Research Article
Volume 1 Issue 1 - 2019
Inadequate Gestational Weight Gain is a Risk Factor for Preterm Delivery in Algerian Pregnant Women
Institute of Nutrition, Food and Food Technologies (INATAA), Laboratory of Nutrition and Food Technologies (LNTA), Constantine 1 University, Algeria
*Corresponding Author: Fouzia Tebbani, Institute of Nutrition, Food and Food Technologies (INATAA), Laboratory of Nutrition and Food Technologies (LNTA), Constantine 1 University, Algeria.
Received: December 04, 2019; Published: December 13, 2019
Abstract
Objectives: Determine the impact of gestational weight gain on the term of pregnancy in an Algerian context. Also use serial prenatal weights to examine whether insufficient gestational weight gain in the first, second and third trimesters is associated with the term of delivery.
Materials and Methods: We conducted a prospective and longitudinal cohort study. We followed for 9 months a cohort of 238 pregnant women, aged 20 to 43 and living in Constantine, Algeria. We followed for 9 months a cohort of 238 Algerian pregnant women, aged 20 to 43, and living in Constantine, Algeria. The starting height and weight as well as the weight at the end of each trimester of pregnancy were measured. Gestational weight gain was rated as consistent, higher or lower than IOM recommendations. Data included age, parity, education and BMI before pregnancy. Pregnancy issues were the term of delivery based on calculated gestational age. Pregnancy outcomes were analyzed in relation to weight gain. The statistics were performed using the SPSS and StatviewTM software.
Results: The mean gestational age was 38.9 ± 2.0 weeks. The rate of prematurity (<37 weeks) was 22.7% and the rate of over-term (≥ 42 weeks) concerned 9.7% of women. The majority of women (72.3%) had an abnormal weight gain at the end of the pregnancy and only 27.7% of them had adequate weight gain. After a multivariate analysis, we were able to show that regardless of the age, parity, socioeconomic level and weight status of women before gestation, excessive weight gain was more frequent in women who gave birth after term, while that insufficient weight gain was more common in those who gave birth prematurely.
Conclusion: Weight gain during pregnancy can determine the term of delivery in pregnant Algerian women. Thus, in our study, whatever the age, parity, socioeconomic level and weight of women before pregnancy, excessive weight gain is more frequent in women who gave birth after term, while taking Inadequate weight is more common in those who have given premature birth.
Key words: Pregnancy ; Excessive weight gain ; INSUFFICIENT weight gain ; Prematurity ; Term overrun.
Introduction
Weight gain during pregnancy is a biological phenomenon that promotes growth and normal development of the fetus. It is a good predictor of the child's health at birth [1]. It has been studied as a predictor of adverse pregnancy outcomes and used as a basic indicator assessing maternal and neonatal health during the prenatal period [2]. The majority of studies on the association between non-optimal gestational weight gain and pregnancy outcomes have been conducted in developed countries [3, 4].
According to the American Institute of Medicine (IOM), to every BMI before pregnancy, corresponds an acceptable weight gain. Undreweight women should gain more weight during pregnancy to meet the energy requirements for fetal growth ; while overweight and obese women should have less weight gain because the reserves available to them would be sufficient for both fetal growth and maternal metabolism [1].
An abnormal weight gain during pregnancy is associated with higher risk for adverse outcome of pregnancy such as preterm birth, low birth weight or caesarian delivery [5, 6]. Post term delivery is more common in women with excessive gestational weight gain [7]. Post term delivery is also associated with increased risk of perinatal complications, including perinatal mortality, birth injury, low Apgar scores, macrosomia, meconium aspiration syndrome, NICU admission and cesarean delivery [8, 9].
In Algeria, to our knowledge, there are no national studies on gestational weight gain in pregnant women. This multidisciplinary subject (related to the health of the mother and the child) is still very little documented because of the lack of studies. To date, there are no national references on weight gain of Algerian pregnant women. Further more, national data on the risks of inadequate gestational weight gain on maternal and child health in the short and long term are missing. This situation justifies our study which is a contribution to a better knowledge about weight gain in pregnant women during pregnancy in Algeria. Our study is the first prospective longitudinal cohort study on the monitoring of weight gain during pregnancy.
The influence of gestational weight gain on term of delivery among Algerian women remains to be elucidated. Thus, the aim of the study is to determine the impact of gestational weight gain on the term of delivery in the Algerian context. Also to use serial antenatal weights to examine whether inadequate GWG in the first, second and third trimesters is associated with term of delivery.
