Research Article
Volume 2 Issue 1 - 2020
Impact of a Daily Probiotic (Lactobacillus casei Shirota) for 12 Months on the Frequency of Diverticulitis Episodes: Feasibility Study in Primary Care.
160 Manor Way, Onslow Village, Guildford, Surrey GU2 7RR, UK
2Department of Nutritional Sciences, Faculty of Health and Medical Sciences, Duke of Kent Building, University of Surrey, Guildford, Surrey GU2 7TE
3Queen's Avenue, Dorchester, DT1 2EP, formerly at Yakult UK Limited, Odyssey Business Park, West End Road, South Ruislip HA4 6QQ, UK
4Department of Clinical Medicine and Ageing, University of Surrey, Guildford, Surrey GU2 7XH, UK.
5Expert Nutrition Works, Whimple, Exeter, EX5 2PP
6Department of Mathematics, University of Surrey, Guildford, Surrey GU2 7XH, UK.
7Nutrition and Dietetic Service (Cambridge and Peterborough NHS Foundation Trust), First Floor, Sackville House, Sackville Way, Cambourne, Cambridgeshire, CB23 6HL
2Department of Nutritional Sciences, Faculty of Health and Medical Sciences, Duke of Kent Building, University of Surrey, Guildford, Surrey GU2 7TE
3Queen's Avenue, Dorchester, DT1 2EP, formerly at Yakult UK Limited, Odyssey Business Park, West End Road, South Ruislip HA4 6QQ, UK
4Department of Clinical Medicine and Ageing, University of Surrey, Guildford, Surrey GU2 7XH, UK.
5Expert Nutrition Works, Whimple, Exeter, EX5 2PP
6Department of Mathematics, University of Surrey, Guildford, Surrey GU2 7XH, UK.
7Nutrition and Dietetic Service (Cambridge and Peterborough NHS Foundation Trust), First Floor, Sackville House, Sackville Way, Cambourne, Cambridgeshire, CB23 6HL
*Corresponding Author: Dr. J. A. A. Nichols, Department of Nutritional Sciences, University of Surrey, 60 Manor Way, Guildford GU2 7RR, UK.
Received: March 16, 2020; Published: March 25, 2020
Background:Some small secondary care studies have shown benefits from taking probiotics in diverticular disease. We investigated the feasibility of conducting a year-long probiotic study in primary care.
Methods:Patients (n=21) with a past history of uncomplicated acute diverticulitis but not currently taking antibiotics were recruited from four general practices. Subjects were asked to take the daily probiotic drink YakultÒ. Probiotic effects were assessed by comparing clinically diagnosed diverticulitis attacks in the 6 months prior to probiotic intervention with the 0-6 and 6-12 month periods after probiotic intervention commenced. Effects on gastrointestinal symptoms were assessed by validated questionnaires at baseline, 6 and 12 months. Diet diaries were also completed.
Results: Two thirds (14/21) of subjects who demonstrated good adherence in taking the daily probiotic improved symptomatically (p=0.025). Clinical presentations with diverticulitis were down 50% during the study period compared with the 12 months period before (7 vs 14 cases; p=0.021). The mean change in gastrointestinal symptom score improved significantly (p <0.001). Diverticulitis episodes did not correlate with body mass index (BMI), diabetic status or dietary fibre.
Conclusions: Taking the daily probiotic was associated with a lower rate of clinical presentation with diverticulitis episodes and improvement in gastrointestinal symptoms. Sufficient information is presented to design a definitive trial. However, we recommend inclusion of a method to validate the diagnosis of diverticulitis. It is feasible to recruit and run a study that requires participants to take a daily probiotic drink for a year in a primary care setting.
LACTOPRoD was registered at ClinicalTrials.gov: NCT01609751 in 2012.
Keywords: Dietary fibre; Diverticulitis; Gastrointestinal symptoms; Lactobacillus casei Shirota; Primary care; Probiotic
Abbreviations: LcS: Lactobacillus casei Shirota; GP: general practitioner; NHS: National Health Service; UAD: uncomplicated acute diverticulitis; CMR: computerised medical records; BMI: body mass index; GIT: gastrointestinal tract; PI: principal investigator; IBS: irritable bowel syndrome.
Introduction
The risk of diverticulitis increases with age [1] and is a considerable burden of disease in the developed world [2]. Trials showing benefits from a daily dose of a probiotic in subjects with a history of diverticulitis have been carried out in hospital clinics but never in a primary care setting. These trials have usually involved a combination of a probiotic and another preventive [3-5]. One trial using a single probiotic without a drug or antibiotic showed significant benefits [6].
