especially in boys.
In IDA, macrophages have reduced bactericidal activity as well as neutrophils showed decreased activity of myeloperoxidase, which is a crucial enzyme involved in the killing process of pathogen. People with IDA suffer from complement deficiency that leads to bacterial infection [10]. In experimental animal, the IgM and IgG antibody responses against sheep erythrocytes determined by hemolytic plaque assay were suppressed in the iron deficient mice [11]. In view of inadequate information on the current situation of IDA and its causes and health effects, this study was aimed to determine its prevalence and investigate certain nutritional, biochemical and immune functions in adolescent school children in Dhaka city, Bangladesh.
Materials and Methods
Study subjects and sample collection
With permission of the school authority, the pupils aged 12 to 15 years and their guardians were informed about the study objectives. A total of 135 full consenting adolescents (70 boys and 65 girls) were enrolled. Their monthly family income, food intake and demographic data were recorded on preformed questionnaire forms. From each study participant, 6 mL of peripheral blood sample was collected, of which 2 mL was transferred in EDTA-tubes and 4 mL was taken in glass tubes, the plasma and serum were separated and preserved at -20°C for analysis.
With permission of the school authority, the pupils aged 12 to 15 years and their guardians were informed about the study objectives. A total of 135 full consenting adolescents (70 boys and 65 girls) were enrolled. Their monthly family income, food intake and demographic data were recorded on preformed questionnaire forms. From each study participant, 6 mL of peripheral blood sample was collected, of which 2 mL was transferred in EDTA-tubes and 4 mL was taken in glass tubes, the plasma and serum were separated and preserved at -20°C for analysis.
Determination of blood cell counts
The fresh blood samples were diluted and the total red blood cells (RBCs) and white blood cells (WBCs) were counted in hemocytometer using an Olympus microscope. For the differential WBC count, a smear of blood was stained with Giemsa’s stain and 200 cells were counted randomly, and the proportions of different leukocytes were determined.
The fresh blood samples were diluted and the total red blood cells (RBCs) and white blood cells (WBCs) were counted in hemocytometer using an Olympus microscope. For the differential WBC count, a smear of blood was stained with Giemsa’s stain and 200 cells were counted randomly, and the proportions of different leukocytes were determined.
Determination of hematological parameters
Hemoglobin concentrations of the fresh blood samples were determined using the reagent Kit (Randox Laboratories Ltd, UK) and following the specified procedure. The hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated following the standard methods.
Hemoglobin concentrations of the fresh blood samples were determined using the reagent Kit (Randox Laboratories Ltd, UK) and following the specified procedure. The hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated following the standard methods.
Assessment of anemia
Hemoglobin concentrations lower than 12.0 g/dL for the adolescent children were considered anemia, as described by Aggett et al [6].
Hemoglobin concentrations lower than 12.0 g/dL for the adolescent children were considered anemia, as described by Aggett et al [6].
Determination of serum iron
Serum sample (1 mL) was mixed with 1 mL concentrated nitric acid and 0.5 mL perchloric acid and heat-digested for 1 hour until the solution became clear to free iron from organic state to inorganic state for the detection. The residue was diluted with distilled water and the iron content was determined using a Varian 240 Atomic Absorption Spectrophotometer.
Serum sample (1 mL) was mixed with 1 mL concentrated nitric acid and 0.5 mL perchloric acid and heat-digested for 1 hour until the solution became clear to free iron from organic state to inorganic state for the detection. The residue was diluted with distilled water and the iron content was determined using a Varian 240 Atomic Absorption Spectrophotometer.
Determination of TIBC
To determine the total iron binding capacity (TIBC) of serum samples, the reagent Kit (Human Diagnostica GmbH, Germany) was used and the recommended procedure was followed.
To determine the total iron binding capacity (TIBC) of serum samples, the reagent Kit (Human Diagnostica GmbH, Germany) was used and the recommended procedure was followed.
Determination of protein
The biuret reagent was used for the determination of serum total protein and the absorbance was measured at 540 nm.