Materials and Methods
Study site and population
We conducted a prospective and longitudinal cohort study. We followed for 9 months a cohort of Algerian pregnant women, aged 20 to 43 years old, residing in the city of Constantine, Algeria. The setting of the study were maternities, antenatal centers and private gynecologists and the study has been carried out from December 2013 to July 2016.
We conducted a prospective and longitudinal cohort study. We followed for 9 months a cohort of Algerian pregnant women, aged 20 to 43 years old, residing in the city of Constantine, Algeria. The setting of the study were maternities, antenatal centers and private gynecologists and the study has been carried out from December 2013 to July 2016.
We excluded women refusing to participate in the study (n = 703, 57.1%) and women with multiple pregnancies (n = 15, 1.2 %) to homogenize and simplify the calculations of rate of weight gain. We further excluded women with gestational diabetes and hypertension (n = 114, 9.3%). We further excluded women who started prenatal care after 13 weeks of pregnancy (n = 110, 8.9%), in order to approximately estimate prepregnancy weight using weight measurements in 1st trimester because we did not have available information on the women’s prepregnancy weight measurements. Finally, we excluded women who missed the weight measurement appointment (n = 51, 4.1%), leaving 238 (19.3%) women available for the analysis. Potentially eligible women were given an informational letter explaining the study and its objectives and requesting their participation. A signed consent was obtained from each study participant.
Data collection procedures
The participants were recruited and followed up longitudinally, once at the end of each trimester of pregnancy. The trimesters were defined as first (less than 16 weeks of amenorrhea), second (16–28 weeks of amenorrhea) and third (29–41 weeks of amenorrhea) [10].
The participants were recruited and followed up longitudinally, once at the end of each trimester of pregnancy. The trimesters were defined as first (less than 16 weeks of amenorrhea), second (16–28 weeks of amenorrhea) and third (29–41 weeks of amenorrhea) [10].
Study variables
Informations on age, parity, education and socioeconomic level were collected through the structured questioner with women. These informations were obtained by face to face interview with each pregnant woman. Level of education was divided into three categories depending on the schooling level: a low level (illiterate and primary), a medium level (middle and secondary plus training) and a high level (university).
Informations on age, parity, education and socioeconomic level were collected through the structured questioner with women. These informations were obtained by face to face interview with each pregnant woman. Level of education was divided into three categories depending on the schooling level: a low level (illiterate and primary), a medium level (middle and secondary plus training) and a high level (university).
For standard of living, we proceeded to calculate a score reflecting the socioeconomic level of women in our population. The approach consisted in assigning a score, which reflected the degree of ease of pregnant women for each of the variables considered as predictor. The establishment indicators selected were: the overall monthly income of the household, the number of active persons per household, which allowed us to define an index of economic coverage given by the number of active people for each person living under the same you. Also, the type of occupancy (owner or tenant), the occupancy rate per household, which was defined by the ratio of household size (number of people) and the number of rooms of the family home, and finally owned property (TV, freezer, stove, bath heater, air conditioner, microwave, washing machine, internet connection and car). The low standard of living score (SNV) was assigned to pregnant women whose total was less than 10 points ; the average SNV for those with total points was between 10 and 15 points and the high SNV group represents women who totaled more than 15 points.
Maternal pre-pregnancy BMI and GWG
Weight and height were measured according to a standard protocol [14, 15]. Pre-pregnancy weight was measured when the pregnant woman consulted at the early first trimester. During pregnancy, weight was measured at the end of each trimester (first, second and third) by using an electronic weighing balance Seca to the nearest 0.1 kg and weight gain of each pregnancy trimester was calculated by subtracting the previous trimester weight from the current trimester weight. Height was measured in centimetres using a Seca toise, with a length of 2 m graduated in centimeters and with a precision of 0.1 cm. Pregnant women were asked to maintain an upright and erect posture with her feet together and the back of her heels touching the pole of the anthropometer. The height was measured when the horizontal headpiece was lowered onto the women’s head.
Weight and height were measured according to a standard protocol [14, 15]. Pre-pregnancy weight was measured when the pregnant woman consulted at the early first trimester. During pregnancy, weight was measured at the end of each trimester (first, second and third) by using an electronic weighing balance Seca to the nearest 0.1 kg and weight gain of each pregnancy trimester was calculated by subtracting the previous trimester weight from the current trimester weight. Height was measured in centimetres using a Seca toise, with a length of 2 m graduated in centimeters and with a precision of 0.1 cm. Pregnant women were asked to maintain an upright and erect posture with her feet together and the back of her heels touching the pole of the anthropometer. The height was measured when the horizontal headpiece was lowered onto the women’s head.