Duration and probiotic strain appear to be critical in determining whether probiotic treatment has benefits [7]. A 12-week trial showed no benefit from a daily probiotic [8] whereas a 12-month trial showed definite benefits [5]. This also cancelled out any seasonal variation [9,10]. There is a need for a trial in prevention of acute episodes of diverticulitis in a primary care setting, particularly as patients with diverticulitis are interested in probiotics [11,12]. We carried out this study to see whether it was feasible to administer a daily dose of Yakult containing the probiotic strain Lactobacillus casei Shirota (LcS) for 12 months and to explore its impact on rate of clinical presentation of diverticulitis. YakultÒ was chosen for its good quality control and a long history of safe use as well as numerous trials that have shown its probiotic efficacy, including several in gastrointestinal disorders [13-17, Supplementary Material].
Methods
The probiotic was supplied to participants free of charge at their general practitioner (GP) surgeries. Potential confounding factors include obesity [18], diabetes [19, 20] and dietary fibre [21], therefore participants were asked to complete 4-day diet diaries. This open-label, uncontrolled study investigated whether patients previously diagnosed with uncomplicated acute diverticulitis (UAD) might benefit from a 12-month probiotic intervention (2013-14). Primary outcome was the number of episodes of diverticulitis during the 0-6 and 6-12 month periods after LcS probiotic intervention compared with the previous 6 months when no probiotic was taken. Secondary outcome measures were: (i) gastrointestinal symptom scores at 0, 6 and 12 months, (ii) correlation between dietary intake and symptoms. Previous trials listed in our introduction suggest an open label trial of at least 20 probiotic naïve subjects should produce useful results.
Subjects and screening
UK general practices have a list-based system, where patients register with one GP supported by computerised medical records (CMR) that can be used for this type of trial [22]. Subjects were recruited from four general practices in Surrey, UK. A list of subjects was generated from CMR searches for people with both confirmed colonic diverticular disease and past prescriptions of antibiotics commonly prescribed for UAD. With the patient present, their GP records were explored to confirm that a diagnosis of acute diverticulitis had been made on at least one occasion.
UK general practices have a list-based system, where patients register with one GP supported by computerised medical records (CMR) that can be used for this type of trial [22]. Subjects were recruited from four general practices in Surrey, UK. A list of subjects was generated from CMR searches for people with both confirmed colonic diverticular disease and past prescriptions of antibiotics commonly prescribed for UAD. With the patient present, their GP records were explored to confirm that a diagnosis of acute diverticulitis had been made on at least one occasion.
Inclusion criteria: one or more UADs in the past 5 years; proven diverticular disease; agreement to collect and consume daily YakultÒ for 12 months; able to keep a diet, symptom and medication diaries; no probiotic gap >1 week; age ≥ 40 and ≤ 75 years at start of trial; informed consent.
Exclusion criteria: Recent history of peptic ulcer; history of inflammatory bowel disease; chronic renal insufficiency; recent major diverticulitis complications; any serious debilitating illness (cancers, cardiovascular disease etc. but not diabetes mellitus); dementias or memory problems; immunosuppressed patients.
During screening, it was discovered that 33% of potential recruits (n=22) had been taking a daily probiotic for more than 2 years because they believed this helped control GIT symptoms. Five of these subjects were monitored and assessed in a separate study (Figure 1, Table 1).
| Dosage schedule | Number of subjects | |
| Actimel* | 1 bottle daily | 2 |
| Actimel* | 1 bottle daily | 1 |
| & Activia yogurt? | 1 yoghurt daily | |
| & Benefiber (wheat dextrins) | 4 Gm daily | |
| Lactobacillus acidophilus capsule | 2-3 daily | 1 |
| Yeo Valley yoghurt³ | 1 daily | 1 |
| Total | 5 |
*Each bottle of Actimel contains 10 billion Lactobacillus casei DN-114001and smaller amounts of L bulgaricus and Streptococcus thermophilus.
?Each yoghurt contains unspecified amounts of Bifidobacterium lactis DN 173 010/CNCM and smaller amounts of L bulgaricus and Streptococcus thermophilus.
?Each yoghurt contains a Bifidobacterium (species not specified) and smaller amounts of L bulgaricus and Streptococcus thermophilus.
Table 1: Probiotics being taken by subjects in the DIY group.
?Each yoghurt contains unspecified amounts of Bifidobacterium lactis DN 173 010/CNCM and smaller amounts of L bulgaricus and Streptococcus thermophilus.
?Each yoghurt contains a Bifidobacterium (species not specified) and smaller amounts of L bulgaricus and Streptococcus thermophilus.
Table 1: Probiotics being taken by subjects in the DIY group.