The biuret reagent was used for the determination of serum total protein and the absorbance was measured at 540 nm.
Determination of cholesterol
The serum total cholesterol was determined by enzymatic end point method using cholesterol assay Kit (Randox Laboratories Ltd, UK) and following the standard protocol.
The serum total cholesterol was determined by enzymatic end point method using cholesterol assay Kit (Randox Laboratories Ltd, UK) and following the standard protocol.
Determination of IgG and complement C3
Serum IgG and complement C3 were determined using reagents for BN ProSpec® system for nephelometry (Siemens Healthcare Diagnostics Products GmbH, Germany).
Serum IgG and complement C3 were determined using reagents for BN ProSpec® system for nephelometry (Siemens Healthcare Diagnostics Products GmbH, Germany).
Assay of myeloperoxidase activity
The plasma myeloperoxidase (MPO) activity can be measured by a colorimetric assay using ortho-dianisidine, according to Bradley et al., 1982 [12], as detailed elsewhere [13].
The plasma myeloperoxidase (MPO) activity can be measured by a colorimetric assay using ortho-dianisidine, according to Bradley et al., 1982 [12], as detailed elsewhere [13].
Assay of bactericidal activity
The complement mediated bactericidal activity was measured according to the procedure detailed earlier [14]. Briefly, complements were allowed to exert bactericidal activity on a standard preparation of Escherichia coli DH5α and the remaining viable cells were assessed by plate count method.
The complement mediated bactericidal activity was measured according to the procedure detailed earlier [14]. Briefly, complements were allowed to exert bactericidal activity on a standard preparation of Escherichia coli DH5α and the remaining viable cells were assessed by plate count method.
Statistical analysis
Data analyses were carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, USA). The statistical methods used were chi square test, Independent samples t-test, Pearson correlation and simple statistical analyses. The results were considered significant when the value of p was <0.05.
Data analyses were carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, USA). The statistical methods used were chi square test, Independent samples t-test, Pearson correlation and simple statistical analyses. The results were considered significant when the value of p was <0.05.
Results
Status of anemia among the studied children
In this study, the approximate proportions of boys:girls were 52:48 and the percentage of total anemic children was 26.67% (36 out of 135). On the basis of gender, 10% of the boys and 44.61% of the girls were anemic (p < 0.001, chi square test, Table 1).
In this study, the approximate proportions of boys:girls were 52:48 and the percentage of total anemic children was 26.67% (36 out of 135). On the basis of gender, 10% of the boys and 44.61% of the girls were anemic (p < 0.001, chi square test, Table 1).
| Parameters | Boys (N = 70) | Girls (N = 65) |
| Anemia status (%) | 10.00 | 44.61*** |
| Age (years) | 13.41 ± 1.44 | 13.00 ± 1.49† |
| Body mass index (BMI, Kg/m2) | 20.57 ± 3.65 | 20.23 ± 4.10† |
| Number of family members | 4.70 ± 0.70 | 4.73 ± 0.95† |
| Pulse rate (beats per minute) | 84.0 ± 9.98 | 83.77 ± 13.33† |
| Monthly family income (×103 Taka) | 55.54 ± 23.90 | 47.83 ± 21.27† |
†Not significant, t-test; ***p<0.001, chi-square test.
Table 1: Evaluation of anemia, nutritional and demographic data.
Table 1: Evaluation of anemia, nutritional and demographic data.
The physical health, nutritional and demographic data
The data of the studied children were recorded on questionnaire forms. There were no major physical health problems except the paleness of eyes in some children which was indication of anemia. However, some girls had gynecological problems. The mean age, body mass index (BMI), pulse rate, number of family member and monthly family income (MFI) of the boys and girls did not differ significantly (Table 1).
The data of the studied children were recorded on questionnaire forms. There were no major physical health problems except the paleness of eyes in some children which was indication of anemia. However, some girls had gynecological problems. The mean age, body mass index (BMI), pulse rate, number of family member and monthly family income (MFI) of the boys and girls did not differ significantly (Table 1).