Pre pregnancy nutritional status was defined and categorized using body mass index [BMI = weight (kg)/height (m2)], according to the Institute of Medicine (IOM) [1] criteria: underweight < 18.5, 18.5-24.9 -normal weight. 25.0- 29.9 -overweight and equal or more than 30 –obese. Total gestational weight gain was estimated by subtracting the pre pregnancy weight from the last weight before the delivery. The recommended gestational weight gain for each nutritional status is given in Table 1.
| Weight status before pregnancy | Pre- pregnancy BMI (kg/m²) | Weight gain during the 1st trimester (kg) | Weight gain during the 2nd and 3rd trimesters (kg) | Total weight gain (kg) |
| Underweight | ≤ 18.5 | 0.5-2 | 5.28 à 6.96 | 12.5 à18 |
| Normal | 18.5-24.9 | 4.20 à 6.0 | 11.5 à 16 | |
| Overweight | 25-29.9 | 2.76 à 3.96 | 7 à 11.5 | |
| Obese | ≥ 30 | 2.04 à 3.24 | 5 à 9 |
Table 1: Recommended range of quarterly and total gestational weight gain (GWG) for pregnant women by pre-pregnancy body mass index (BMI) [1].
Gestational weight gain in relation to pre-pregnancy BMI was divided in two groups of normal and abnormal based on the recommendations of IOM [1]. Accordingly, the normal was defined as weight gain within the suggested gain and abnormal one as above or below the recommendations.
Outcomes
Outcome of interest was term of delivery based on the calculated gestational age. Gestational age is the only criterion used to identify the term delivery in weeks of amenorrhea (WA). It was estimated as the difference between the first day of the last menstrual period (LMP) and the date of birth. It was confirmed or corrected from an early ultrasound report at the first consultation. In case of discrepancy between the estimation by the LMP and the ultrasound, the decision was to use ultrasound evaluation that was conducted by the gynecologist in early pregnancy [11, 12, 13]. A pre-term delivery was defined as any delivery occurring before 37 weeks of amenorrhea (WA) and was considered as a premature delivery. A term delivery was any delivery occurring between 37 and 41 weeks. A post-term delivery occured when gestational age was greater than or equal to 42 WA.
Outcome of interest was term of delivery based on the calculated gestational age. Gestational age is the only criterion used to identify the term delivery in weeks of amenorrhea (WA). It was estimated as the difference between the first day of the last menstrual period (LMP) and the date of birth. It was confirmed or corrected from an early ultrasound report at the first consultation. In case of discrepancy between the estimation by the LMP and the ultrasound, the decision was to use ultrasound evaluation that was conducted by the gynecologist in early pregnancy [11, 12, 13]. A pre-term delivery was defined as any delivery occurring before 37 weeks of amenorrhea (WA) and was considered as a premature delivery. A term delivery was any delivery occurring between 37 and 41 weeks. A post-term delivery occured when gestational age was greater than or equal to 42 WA.
Statistical analyses
Statistical analyses were performed using StatView software version5 (Abacus Concepts TM, Berkeley, USA) and SPSS. Continuous variables were described using mean ± standard deviation and categorical variables were described using frequencies. Underweight (BMI < 18.5 kg/m2) and normal weight women (BMI 18.5–24.9 kg/m2) were analyzed together because only 2.1% of the eligible sample was under weight.
Statistical analyses were performed using StatView software version5 (Abacus Concepts TM, Berkeley, USA) and SPSS. Continuous variables were described using mean ± standard deviation and categorical variables were described using frequencies. Underweight (BMI < 18.5 kg/m2) and normal weight women (BMI 18.5–24.9 kg/m2) were analyzed together because only 2.1% of the eligible sample was under weight.
Differences between groups were tested for statistical significance by using Chi square test for proportions, Student’s t-test, or one-way ANOVA for continuous variables and multiple comparisons.
Multivariate analyses were done to find out the association between GWG and term of delivery and were adjusted for some maternal characteristics such as age, parity, education, socioeconomic level and BMI. Comparisons were made between recommended weight gain, less than recommended weight gain, and more than recommended weight gain in each BMI class. A value of P < 0.05 was considered significant.
Results
A total of 238 women participated in the study with a mean age of 30.1 ± 4.9 years. Of those, only 15.5 % were over 35 years old and 84.5 % were between 20 and 35 years old. The majority of women were primiparous (36.6 %). Mean pre-pregnancy BMI was 27.2 ± 5.2 kg/m2. Before pregnancy, 63.9 % of women were overweight (of which 23.1 % obese) and only 36.1 % of them had normal weight status before pregnancy. None of the women was underweight before pregnancy. Further demographic information was listed on Table 2.