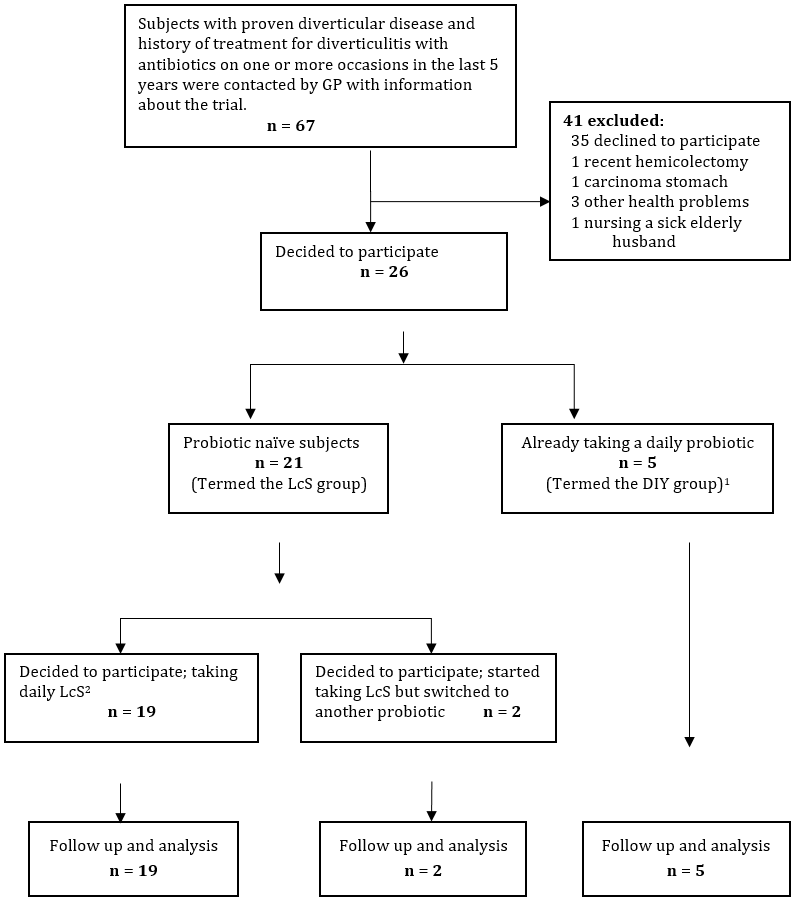
1DIY (Do-it-yourself) group were patients who had already decided of their own volition to purchase a probiotic and had already been taking a daily dose for more than one year.
2LcS: Lactobacillus casei Shirota probiotic
Figure 1: Recruitment flow diagram.
2LcS: Lactobacillus casei Shirota probiotic
Figure 1: Recruitment flow diagram.
Patients were seen and assessed by the principal investigator (PI) at a booking-in clinic. After a full explanation of the study and with their agreement to participate, suitable subjects were asked to read and sign a consent form. General health, drug history and BMI were recorded. Blood samples were taken for HbA1c measurement, as high blood sugar may be an adverse confounding factor [19,20].
Participants were helped to complete a validated gastrointestinal (GIT) symptom score questionnaire [23], which was repeated at 6 and 12 months. Over the period of the study, diverticulitis episodes were tracked from the subjects’ clinical records. There was no rapid access to imaging [24, 25], therefore diagnosis of diverticulitis was based on the following clinical criteria which were agreed by all the GPs in the participating primary care centres [26] and have been shown to be accurate in at least 66% of cases [27]:
- History of diverticulosis confirmed by investigation (usually colonoscopy)
- Low abdominal pain and persistent left iliac fossa tenderness
- Temperature above 37°C (usually above 37.3°C) [27-29].
- Records showed a history of an appropriate antibiotic prescription.
Participants were also asked to record details of their GIT symptoms and general health on monthly forms. These were returned in a Freepost envelope together with YakultÒ bottle tops. Participants were reviewed by the PI at 6 and 12 months. This involved subjects attending a research clinic at the primary care premises; if this was not possible then they were reviewed by telephone (Figure 1).
Participants were asked to complete validated diet diaries (30) at the start of the study, after six months and at the end of the study. Diaries were analysed for dietary fibre and other individual nutrients using Dietplan 6 (Forestfield Software Ltd).
The clinical event rate for diverticulitis was calculated for the 12 months and 6 months before starting LcS and during the 1-6 and 6-12 month periods taking daily LcS. Changes in these values were tested for significance using the Wilcoxon matched pairs test. The proportion of subjects that were free of diverticulitis in the same three 6-month periods was estimated and McNemar’s test of paired proportions was used to test for significance. Changes in the mean GIT symptom scores recorded at start, 6 months and 12 months, with 95% confidence limits, were calculated and the paired t-test was used to assess the significance of any changes to these values. For potential confounding factors, including HbA1c, BMI and a range of nutrient intakes, the Pearson correlation coefficient was calculated for correlations between these values and the diverticulitis attack rate and the GIT symptom score.
Results
There were no significant reports of LcS side effects.