Evaluation of hematological parameters
The hemoglobin concentrations in the boys varied from 9.56 to 17.61 g/dL while the value in the girls ranged from 8.68 to 15.11 g/dL and their mean value was significantly lower (p< 0.001, Table 2). Similarly, the RBC count and hematocrit value of the girls were significantly lower. The MCV, MCH and MCHC of the girls were lower while the total and differential WBC counts in all of the boys and girls fell within the normal range.
The hemoglobin concentrations in the boys varied from 9.56 to 17.61 g/dL while the value in the girls ranged from 8.68 to 15.11 g/dL and their mean value was significantly lower (p< 0.001, Table 2). Similarly, the RBC count and hematocrit value of the girls were significantly lower. The MCV, MCH and MCHC of the girls were lower while the total and differential WBC counts in all of the boys and girls fell within the normal range.
| Variables | Boys (N = 70) | Girls (N = 65) |
| Hemoglobin (g/dL) | 13.75 ± 1.64 | 12.21 ± 1.52*** |
| RBC count (109 cells/mL) | 4.70 ± 0.67 | 4.29 ± 0.70*** |
| Hematocrit (%) | 45.05 ± 3.87 | 40.45 ± 4.15*** |
| MCV (fL) | 97.56 ± 14.26 | 96.98 ± 18.91† |
| MCH (pg) | 29.77 ± 4.94 | 29.00 ± 5.73† |
| MCHC (g/dL) | 30.62 ± 3.63 | 30.04 ± 3.55† |
| WBC count (106 cells/mL) | 8.61 ± 2.24 | 8.83 ± 2.04† |
| Neutrophil (%) | 57.57 ± 7.38 | 57.53 ± 5.67† |
| Lymphocyte (%) | 38.98 ±6.85 | 38.98±5.31† |
| Monocyte (%) | 2.64 ± 1.20 | 2.71±.94† |
| Eosinophil (%) | 0.79 ± 0.55 | 0.67±0.75† |
| Basophil (%) | 0.06 ± 0.15 | 0.06±0.15† |
Values are mean ± SD. †Not significant; *** p<0.001
Table 2: Comparison of hematological parameters between the boys and girls.
Table 2: Comparison of hematological parameters between the boys and girls.
Food consumption by an individual boy and girl
The total amount of protein- and mineral-rich cooked food items (meat - chicken, beef or mutton servings; fish - medium pieces; eggs - standard sized hen egg; milk - cups; vegetables - servings; lentil soups - cups) consumed per week by an individual boy and girl were carefully recorded on questionnaire forms. The results showed lower consumption by the girls than boys of fish (395/450 g), milk (486/592 mL), egg (233/250 g) and pulses (690/730 mL) but consumption of meat (828/835 g) and vegetables (258/260 g) were similar.
The total amount of protein- and mineral-rich cooked food items (meat - chicken, beef or mutton servings; fish - medium pieces; eggs - standard sized hen egg; milk - cups; vegetables - servings; lentil soups - cups) consumed per week by an individual boy and girl were carefully recorded on questionnaire forms. The results showed lower consumption by the girls than boys of fish (395/450 g), milk (486/592 mL), egg (233/250 g) and pulses (690/730 mL) but consumption of meat (828/835 g) and vegetables (258/260 g) were similar.
Food consumption by anemic and non-anemic girls
The percentages of anemic girls who ate protein-rich food items in seven days of a week were much lower than the percentages of non-anemic girls (meat: 18/38%, fish: 18/40%, pulses: 50/60%). However, the percentage of anemic girls who ate eggs in seven days of a week was higher than the non-anemic girls (27/15%).
The percentages of anemic girls who ate protein-rich food items in seven days of a week were much lower than the percentages of non-anemic girls (meat: 18/38%, fish: 18/40%, pulses: 50/60%). However, the percentage of anemic girls who ate eggs in seven days of a week was higher than the non-anemic girls (27/15%).
Levels of serum iron
It was found that the mean ± SD serum iron level in the boys (N=44) was 153.84 ± 48.51 µg/dL while the value in the girls (N=56) was 128.71 ± 26.22 µg/dL (p<0.01).