The mean gestational age was 38.9 ± 2.0 weeks. The lowest gestational age was 27.0 weeks and the longest gestational age was 43.0 weeks. The majority of women (67.6 %) had a normal-term (> 37 weeks). The prematurity rate (< 37SA) was 22.7 % and the term overrun (≥ 42 weeks) concerned 9.7 % of women in our population (Table 2).
| Number (%) | Mean ± SD | |
| Maternal age (years) | ||
| 20-35 | 201 (84.5) | 30.1 ± 4.9 |
| > 35 | 37 (15.5) | |
| Parity | ||
| Nuliparity | 82 (34.5) | 1.0 ± 0.9 |
| Primiparity | 87 (36.6) | |
| multiparity | 69 (29.0) | |
| Maternal education | ||
| Low | 49 (20.6) | |
| Average | 96 (40.3) | |
| High | 93 (39.1) | |
| Socio-economic level | ||
| Low | 79 (33.2) | |
| Average | 115 (48.3) | |
| High | 44 (18.5) | |
| Pre-pregnancy BMI (kg/m2) | ||
| Normal (18.5-24.9) | 86 (36.1) | 27.2 ± 5.2 |
| Overweight (25-29.9) | 97 (40.8) | |
| Obese (≥30) | 55 (23.1) | |
| Gestational age at delivery (weeks) | ||
| Pre-Term | 54 (22.7) | 38.9 ± 2.0 |
| Full-Term | 161 (67.6) | |
| After-Term | 23 (9.7) | |
Data are mean ± SD or n (%) unless otherwise specified
BMI: body mass index
GWG: gestational weight gain.
Table 2: Characteristic of women included in the study.
BMI: body mass index
GWG: gestational weight gain.
Table 2: Characteristic of women included in the study.
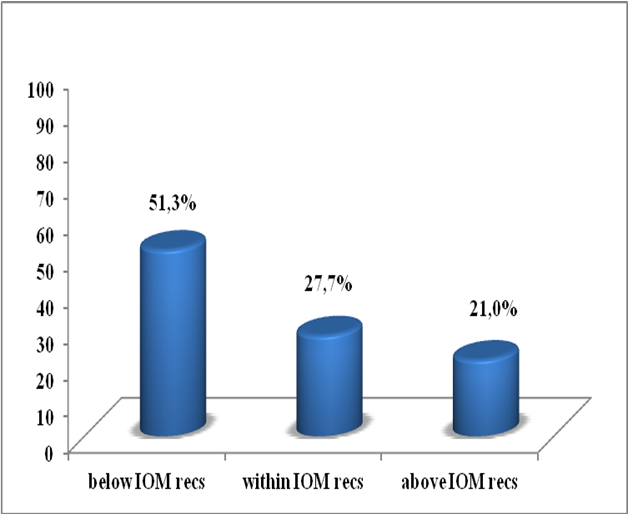
The mean overall gestational weight gain was 8.6 ± 5.6 kg. The mean rate of GWG in 1st, 2nd and 3rd trimester was respectively 1.0 ± 3.3 kg, 4.4 ± 2.9 kg and 3.2 ± 2.4 kg (Table 3). According to the 2009 IOM recommendations, we categorized weight gain into three categories: insufficient, adequate and excessive. The majority of women (72.3 %) had an abnormal weight gain at the end of pregnancy (of which 51.3 % had an insufficient weight gain and 21.0 % had an excessive weight gain) and only 27.7 % of them had an adequate weight gain (Figure 1).
| Mean ± SD | Min | Max | |
| 1er trimester weight gain (kg) | 1.0 ± 3.3 | -8.4 | 10.0 |
| 2ème trimester weight gain (kg) | 4.4 ± 2.9 | -2.3 | 12.8 |
| 3ème trimester weight gain (kg) | 3.2 ± 2.4 | -5.7 | 10.3 |
Table 3: Average weight gain of pregnant women during the three trimesters of pregnancy.
Excessive weight gain was significantly more frequent (p < 0.0001) in obese women (30.9%) compared to overweight (20.6%) and normoponderal women (15.1%). And insufficient gain was more common (p < 0.0001) in obese (65.5%) and normoponderal women (55.8%) (Table 4). Meanwhile, the mean weight gain in the four pre pregnancy BMI groups (18.5 -24.9, 25.0-29.9, ≥ 30) was respectively 11.0 ± 5.2 kg, 8.5 ± 5.2 kg and 5.1 ± 5.1 kg.
| Maternal weight gain | Pre-pregnancy BMI | p | |||||
| Obese | Overweight | Normal | |||||
| N | % | N | % | N | % | ||
| Above IOM recs | 17 | 30.9 | 20 | 20.6 | 13 | 15.1 | <0.0001 |
| Within IOM recs | 2 | 3.6 | 39 | 40.2 | 25 | 29.1 | |
| Below IOM recs | 36 | 65.5 | 38 | 39.2 | 48 | 55.8 | |
BMI, body mass index; IOM, Institute of Medicine.