Subject characteristics
Out of a total of 67 potential subjects identified from GP records 41 (62%) were excluded from participation (Figure 1).
Out of a total of 67 potential subjects identified from GP records 41 (62%) were excluded from participation (Figure 1).
| Demographic data | |
| Gender male/ | 8 (38.1%) |
| Married/ | 15 (71.4%) |
| Age in years at start of study | 64.4 (SD 7.5) |
| Body mass index [kg/m2] | 26.6 (SD 5.4) |
| Medical parameters | |
| Mean HbA1c% at start of study | 5.5% (SD 0.50) |
| Diabetic at start of study | 3 (14.3%) |
| Diabetic and pre-diabetic at start of study | 5 (23.8%) |
| Cardiovascular conditions | 14 (66.7%) |
| Asthma and COPD | 3 (14.3%) |
| Other GIT conditions | 6* (28.6%) |
| Arthritis and/or spinal disorders | 6 (28.6%) |
| Hypothyroidism | 1 (4.8%) |
*5 had acid reflux and 1 had ill defined symptoms treated with an anti-emetic
Table 2: Demographic and medical details of probiotic-naïve subjects at start of project (n = 21).
Table 2: Demographic and medical details of probiotic-naïve subjects at start of project (n = 21).
Table 2 summarises demographic and medical details of the group of 21 probiotic-naïve subjects recruited between June and November 2013 and who agreed to take the LcS probiotic for 12 months. Two switched to acidophilus capsules but were still included in this group for analysis on the basis of intention to treat.
Most (67%, n=14) showed good adherence with taking the probiotic every day for 12 months, as assessed by the number of Yakult® bottle tops returned. There were no losses or exclusions after recruitment.
Diverticulitis episodes
A total of 7 episodes of clinically diagnosed diverticulitis were recorded over 12 months. There were no diverticular complications requiring secondary care.
A total of 7 episodes of clinically diagnosed diverticulitis were recorded over 12 months. There were no diverticular complications requiring secondary care.
| Diverticulitis free subjects | % free from diverticulitis | Attacks of diverticulitis | ||
| 1 episode | 2 episodes | |||
| The 12 months period before starting LcS* | 8 | 38.1% | 10 | 3 |
| The 6 months period before starting LcS | 11 | 52.4% | 10 | 0 |
| 0-6 months of LcS | 16 | 76.2% | 4 | 1 |
| 6-12 months of LcS | 19 | 90.5% | 2 | 0 |
| 0-12 months of LcS* | 14 | 66.7% | 6 | 1 |
*Wilcoxon Matched Pairs test: p=0.021
Table 3a: Changes in attack rate for diverticulitis 2013-14 on the basis of LcS intention to treat for probiotic naïve subjects (n = 21).
Table 3a: Changes in attack rate for diverticulitis 2013-14 on the basis of LcS intention to treat for probiotic naïve subjects (n = 21).
| Diverticulitis free subjects | % free from diverticulitis | Attacks of diverticulitis | ||
| 1 episode | 2 episodes | |||
| The 12 months period before starting LcS? | 5 | 23.8% | 7 | 2 |
| The 6 months period before starting LcS | 8 | 57.1% | 6 | 0 |
| 0-6 months of LcS | 10 | 71.4% | 3 | 1 |
| 6-12 months of LcS | 13 | 92.9% | 1 | 0 |
| 0-12 months of LcS? | 9 | 64.3% | 4 | 1 |
Table 3b: Changes in attack rate for diverticulitis 2013-14 for probiotic naïve LcS subjects with good compliance only (n = 14).
Tables 3a and 3b demonstrate a reduction in diverticulitis attacks associated with the 12 months intervention (Wilcoxon Matched Pairs test: p=0.021) and for the good adherence sub-group of 14 subjects (Wilcoxon Matched Pairs test: p=0.025).
There was an increase in the proportion free of diverticulitis (McNemar’s test p=0.008) for all LcS subjects (n=21) and for subjects with good adherence (n=14; McNemar’s test: p=0.063). We estimated that this preventive effect could save the NHS approximately £1,300,000/year (supplementary material).