It was found that the mean ± SD serum iron level in the boys (N=44) was 153.84 ± 48.51 µg/dL while the value in the girls (N=56) was 128.71 ± 26.22 µg/dL (p<0.01).
Levels of serum TIBC
The mean serum TIBC in the boys was 461.50 ± 145.53 µg/dL and the corresponding value in the girls was 386.13 ± 78.65 µg/dL, which was significantly lower (p<0.01, Figure 1). Further, the anemic girls had higher TIBC (400.36 ± 87.82 µg/dL, N=20) than the non-anemic girls (377.24 ± 72.37 µg/dL, N=32). A similar result was found in the anemic and non anemic boys.
The mean serum TIBC in the boys was 461.50 ± 145.53 µg/dL and the corresponding value in the girls was 386.13 ± 78.65 µg/dL, which was significantly lower (p<0.01, Figure 1). Further, the anemic girls had higher TIBC (400.36 ± 87.82 µg/dL, N=20) than the non-anemic girls (377.24 ± 72.37 µg/dL, N=32). A similar result was found in the anemic and non anemic boys.
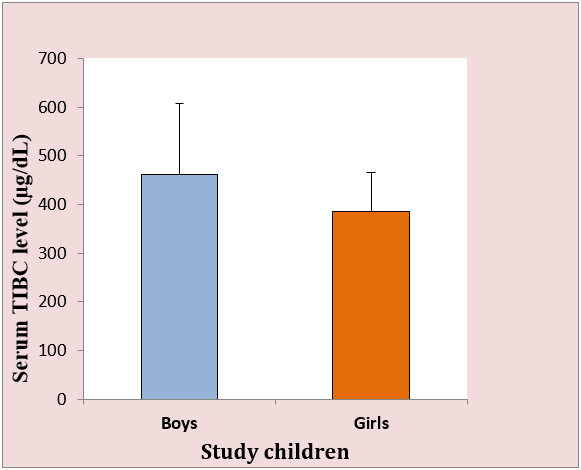
Figure 1: Serum TIBC levels in the boys and girls. The mean TIBC level 386.13 ± 78.65 µg/dL in the girls was significantly lower than 461.50 ± 145.53 µg/dL in the boys (p < 0.01).
Levels of total protein and cholesterol
The mean total protein in serum of the girls (7.54 ± 0.39 g/dL) was significantly lower (p<0.01) than that of the boys (7.79 ± 0.28 g/dL) while the cholesterol levels of the boys (170.87 ± 31.75 mg/dL) and girls (168.47 ± 23.56 mg/dL) did not vary significantly.
The mean total protein in serum of the girls (7.54 ± 0.39 g/dL) was significantly lower (p<0.01) than that of the boys (7.79 ± 0.28 g/dL) while the cholesterol levels of the boys (170.87 ± 31.75 mg/dL) and girls (168.47 ± 23.56 mg/dL) did not vary significantly.
Gynecological data of the girls
The total girls (N=65) was divided into three subgroups to investigate whether menstrual blood loss (MBL) had any effect on the content of hemoglobin in blood. The subgroups were girls with: (i) no or low MBL, (ii) normal to moderately heavy MBL, and (iii) heavy MBL. There were 15 girls who did not have commencement of their menstruation cycle at the time of the study; they were included in subgroup (i). The mean hemoglobin concentrations in the subgroups were 13.23 ± 0.84 g/dL (N=40), 10.69 ± 0.79 g/dL (N=14), and 10.45 ± 0.81 g/dL (N=11), respectively. It was found that girls with heavy MBL had significantly lower hemoglobin (p<0.001) than girls in subgroup (i).
The total girls (N=65) was divided into three subgroups to investigate whether menstrual blood loss (MBL) had any effect on the content of hemoglobin in blood. The subgroups were girls with: (i) no or low MBL, (ii) normal to moderately heavy MBL, and (iii) heavy MBL. There were 15 girls who did not have commencement of their menstruation cycle at the time of the study; they were included in subgroup (i). The mean hemoglobin concentrations in the subgroups were 13.23 ± 0.84 g/dL (N=40), 10.69 ± 0.79 g/dL (N=14), and 10.45 ± 0.81 g/dL (N=11), respectively. It was found that girls with heavy MBL had significantly lower hemoglobin (p<0.001) than girls in subgroup (i).