Comparison performed using Chi-square test.
Table 4: Maternal weight gain by pre-pregnancy BMI category.
Comparison performed using Chi-square test.
Table 4: Maternal weight gain by pre-pregnancy BMI category.
As it was shown in Table 5, the percentage of inadequate gestational weight gain was higher among women who gave a pre-term birth (p = 0.001), compared with women in other groups (68.5 % versus 49.1 % and 26.1 %) while the percentage of excessive gestational weight gain was higher among women who gave a post-term birth (p = 0.001), compared with women in other groups (47.8 % versus 21.1 % and 9.3 %). For weight gain in each trimester of pregnancy, we have found that women with insufficient gain in the 2nd (p = 0.0002) and 3rd (p = 0.0086) trimesters were more likely to gave a pre term delivery. While, women with excessive gain in the 2nd (p = 0.0002) and 3rd (p = 0.0086) trimesters were more likely to gave a post term delivery. For weight gain in the 1st trimester of pregnancy, no significant difference was observed. Further data on parity, age, education and pre-gestational status divided by the three groups was given in Table 5.
| Full-Term (%) | Pre-Term (%) | Post-Term (%) | P | |
| Maternal age (years) | ||||
| 20-35 | 86.3 | 81.5 | 78.3 | 0.47 |
| > 35 | 13.7 | 18.5 | 21.7 | |
| Parity | ||||
| Nuliparity | 33.5 | 40.7 | 26.1 | 0.3 |
| Primiparity | 39.8 | 29.6 | 30.4 | |
| multiparity | 26.7 | 29.6 | 43.5 | |
| Maternal education | ||||
| Low | 21.7 | 18.5 | 17.4 | 0.4 |
| Average | 36.0 | 48.1 | 52.2 | |
| High | 42.2 | 33.3 | 30.4 | |
| Socio-economic level | ||||
| Low | 31.1 | 35.2 | 43.5 | 0.5 |
| Average | 48.4 | 51.9 | 39.1 | |
| High | 20.5 | 13.0 | 17.4 | |
| Pre-pregnancy BMI (kg/m2) | ||||
| Normal (18.5-24.9) | 34.2 | 44.4 | 30.4 | 0.26 |
| Overweight (25-29.9) | 44.7 | 27.8 | 43.5 | |
| Obese (≥ 30) | 21.1 | 27.8 | 26.1 | |
| Total GWG (kg) | ||||
| Below IOM recs | 49.1 | 68.5 | 26.1 | 0.001 |
| WithinIOM recs | 29.8 | 22.2 | 26.1 | |
| Above IOM recs | 21.1 | 9.3 | 47.8 | |
| 1st trimester GWG (kg) | ||||
| Below IOM recs | 44.7 | 42.6 | 43.5 | 0.49 |
| WithinIOM recs | 17.4 | 14.8 | 4.3 | |
| Above IOM recs | 37.9 | 42.6 | 52.2 | |
| 2nd trimester GWG (kg) | ||||
| Below IOM recs | 2.92 | 57.4 | 17.4 | 0.0002 |
| WithinIOM recs | 28.6 | 9.3 | 17.4 | |
| Above IOM recs | 42.2 | 33.3 | 65.2 | |
| 3rd trimester GWG (kg) | ||||
| Below IOM recs | 43.5 | 69.8 | 39.1 | 0.0086 |
| WithinIOM recs | 29.2 | 13.2 | 21.7 | |
| Above IOM recs | 27.3 | 17.0 | 39.1 | |
Data are (%) unless otherwise specified
Comparison performed using Chi-square test.
Table 5: Materanl characteristics based on term of delivery.
Comparison performed using Chi-square test.
Table 5: Materanl characteristics based on term of delivery.
After a multivariate analysis, we were able to show that regardless of age, parity, socio-economic level and pre-gestational weight status of women, excessive weight gain was more common among women who gave birth after term, while insufficient weight gain was more common among those who gave birth before term.