Gastrointestinal symptoms
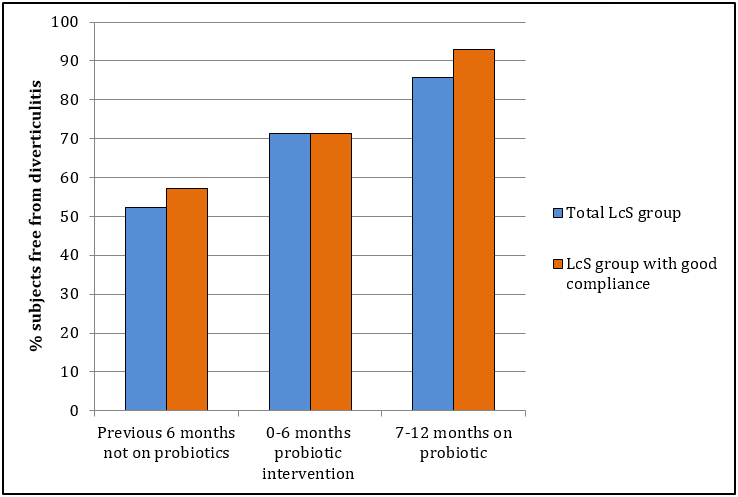
Figure 2: Proportion of subjects taking LcS (L casei Shirota) probiotic, who were free of episodes of diverticulitis. A statistically significant improvement from the first 6 month period compared with the final 6 month period for all 21 LcS subjects and the 14 subjects showing good compliance in taking the daily probiotic (p < 0.01 for both).
There was an improvement in mean GIT symptom scores over the 12-month course of probiotic intervention (Figure 3): start score 25.8 (95% CI 20.4-31.2), 6 month score 19.6 (95% CI 14.4-24.8; paired t-test: p=0.012), 12 month score 15.8 (95% CI 10.6-21.0; p<0.001) and for subjects with good compliance (n=14): mean start score 25 (95% CI 18.6-31.4), 6 month score 18.1 (95% CI 13.3-22.9; p=0.027), 12 month score 14.7 (95% CI 10.9-19.2; p=0.002). There was a mean change of -10.05 (i.e. an improvement) from 0-12 months (SD=10.47). The 14 LcS subjects with good adherence exhibited a mean change of -10.29 (SD=9.79).
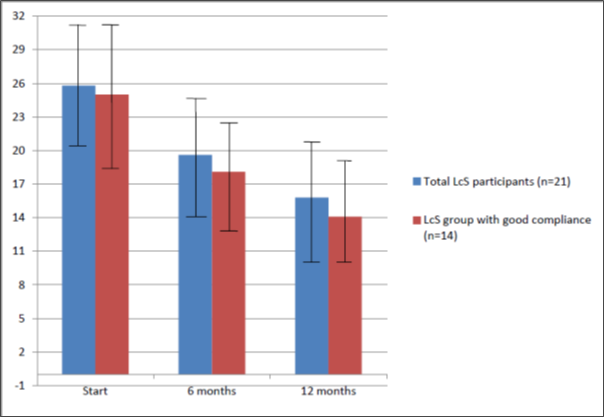
Figure 3: Improvement in mean gastrointestinal symptoms (GIT with 95% confidence intervals) observed in probiotic-naïve patients with diverticulitis taking L. casei Shirota (LcS) 2013-14. Assessment was based on Bovenschen gastrointestinal symptom scores (normal range <8).
Diabetes, BMI and other chronic conditions as confounders
Three participants had diabetes at recruitment and two were pre-diabetic (HbA1c 6-6.49%). There was no statistically significant correlation between HbA1c and diverticulitis attack rate in the last 12 months or GIT symptom score. BMI was also not associated with attack rate or GIT symptom score. The commonest chronic condition under treatment was cardiovascular disease but there was no correlation between this and diverticulitis or GIT symptom score (supplementary material).
Three participants had diabetes at recruitment and two were pre-diabetic (HbA1c 6-6.49%). There was no statistically significant correlation between HbA1c and diverticulitis attack rate in the last 12 months or GIT symptom score. BMI was also not associated with attack rate or GIT symptom score. The commonest chronic condition under treatment was cardiovascular disease but there was no correlation between this and diverticulitis or GIT symptom score (supplementary material).
Effect of diet on risk of diverticulitis and on GIT symptom score
Only 57.7% of all participants completed all their diet diaries. There was no correlation between nutrient intakes and frequency of diverticulitis episodes. Correlation data are summarised in Table 4. There were statistically significant negative Pearson correlation coefficients between GIT symptom scores and dietary fibre (Southgate method r= -0.56; AOAC method r= -0.46) and several other nutrients. However, using repeated measures of ANOVAs with LcS as a factor and each dietary variable as a covariate to model GIT symptom scores revealed no statistically significant dietary variables, with the exception of delta-tocopherol (p=0.008, fitted parameter -20.45, indicating a drop of 20.45 points on the GIT symptoms scale for every increase of 1 point in delta-tocopherol).