Levels of IgG and complement C3
The mean level of serum IgG in the girls (N=34) was lower than in the boys (N=30). Notably, in anemic children (N=15, boys: 02 and girls: 13) the mean ± SD of IgG was 12.89 ± 3.09 g/L, which was lower than the whole groups of boys or girls. Similar to IgG, the mean level of complement component C3 in the girls was lower than in the boys (Table 3).
The mean level of serum IgG in the girls (N=34) was lower than in the boys (N=30). Notably, in anemic children (N=15, boys: 02 and girls: 13) the mean ± SD of IgG was 12.89 ± 3.09 g/L, which was lower than the whole groups of boys or girls. Similar to IgG, the mean level of complement component C3 in the girls was lower than in the boys (Table 3).
| Variables | Boys (N = 30) | Girls (N = 34) |
| Immunoglobulin G (g/L) | 14.28 ± 3.62 | 13.85 ± 2.50† |
| Complement protein C3 (g/L) | 1.44 ± 0.32 | 1.40 ± 0.14† |
| Myeloperoxidase activity (U/mg) | 13.28 ± 3.56 | 12.68 ± 2.68† |
†Not significant.
Table 1: Levels of IgG and C3, and MPO activity in boys and girls.
Table 1: Levels of IgG and C3, and MPO activity in boys and girls.
Assessment of MPO activity
It was found that the mean MPO activity, 12.68 U/mg, in the girls was lower than 13.28 U/mg in the boys (Table 3).
It was found that the mean MPO activity, 12.68 U/mg, in the girls was lower than 13.28 U/mg in the boys (Table 3).
Evaluation of bactericidal activity
The numbers of colonies grown on agar plates varied from 65.8-1245.7×106 cfu/mL when E. coli DH5α were grown in PBS medium (control). On the other hand, the number of colonies varied from 0.2-52.9×106 cfu/mL and 0.3-88.5×106 cfu/mL after treatment of the bacterial cells with plasma complements from the boys and girls, respectively. Compared to PBS medium, complements from both the boys and girls exhibited highly significant bactericidal activities (p < 0.001, both). However, the percentages of bacterial cells killed by the boys varied from 85.8 to 100 with the mean value of 96.0 and that for the girls varied from 86.4 to 99.9 with a mean value of 94.6, which was significantly lower (Table 4). Furthermore, complements from the anemic children showed lower bactericidal activity compared to the non-anemic children.
The numbers of colonies grown on agar plates varied from 65.8-1245.7×106 cfu/mL when E. coli DH5α were grown in PBS medium (control). On the other hand, the number of colonies varied from 0.2-52.9×106 cfu/mL and 0.3-88.5×106 cfu/mL after treatment of the bacterial cells with plasma complements from the boys and girls, respectively. Compared to PBS medium, complements from both the boys and girls exhibited highly significant bactericidal activities (p < 0.001, both). However, the percentages of bacterial cells killed by the boys varied from 85.8 to 100 with the mean value of 96.0 and that for the girls varied from 86.4 to 99.9 with a mean value of 94.6, which was significantly lower (Table 4). Furthermore, complements from the anemic children showed lower bactericidal activity compared to the non-anemic children.
| Study children | Bactericidal activity (%) | Statistics (p-value) |
| Boys (N=68) | 96.0 ± 3.5 | ?0.05 |
| Girls ( N=63) | 94.6 ± 3.3 | |
| Non anemic girls ( N = 36) | 94.7 ± 2.8 | NS |
| Anemic girls ( N=27) | 94.4 ± 3.9 |
Values are mean ± SD. NS: Not significant.
Table 4: Complement mediated bactericidal activity in children.
Table 4: Complement mediated bactericidal activity in children.