Discussion
In our study, we were able to demonstrate that obesity before pregnancy is a risk factor for both excessive and insufficient gestational weight gain. Similar associations have been reported in other studies examining the relationship between pre-pregnancy BMI and weight gain during pregnancy. They found rates of excessive gestational weight gain in obese women ranging from 44.0 % to 69.0 % [16, 17, 18]. Being obese before pregnancy and having excessive gestational weight gain are important issues that have been associated with an increase in adverse outcomes for pregnant women and their children in the short and long term [19]. Also, our results are consistent with other studies that have found that high maternal BMI before pregnancy is associated with low weight gain during pregnancy [16, 20, 21]. Inadequate weight gain in obese women could be explained by medical care and / or women's monitoring of their weight gain to avoid gaining too much weight [21]. However, information on the ideal gestational weight gain for each woman based on her starting BMI, if delivered, is among a significant amount of messages to be transmitted to the woman during her pregnancy. Thus, in our population, the majority of women (78.7 %) did not know the proper value of weight gain during pregnancy and the real impacts of abnormal weight gain (excessive or insufficient) on their health and that of their child. This could explain the high percentage of excessive and insufficient gain observed in obese women.
The average term of pregnancy is 38.9 ± 2.0 Week of Amenorrhea (WA). The majority of women (67.6 %) had normal-term (> 37 weeks). The prematurity rate (< 37 SA) is 22.7 % and the term surpassing rate (> 41SA) concerns 9.7 % of women. According to the World Health Organisation, the rate of prematurity in the world varies between 5.0 % and 18.0 % [11]. In Constantine (Algeria), in 2002, TOUATI [22] observed a preterm birth rate of 6.2 %. Our results are far superior to the different observations.
In terms of weight gain, women with low weight gain were significantly more likely (68.5 %) to have delivered prematurely, compared to women with full (49.1 %) and post term (26.1 %) (p = 0.001). these findings can be explained by the fact that in women with insufficient weight gain, the nutritional needs of the fetus are no longer covered by the mother, that’s why we have noted a pre term delivery in this group of women. Several studies have found that prematurity is associated with insufficient gestational weight gain [23, 24]. XINXO et al. [25] et HAN et al, [26], reported that women with low weight gain had a 1.8-fold risk of premature delivery. A meta-analysis found that insufficient gestational weight gain was associated with an increase in premature births [27]. In a systematic review, researchers found that of 13 studies, 11 showed a significant association between low weight gain and prematurity, mainly when the weight gain was lower in the third trimester of pregnancy [28]. Similarly, the study by HICKEY et al. [29], found that less weight gain than IOM's recommendations during the third trimester of pregnancy is associated with an increased risk of preterm birth. The study by ROMAN et al. [30], showed that prematurity is found to be significantly greater in women with insufficient weight gain during pregnancy regardless of BMI. Weight gain during pregnancy is linked to premature delivery. A meta-analysis of 13 studies concluded that insufficient weight gain is associated with an increased risk of prematurity [28]. The rate of preterm delivery increases with weight gain of less than 0.34 kg per week from 20th to 36th SA [31].
We have also found that women with insufficient gain in the 2nd and 3rd trimesters were more likely to gave a pre term delivery. For weight gain in the 1st trimester of pregnancy, no significant difference was observed. Similarly, the study by HICKEY et al. [29], found that insufficient weight gain than IOM's recommendations during the third trimester of pregnancy is associated with an increased risk of preterm birth. The same authors showed that insufficient weight gain in the first trimester was not associated with prematurity (HIEKEY et al. 1995). Several studies have reported an association between low gestational weight gain (GWG) and increased risk of preterm birth [26, 23]. However, a simulation study demonstrated that conventional measures of GWG historically examined (i.e., total GWG or average rate of GWG) may have biased observed associations between GWG and preterm birth due to the inherent correlation between GWG and gestational age [32]. GWG is not linear throughout pregnancy. Instead, it is slower during the first trimester and then increases at a relatively linear rate in the second and third trimesters [1]. Thus, a woman who delivers earlier will have a lower total and average rate of GWG than a woman who delivers later. Most studies have been limited by the availability of only two weight measures (pregravid or early pregnancy weight and weight at delivery) and therefore have examined total GWG or average rate of GWG across the entire pregnancy [26].
To reduce bias associated with gestational age, some studies have examined rate of GWG in the second and third trimesters only by assuming a constant GWG in the first trimester among all pregnancies. This, however, also assumes that first trimester gain is less informative and few studies have specifically examined first trimester GWG. An alternative approach to resolve the inherent bias with gestational age is to examine GWG up to a given gestational week achieved by all pregnancies and determine whether GWG differs among those who eventually delivered pre-term or at term. Furthermore, it has been suggested that the pattern and timing of GWG over the course of pregnancy, not just the total amount, may be an important predictor of preterm birth, particularly for spontaneous preterm labor [33, 34]. Few studies, however, have used serial weight measures to examine GWG patterns throughout pregnancy [33, 28].