Only 57.7% of all participants completed all their diet diaries. There was no correlation between nutrient intakes and frequency of diverticulitis episodes. Correlation data are summarised in Table 4. There were statistically significant negative Pearson correlation coefficients between GIT symptom scores and dietary fibre (Southgate method r= -0.56; AOAC method r= -0.46) and several other nutrients. However, using repeated measures of ANOVAs with LcS as a factor and each dietary variable as a covariate to model GIT symptom scores revealed no statistically significant dietary variables, with the exception of delta-tocopherol (p=0.008, fitted parameter -20.45, indicating a drop of 20.45 points on the GIT symptoms scale for every increase of 1 point in delta-tocopherol).
| Daily intake of nutrients | Bivariate correlations | ||
| Start (n=21) | 6 Months (n=16) | 12 Month (n=16) | |
| Delta tocopherol (mg/day) | -0.4 | -0.57** | -0.53* |
| Beta carotene (microgram/day) | -0.49* | -0.43 | -0.2 |
| Fibre (Southgate method) (mg/day) | -0.33 | -0.29 | -0.56* |
| Folate (microgram/day) | -0.49* | -0.46* | -0.41 |
| Potassium (mg/day) | -0.47* | -0.42 | -0.3 |
| Dietary fibre AOAC method (gm/day) | -0.46* | -0.45 | -0.45 |
| Gamma tocopherol (mg/day) | -0.37 | -0.46* | -0.27 |
| Vitamin A (IU/day) | -0.46* | -0.33 | -0.51* |
| Non-starch polysaccharide (mg/day) | -0.45* | -0.39 | -0.39 |
| Available carbohydrate (gm/day) | -0.41 | -0.44* | -0.15 |
| Calcium (mg/day) | -0.41 | -0.43 | -0.17 |
| Omega 3 fatty acids (mg/day) | -0.31 | -0.4 | -0.07 |
| Soluble non-cellulose (gm/day) | -0.33 | -0.35 | -0.47 |
| Magnesium (mg/day) | -0.32 | -0.4 | -0.26 |
| Vitamin B6 (mg/day) | -0.38 | -0.32 | 0.26 |
| Beta tocopherol (mg/day) | -0.39 | -0.035 | -0.004 |
| Alpha tocopherol (gm/day) | -0.37 | -0.32 | -0.32 |
| Cellulose (gm/day) | -0.26 | -0.3 | -0.4 |
| Zinc (mg/day) | -0.34 | -0.27 | -0.07 |
| Vitamin D (microgram/day) | 0.12 | 0.02 | 0.32 |
| Total vitamin E (mg/day) | -0.28 | -0.16 | 0.08 |
| Selenium (microgram/day) | -0.26 | -0.01 | -0.24 |
| Protein intake/Kg weight (gm/day) | -0.24 | -0.17 | -0.08 |
| Vitamin C (gm/day) | -0.03 | -0.05 | -0.25 |
| Fructose (gm/day) | -0.21 | -0.15 | -0.19 |
**0.01 > p >= 0.001
*0.05 > p >= 0.01
Table 4: Correlations between GIT symptom scores and nutrient intakes.
*0.05 > p >= 0.01
Table 4: Correlations between GIT symptom scores and nutrient intakes.
Participants’ feedback on probiotic intervention
This reflected that LcS intervention had resulted in fewer diverticulitis attacks and helped to control their GIT symptoms; 58% felt they had benefitted from the 12-month probiotic course and intended to continue taking a daily probiotic. Two more expressed a wish to do so but could not afford it.
This reflected that LcS intervention had resulted in fewer diverticulitis attacks and helped to control their GIT symptoms; 58% felt they had benefitted from the 12-month probiotic course and intended to continue taking a daily probiotic. Two more expressed a wish to do so but could not afford it.
Discussion
Our results suggest that it is feasible to conduct a 12-month study on the use of probiotics in diverticular disease. The effect of a daily dose of LcS in preventing diverticulitis was associated with a change in presentation and lower GIT symptom scores. However, our study does not prove a causal link. Symptom improvement and the changes to presentation rate could be a placebo effect.
Stollman et al. tested Bifidobacterium infantis combined with mesalamine against placebo [8] and Tursi et al. tested Lactobacillus casei subspecies DG24 against placebo [5]. When compared with the active (probiotic) intervention, the Stollman trial showed a marked placebo effect whilst the Tursi trial did not. This may be because the Stollman trial only administered their probiotic for 3 months whereas Tursi et al. administered their probiotic for a full 12 months. Recruitment from primary care has the advantage that most UAD occurs in the primary care setting. In the present trial, subjects were recruited up to the age of 75 years because completing feedback forms and diet diaries is a demanding task for older patients. A future trial could include patients aged >75 years if a simple food frequency questionnaire could be used. Some form of dietary monitoring is necessary due to potential confounding factors [18-21, 31].
Another complicating factor is that diverticulitis overlaps with irritable bowel syndrome (IBS) and both are causally linked to low dietary fibre [32]. Several participants reported symptoms of food intolerance that sounded more like IBS than diverticulitis. At the end of the study, four participants who seemed to have IBS were referred back to their GP with the recommendation that they see a dietician. In view of these observations and evidence of probiotic benefit for management of IBS symptoms [33-35], it is recommended that any future trial should include screening out of coeliac disease and IBS at recruitment. This study is pertinent to NHS costs and antibiotic policy [36, 37].