Discussion
Anemia is known to be a significant global problem as it affects 305 million (25.4%) of school age children. In developing countries, the prevalence of anemia among school age children is 40%. Lack of awareness among the mothers about the problem coupled with their low educational status, poor nutritional practices and unhealthy food habits, low iron bioavailability of the diet, decreased physical activities, malaria and parasitic infestations are additional factors associated with lower hemoglobin level in children [15].
The present study conducted to investigate the prevalence of iron deficiency anemia in adolescent children from a leading school in Dhaka city showed that 26.67% of the participants and 44.61% of the girls were suffering from IDA. These findings support the previous observations of anemia prevalence among adolescent children [2] and girls [9]. However, our finding of IDA in 10% of adolescent boys was much lower than 31% reported earlier [9]. We found the socioeconomic background and family size of the girls were similar to those of the boys. However, the mean MFI of the girls was lower than that of the boys.
In IDA, the amount of hemoglobin within each RBC is significantly decreased; RBC is smaller in size than normal (hypochromic). In addition, iron deficiency is associated with lower MCV and MCHC [16]. The present study also found lower amount of hemoglobin within RBC (MCH), lower MCV and MCHC in girls in addition to significantly lower hemoglobin, RBC count and hematocrit than the boys. Further, the serum iron level of the girls was significantly lower than the boys, which could be due to inadequate iron intake, blood loss or impaired absorption of iron.
Once iron is translocated across the epithelial barrier, it is coupled to transferrin, which delivers iron to tissues throughout the body. Since TIBC measures the amount of iron bound with transferrin, increased TIBC indicates lower level of iron in the blood [17]. The present study found significantly lower level of TIBC in girls than the boys, while the anemic children had higher TIBC than the non-anemic children. This finding supported a previous observation that transferrin or TIBC measurement was better indicator in predicting iron deficiency [18].
Previous researches reported that in severe IDA (hemoglobin <8.0 g/dL) cholesterol level was decreased, which could be treated by iron supplementation [19-20]. It has been reported that iron deficient state reduces the activity of thyroid peroxidase and other enzymes that contribute to cholesterol synthesis [21], which suggested that IDA might have lowered the level of serum cholesterol in the girls. This study found serum total protein levels of the girls were significantly lower, which could be due to lower protein-rich food intake by the girls. Notably, upon supplementation with protein-rich food, the serum iron level was increased in children with IDA, indicating that dietary proteins helped in the management of IDA [22].
Adolescence is a critical period of life when iron requirement is high because of growth and muscle development [23]. Adolescent children often show eating disorders like refusal to eat, preference for excessive weight-loss diets, skipping meals etc. From the nutritional data, it was found that the mean weekly consumption of protein- and mineral-rich food items such as fish, egg, milk, pulses etc. were lower by the girls. During health examination, some boys and girls expressed unwillingness to consume fish and milk due to characteristic smell of the food items. However, the food intake of the children did not correlate with their MFI.
Heavy menstrual blood loss (MBL) is one of the most common adolescent gynecologic complaints which can be associated with significant morbidity. Worldwide, about 12 to 37% young women are suffering from heavy MBL [24]. In this study, 11 out of 65 girls (about17%) had heavy MBL. The mean hemoglobin level of these girls was significantly lower (p<0.001) than girls with normal MBL, which supports the observation of a previous study [25]. This finding of the present study suggests that heavy MBL is one of the most important factors contributing to IDA.
Myeloperoxidase, a heme containing enzyme involved in the production of hypochlorite, is a strong cytotoxic oxidant important in the destruction of foreign pathogens such as bacteria. Decreased polymorphonuclear leukocyte MPO activity was found in adults with IDA compared to the controls [26]. In the current study, although the plasma MPO activity in the girls was lower than the boys but the difference was not significant. This finding was similar to another study carried out in children with IDA that showed polymorphonuclear leukocytes MPO activity to be within the normal limits [27].