In the other hand, women in our study with total excessive weight gain and excessive gain in the 2nd and 3rd trimesters were significantly more frequent (47.8 %) to have delivered after term, compared to women with full (21.1 %) and pre term (9.3 %) (p = 0.001). Our results are similar to those found by DERUELLE et al. [35], who suggested that excessive weight gain is a risk factor for a term exceeding. Other studies have also shown that overtime delivery is more common in women with excess weight gain [36, 37]. Thus, gestation duration is significantly higher in women with excessive weight gain compared to women with reasonable weight gain [38]. Our findings can be explained by the fact that in women with excessive gestational weight gain nutritional needs of the fetus were still be covered by the mother, that’s why we have noted a post term delivery in this group of women.
Conclusion
Gestational weight gain can determine the term of delivery among the Algerian women. Thus, in our study, regardless of age, parity, socio-economic level and pre-pregnancy weight status of women, excessive weight gain is more common among women who gave birth after term, while insufficient weight gain is more common among those who gave birth prematurely. These findings place the maternal gestational weight gain as potential predictor of term of delivery. Gestational weight gain assessments should be part of routine prenatal visits to avoid inadequate weight gain, whether excessive or insufficient.
References
- IOM. (2009). Weight Gain during Pregnancy: Reexamining the Guidelines; Committee to Reexamine IOM Pregnancy weight Guidelines; Sponsor Briefing May 27.
- Hernandez DC. (2012). Gestational weight gain as a predictor of longitudinal body mass index transitions among socioeconomically disadvantaged women. J Womens Health (Larchmt) 21: 1082-90.
- Sullivan EA, Dickinson JE, Vaughan GA, Peek MJ, Ellwood D, Homer CS et al. (2015) Maternal super-obesity and perinatal outcomes in Australia: a national population-based cohort study. BMC Pregnancy and Childbirth. 15:322.
- Walsh J, McGowan C, Mahony R, Foley M, Mcauliffe F. (2014). Obstetric and Metabolic Implications of Excessive Gestational Weight Gain in Pregnancy. Obesity | VOLUME 22 | NUMBER 7.
- Dietz PM, Callaghan WM, Cogswell ME, Morrow B, Ferre C, Schieve LA. (2006). Combined effect of prepregnancy body mass index and weight gain during pregnancy on the risk of preterm delivery. Epidemiology. 17(2): 170-177.
- Hauger MS, Gibbons L, Vik T, Belizan JM. (2008). Pre pregnancy weight status and risk of adverse pregnant outcome. Acta Obstet Gynecol Scand. 87(9): 953-959).
- Johnson JW, Longmate JA, Frentzen B. (1992). Excessive maternal weight and pregnancy outcome. Am J Obstet Gynecol. 167 (2): 353-70.
- Norwitz ER, Snegovskikh VV, Caughey AB. (2007) Prolonged pregnancy. When should we intervene? Clin Obstet Gynceol 50(2): 547.
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i–xii, 1–253.).
- CDC. Pregnancy nutrition surveillance nation. Summary of trends in maternal health indictors 2011. http://www.cdc.gov/pednss/pnss_tables/tables_numeric.htm. Retrieved February 25, 2014.
- WHO. (2016) Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland:World Health Organization.
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo Jh et al. (2010). "The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity." Bull World Health Organ, 88 (1): 31-38.
- WHO. "WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths 1977. Modifications recommended by FIGO as amended October 14, 1976.
- Cogill B. (2003). Guide to measuring anthropometric indicators. Technical assis- tance project for food and nutrition. Washinghton, DC: Academy for Development and Education. p. 104.
- WHO, (1995). Expert Committee. Physical status: the use and interpretation of anthropometry. Geneva: WHO, technical report; [series 854].
- Tebbani F, Oulamara H, Agli A. (2018). Effects of gestational weight gain on pregnancy complications. Nutrition clinique et métabolisme 32 pp. 28-33.
- Akgun N, Keskin HL, Ustuner I, Pekcan G, Avsar AF. (2017). Factors affecting pregnancy weight gain and relationships with maternal/fetal outcomes in Turkey. Saudi Med J Vol. 38 (5): 503-508.
- Boyle A, Timofeev J, Halscott T, Desale S, Driggers RW, Ramsey PS. (2014). Is 40 the new 30? pregnancy outcomes by degree of weight gain among obesity subclasses. Obstet Gynecol. 123 Suppl 1:41S.