Power calculations indicated that in order to detect a decrease of 25% in diverticulitis attack rate (Table 2a), with size=5% and power=80%, a minimum of 49 subjects per treatment group would need to be recruited for any future placebo versus probiotic RCT. Therefore, allowing for 33.3% dropouts, a definitive study would require 148 recruits. Evidence suggests that 2/3 of episodes of UAD can be accurately diagnosed on purely clinical grounds by an experienced GP [25]. However, ultrasound provides the gold standard for future research and we would recommend its inclusion in future trials [27].
Conclusions
Although underpowered to determine confounders, the study showed the feasibility of taking a simple, commercially available, daily probiotic for prevention of diverticulitis. This effect should be tested in a definitive trial.
Declarations
Ethics approval and consent to participate: The protocol was approved by the ethical committees of Surrey NHS (REC Reference 12/LO/1363) and the University of Surrey (REC Reference EC/2012/141/FHMS). LACTOPRoD was registered at ClinicalTrials.gov: NCT01609751 in 2012. All participants were fully informed of the details of the trial with a fact sheet posted before attendance at the research clinic. At first attendance they were asked to read and sign a consent form, which was countersigned by the PI.
Ethics approval and consent to participate: The protocol was approved by the ethical committees of Surrey NHS (REC Reference 12/LO/1363) and the University of Surrey (REC Reference EC/2012/141/FHMS). LACTOPRoD was registered at ClinicalTrials.gov: NCT01609751 in 2012. All participants were fully informed of the details of the trial with a fact sheet posted before attendance at the research clinic. At first attendance they were asked to read and sign a consent form, which was countersigned by the PI.
Consent for publication: Not applicable.
Transparency Declaration
As the lead author, I affirm that this manuscript is an honest, accurate, and transparent account of the study being reported. The reporting of this work is compliant with STROBE2 guidelines. I affirm that no important aspects of the study have been omitted and thttps://figshare.com/hat any discrepancies from the study as planned (ClinicalTrials.gov) have been explained. The raw data for this study is available at Fig share: https://figshare.com/
As the lead author, I affirm that this manuscript is an honest, accurate, and transparent account of the study being reported. The reporting of this work is compliant with STROBE2 guidelines. I affirm that no important aspects of the study have been omitted and thttps://figshare.com/hat any discrepancies from the study as planned (ClinicalTrials.gov) have been explained. The raw data for this study is available at Fig share: https://figshare.com/
References
- Bharucha AE, Parthasarathy G, Ditah I, Fletcher JG, Ewelukwa, Pendlimari R, et al. (2015). Temporal trends in the incidence and natural history of diverticulitis: A population-based study. Am J Gastroenterol,110: 1589-96.
- Reddy VB, Longo WE (2013). The burden of diverticular disease on patients and healthcare systems. Gastroenterol Hepatol (N Y), 9: 21-7.
- Giaccari S, Tronci S, Falconieri M, Ferrieri A (1993). Long-term treatment with rifaximin and lactobacilli in post-diverticulitic stenoses of the colon. Riv Eur Sci Med Farmacol, 15: 29-34.
- Lahner E, Bellisario C, Hassan C, Zullo A, Esposito G, Annibale B. (2016). Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis, 25: 79-86.
- Tursi A, Brandimarte G, Elisei W, Picchio M, Forti G, Pianese G, Rodino S, D’Amico T, Sacca N, Portincasa P, Capezzuto E, Lattanzio R, Spadaccini A, Fiorella S, Polimeni F, Polimeni N, Stoppino V, Stoppino G, Giorgetti GM, Aiello F, Danese S. (2013). Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease – a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Therap, 38: 741–751.
- Fric P, Zavoral M. (2003). The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol, 15: 313-5.
- Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Jarvenpaa S, et al. (2008). Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol 14: 2029-36.
- Stollman N, Magowan S, Shanahan F, Quigley EM, Group DI. (2013). A randomized controlled study of mesalamine after acute diverticulitis: results of the DIVA trial. J Clin Gastroenterol, 47: 621-9
- Maguire LH, Song M, Strate LL, Giovannucci EL, Chan AT. (2015). Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surgery, 150(1): 74-7.
- Ricciardi R, Roberts PL, Read TE et al. (2011). Cyclical increase in diverticulitis during the summer months. Archives Surg, 146(3): 319-23.
- Nichols JAA, Thomas LV (2010). Survey of incidence of diverticular disease, dietary advice and probiotic advice in three Surrey practices. Proc Nutr Soc, 69: E109.
- Johnson N, Thomas LV, Jordan D (2016). Probiotics: assessing health professionals’ knowledge and understanding. Gastrointestinal Nursing, 14: 27-32.