The present study assessed the immune functions of the adolescent children. Due to the limitation of samples, it was not possible to determine IgG and C3 levels in each sample. We found the mean serum level of IgG was lower in the girls than the boys and in anemic children than the non-anemic children. Some previous studies reported lower IgG levels in children with IDA compared to the controls [10, 28], which were consistent with the findings of the present study. Further, there was no difference in the mean levels of complement C3 in the boys and girls, which supported the observation of a previous study on iron deficient children [29].
This study found that the girls had significantly lower complement-mediated bactericidal activity than the boys, and the anemic children showed lower bacterial killing than the non-anemic children. It is important to recall here that the anemic children has lower levels of serum IgG which is an important opsonin that help complement function in exhibiting bactericidal activity, which may be affected in its deficiency. Lower bactericidal activity in the girls, particularly anemic children, might increase their susceptibility to bacterial infection.
Conclusion
This study found 26.67% of the adolescent school children in Dhaka city were anemic and the prevalence of anemia was significantly higher in the girls, 44.61%, who had significantly lower hemoglobin, hematocrit, RBC count, serum iron and TIBC than the boys. The overall intake of protein- and mineral-rich food items was lower by the girls, which was largely due to poor dietary habit. About 17% of the girls suffered heavy MBL which was associated with anemia. The girls had lower IgG, complement C3, MPO activity and significantly lower bactericidal activity, suggesting impaired immune function. Further, the anemic children had lower serum IgG and bactericidal activity than the non-anemic children, indicating that iron deficiency anemia might be responsible for a decreased humoral immunity.
Acknowledgements
This work was supported by the University Grants Commission of Bangladesh.
This work was supported by the University Grants Commission of Bangladesh.
Ethics Approval
The present study was approved by the Ethical Review Committee of the Faculty of Biological Sciences, University of Dhaka, Bangladesh.
The present study was approved by the Ethical Review Committee of the Faculty of Biological Sciences, University of Dhaka, Bangladesh.
Conflict of Interest
No conflict of interest.
No conflict of interest.
References
- Ramzi, M, Haghpanah, S, Malekmakan, L, Cohan, N, Baseri, A., Alamdari, A, Zare, N. (2011). Anemia and iron deficiency in adolescent school girls in Kavar urban area, Southern Iran. Iran Red Crescent Med J, 2011(2): 128-133.
- Balci, YI, Karabulut, A, Gurses, D, Covut, I. E. (2012). Prevalance and risk factors of anemia among adolescents in Denizil, Turkey. Iran J Pediatr, 22(1): 77- 81.
- World Health Organization (2001). Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers.
- Beard, JL. (2001). Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr, 131(2S-2): 568S-579S.
- Ilangovan, M, Prabha, DC, Narayanasamy, K, Nirmala Devi, N, Rathi, MA. (2015). Impact of the supplemented dietary iron in the biological cycle among the adolescent girls. History, 15(46): 92-98.
- Aggett PJ. Iron. In Present Knowledge in Nutrition. Erdman JW, Macdonald IA, Zeisel SH, Eds. (2012). 10th edition. Wiley-Blackwell, Washington, DC. pp 506-520.
- Qamar, K, Saboor, M, Qudsia, F, Khosa, SM, Moinuddin, Usman, M. (2015). Malabsorption of iron as a cause of iron deficiency anemia in postmenopausal women. Pak J Med Sci, 31(2): 304-308.
- Keskin, Y, Moschonis, G, Dimitriou, M, Sur, H, Kocaoglu, B, Hayran, O, Manios, Y. (2005). Prevalence of iron deficiency among school children of different socio-economic status in urban Turkey. Eur J Clin Nutr, 59(1): 64-71.
- Helen Keller International (2006). The burden of anemia in rural Bangladesh: the need for urgent action. Nutrition Surveillance Project Bull, 16: 1-4.
- Feng, XB, Yang, XQ, Shen, J. (1994). Influence of iron deficiency on serum IgG subclass and pneumococcal polysaccharides specific IgG subclass antibodies. Chinese Med J, 107(11): 813-816.