- Guilloty N, Soto R, Anzalota L, Rosario Z, Cordero JF, Palacios C. (2015). Diet, Pre-pregnancy BMI, and Gestational Weight Gain in Puerto Rican Women. Matern Child Health J. November ; 19(11): 2453–2461.
- Sato A, Fujimori E. (2012). Nutritional status and weight gain in pregnant women. Rev. Latino-Am. Enfermagem May.-June; 20 (3):462-8.
- Heude B, Thiébaugeorges O, Goua V, Forhan A, Kaminski M, Foliguet B et al. (2011). Pre-pregnancy body mass index and weight gain during pregnancy: relations with gestational diabetes and hypertension, and birth outcomes. Matern Child Health J. 16:355-63.
- Touati D. (2011). Statut nutritionnel et sociodémographique d’une cohorte de femmes enceintes d’el Khroub (Constantine, Algerie). Répercussions sur le poids de naissance du nouveau-né. Thèse de Doctorat en sciences alimentaires Spécialité : Nutrition Humaine. N° d’ordre. 64/TS/2011 N° de Série : 02/INAT/2011.
- Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J et al. (2008). Outcomes of maternal weight gain. Evid Rep Technol Assess. 168: 1-223.
- Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. (2007). Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol 21:5–14.
- Xinxo S, Bimbashi AZ, Kakarriqi E, Zaimi E. (2013). Association between maternal nutritional status of pre pregnancy, gestational weight gain and preterm birth. Mater Sociomed. 25: 6-8.
- Han Z, Lutsiv O, Mulla S, Rosen A, Beyene J, McDonald SD. (2011). Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 90: 935-54.
- Goldstein FR, Boyle AJ, Rode L, Abell SK, Misso M, Kim YJ et al. (2017). Association of Gestational Weight Gain with Maternal and Infant Outcomes. A Systematic Review and Meta-analysis. JAMA. 317(21):2207-2225.
- Carmiehael SL, Abrams B. (1997). A critical review of the relationship between gestational weight gain and preterm delivery. Obstet Gyneeol. 89(5 Pt 2): p. 865-73.
- Hiekey CA, Cliver SP, Mcneal SF, Hoffman HJ, Goldenberg RL. (1995). Prenatal weight gain patterns and spontaneous preterm birth among nonobese black and white women. Obstet Gyneeol, 85(6): p. 909-14.
- Roman H, Goffinet F, Hulsey TF, Newman R, Robillard PY, Hulsey TC. (2008). Maternal body mass index at delivery and risk of caesarean due to dystocia in low risk pregnancies. Acta Obstet Gynecol Scand 87(2): 163-70.
- Théron GB, Thompson ML. The usefulness of weight gain apart to identify women who will develop preeclampsia. Ear Gynaecol Biol 1998; 78: 47-51.
- Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. (2012). The bias in current measures of gestational weight gain. Paediatric and Perinatal Epidemiology. 26(2):109–116. [PubMed: 22324496].
- Rudra CB, Frederick IO, Williams MA. (2008). Pre-pregnancy body mass index and weight gain during pregnancy in relation to preterm delivery subtypes. Acta Obstetricia et Gyne-cologica Scandinavica. 87(5):510–517.
- Siega-Riz AM, Adair LS, Hobel CJ. (1996). Maternal underweight status and inadequate rate of weight gain during the third trimester of pregnancy increases the risk of preterm delivery. Journal of Nutrition. 126(1): 146–153.
- Deruelle P, Houffin-Debarge V, Vaast P, Delville N, Helou N, Subtil D et al. (2004). Effets maternels et foetaux d'une prise de poids excessive au cours de la grossesse dans une population de patientes de poids normal avant la grossesse. Gynécologie Obstétrique et Fertilité 32 : 398-403.
- Hamon C, Fanello S, Catala L, Parot E. (2005). Conséquences de l’obésité maternelle sur le déroulement du travail et l’accouchement. Rev Sage-Femme. 4: 172-177.
- Johnson JW, Longmate JA, Frentzen B. (1992). Excessive maternal weight and pregnancy outcome. Am J Obstet Gynecol 167 (2): 353-70.
- Scholl T, Hediger M, Schall J, Ances I, Smith W. (1995). Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstetrics & Gynecology, 86: 423-427.
Citation: TEBBANI Fouzia, OULAMARA Hayet and AGLI Abdenacer. (2019). Inadequate Gestational Weight Gain is a Risk Factor for Preterm Delivery in Algerian Pregnant Women. Journal of Gynaecology and Paediatric Care 1(1).
Copyright: © 2019 Fouzia Tebbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.