- Spanhaak S, Havenaar R, Schaafsma G (1998). The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur J Clin Nutr, 52: 899-907.
- Tsuji H, Chonan O, Suyama Y, Kado Y, Nomoto K, Nanno M, et al. (2014). Maintenance of healthy intestinal microbiota in women who regularly consume probiotics. Int J Probiotics & Prebiotics, 9:31-8.
- Krammer HJ, von Seggem H, Schaumberg J, Neumer F (2011). Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctol, 33: 109-13.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ. (2003). Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol, 17: 655-9.
- Tuohy KM, Pinart-Gilberga M, Jones M, Hoyles L, McCartney AL, Gibson GR (2007). Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. J Appl Microbiol, 102: 1026-32.
- Strate LL,. Liu YL, Aldoori WH, Syngal S, Giovannucc E. (2009). Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterol,136: 115–122.
- Schowengerdt CG, Hedges GR, Yaw PB, Altemeier WA (1969). Diverticulosis, diverticulitis, and diabetes. A review of 740 cases. Arch Surg, 98: 500-4.
- Esteve E, Ricart W, Fernandez-Real JM (2011). Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: did gut microbiote co-evolve with insulin resistance? Current Opinion In Clini Nutr And Metabol Care,14: 483-90.
- Crowe FL, Appleby PN, Allen NE, Key TJ (2011). Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ, 343: d4131.
- de Lusignan S, van Weel C (2006). The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract, 23(2): 253-63.
- Bovenschen HJ, Janssen MJ, van Oijen MG, Laheij RJ, van Rossum LG, Jansen JB (2006). Evaluation of a gastrointestinal symptoms questionnaire. Dig Dis Sci, 51: 1509-15.
- Helou N, Abdalkadar M, Abu-Ristum RS (2013). Sonography: first line modality in the diagnosis of acute colonic diverticulitis. J Ultrasound Med, 32:1689-1694.
- Clinical features of acute diverticulitis. GP Notebook. 2017. http://www.gpnotebook.co.uk/simplepage.cfm?ID=1469710366&linkID=12794&cook=yes (accessed March 2019).
- Tursi A, Brandimarte G, Giorgetti G, Elisei W, Maiorano M, Aiello F (2008). The clinical picture of uncomplicated versus complicated diverticulitis of the colon. Dig Dis Sci, 53: 2474-9.
- Andeweg CS, Wegdam JA, Groenewoud J, van der Wilt GJ, van Goor H, Bleichrodt RP (2014). Toward an evidence-based step-up approach in diagnosing diverticulitis. Scand J Gastroenterol, 49(7): 775-84.
- Almerie MQ, Simpson J (2015). Diagnosing and treating diverticular disease. Practitioner, 259:29-33, 3.
- Alonso S, Pera M, Pares D, Pascual M, Gil MJ, Courtier R, et al (2010). Outpatient treatment of patients with uncomplicated acute diverticulitis. Colorectal Dis, 12:e278-82.
- Bigham SA, Welch AA, McRaggart A, Mulligan AA, Runswick SA, Luben R, Oakes S, Khaw KT, Wareham N, Day NE (2001). Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr, 4(3): 847-58.
- Staudacher HM, Irving PM, Lomer MC, Whelan K (2014). Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol, 11: 256-66.
- Wang YR, Talley NJ, Picco MF (2011). Overlap: irritable bowel syndrome, inflammatory bowel disease, and diverticular disease. J Clin Gastroenterol, 45:S36-S42.
- Moraes-Filho JP, Quigley EM (2015). The intestinal microbiota and the role of probiotics in irritable bowel syndrome: a review. Arquiv Gastroenterol, 52: 331-8.
- Tilley L, Keppens K, Kushiro A, Takada T, Sakai T, Vaneechoutte M, et al (2014). A probiotic fermented milk drink containing Lactobacillus casei strain Shirota improves stool consistency of subjects with hard stools. Int J Probiotics & Prebiotics, 9: 23-30.
- NICE. Irritable bowel syndrome in adults: diagnosis and management. NICE (National Institute of Clinical Excellence and Health) Clinical Guidance CG61 (2008, updated 2015).
- Gray A. (2005). Population ageing and health care expenditure. Ageing Horizons. 2: 15-20.
- O'Neill J. Tackling drug-resistant infections globally: Final report and recommendations. Wellcome Trust, UK. 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed March 2020).
Citation: John Nichols., et al. (2020). Impact of a Daily Probiotic (Lactobacillus Casei Shirota) for 12 Months on the Frequency of Diverticulitis Episodes: Feasibility Study in Primary Care. Archives of Nutrition and Public Health 2(1). DOI: 10.5281/zenodo.3755563
Copyright: © 2020 John Nichols. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