- Omara, FO, Blakley, BR. (1994). The IgM and IgG antibody responses in iron-deficient and iron-loaded mice. Biol Trace Elem Res, 46(1-2): 155-161.
- Bradley, PP, Priebat DA, Christensen RD, Rothstein G. (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Inves Dmatol, 78(3):206-9.
- Choudhury TZ, Kamruzzaman M, Islam LN. (2018). Investigation of the cellular and soluble markers of inflammation for the assessment of cardiovascular risk in patients with acute coronary syndrome in Bangladesh. Int J Immunol Stud, 2(1).
- Islam, LN, Hossain, M, Zahid, MSH. (2006). Complement mediated bactericidal activity and humoral immune response in type 2 diabetes mellitus. Int J Diabetes Metab, 14(2): 92-97.
- Assefa, S, Mossie, A, Hamza, L. (2014). Prevalence and severity of anemia among school children in Jimma Town, Southwest Ethiopia. BMC Hematology, 14(1): 1.
- Turgeon, ML. (2011). Clinical Laboratory Science: The Basics and Routine Techniques. 6th edition. Elsevier Mosby, China.
- Deokar, SA, Pooja, SKR, Amruta, AB, Anita, BR. (2013). Study of biochemical markers in iron deficiency anemia. Int J Res Med Sci, 1(4): 541-544.
- Hawkins, RC. (2007). Total iron binding capacity or transferrin concentration alone out performs iron and saturation indices in predicting iron deficiency. Clin Chim Acta, 380(1-2): 203-207.
- Ohira, Y, Edgerton, VR, Gardner, GW, Senewiratne, B. (1980). Serum lipid levels in iron deficiency anemia and effects of various treatments. J Nutr Sci Vitaminol, 26(4): 375-379.
- Choi, JW, Kim, SK, Pai, SH. (2001). Changes in serum lipid concentrations during iron depletion and after iron supplementation. Ann Clin Lab Sci, 31(2): 151–156.
- Hess, SY, Zimmermann, MB, Arnold, M, Langhans, W, Hurrell, RF. (2002). Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr, 132(7): 1951-1955.
- Baker, RD, Greer, FR. (2010). Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics, 126(5): 1040-1050.
- Mesias, M, Seiquer, I, Navarro, MP. (2013). Iron nutrition in adolescence. Crit Rev Food Sci Nutr, 53(11): 1226-1237.
- Friberg, B, Orno, AK, Lindgren, A, Lethagen, S. (2006). Bleeding disorders among young women: a population-based prevalence study. Acta Obstet Gynecol Scand, 85(2): 200-206.
- Barr, F, Brabin, L, Agbaje, S, Buseri, F, Ikimalo, J, Briggs, N. (1998). Reducing iron deficiency anaemia due to heavy menstrual blood loss in Nigerian rural adolescent. Public Health Nutr, 1(4): 249-257.
- Turgeon-O'Brien, H, Amiot, J, Lemieux, L. Dillon, JC. (1985). Myeloperoxidase activity of polymorphonuclear leukocytes in iron deficiency anemia and anemia of chronic disorders. Acta Haematol, 74(3): 151-154.
- Yetgin, S, Altay, C, Cilic, G, Lalei, Y. (1979). Myeloperoxidase activity and bactericidal function of PMN in iron deficiency. Acta Haematol, 61(1): 10-14.
- Ekiz, C, Agaoglu, L, Karakas, Z, Gurel, N, Yalcin, I. (2005). The effect of iron deficiency anemia on the function of the immune system. Hematolo J, 5(7): 579-583.
- Galan, P, Davila, M, Mekki, N, Hercberg, S. (1998). Iron deficiency, inflammatory processes and humoral immunity in children. Int J Vitam Nutr Res, 58(2): 225-230.
Citation: Laila N. Islam, Nusrat Jahan Nova, Badhan Rani Dey, Naznin Nahar Nipa, Md. Omar Faruk. (2019). Iron Deficiency Anemia in Adolescent School Children: Effects on Biochemical and Immune Functions. Archives of Nutrition and Public Health 1(2).
Copyright: © 2019 Laila N. Islam. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
