Research Article
Volume 1 Issue 1 - 2018
Effects of Cadmium Exposure on Growth and Survival and Accumulation in Various Organs of Nile Tilapia (Oreochromis niloticus, Linnaeus)
1Department of Genetics & Fish Breeding, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
2Department of Fisheries Biology & Aquatic Environment, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
2Department of Fisheries Biology & Aquatic Environment, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
*Corresponding Author: Zinia Rahman, Department of Genetics & Fish Breeding, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh.
Received: December 12, 2018; Published: December 29, 2018
Abstract
Pollution and food safety are concerning issues in recent years. For that, the present investigation was aimed to evaluate the effects of Cadmium (Cd) exposure on growth and survival and accumulation in various organs of Nile tilapia (Oreochromis niloticus). In six treatments, six various doses of Cd was applied daily. All treatment barrels got 100% mortality within the 3rd week of Cd exposure. 100% mortality was observed in day 16 where the highest Cd dose was applied. Fish growth was found higher in control, where no Cd was exposed, compared to other treatments where Cd was exposed. Data showed the order of Cd accumulation in various organs was: liver > kidney > gonad > gills > muscles in all treatments. Highest amount of Cd accumulation was found in liver 1.743 ± 0.049 mg/kg which is the main site for toxicant bioaccumulation and storage and lowest concentration was found in muscles 0.004 ± 0.0021 mg/kg as muscles do not actively accumulate metals. This study indicates that all these concentrated metals in different parts of fish body could be concentrated into human body, if they are consumed for long time and if so happen; there will be a massive health risk for us.
Keywords: Cadmium; Toxicity; Oreochromis niloticus; Mortality rate; Bioaccumulation
Introduction
Industrial development in the developing and developed countries has been resulted in heavy metals (HMs) contamination of local waters. The term HMs is a general collective term which applies to a group of metals and metalloids with atomic density greater than 4 g/cm3 or 5 times or more greater than water. HMs is non-biodegradable and once discharged into waterbodies, they can either be adsorbed on sediment particles or accumulated in aquatic organisms. HMs discharging into river or any aquatic environment can change both aquatic species diversity and ecosystems, due to their toxicity. Toxicity with HMs is due to disrupt the function of essential biological molecules such as protein, enzymes and DNA as metals lead to displacement of an essential metal cofactor of the enzyme and interaction of the metallic ions with DNA which proven to be carcinogenic to animals and humans [1]. In addition, digestive, renal, nervous, endocrine, reproductive and respiratory system defect were also, confirmed as a result of HMs exposure [2].
Bioaccumulation is a process by which the chemicals are taken up by an organism either directly from exposure to a contaminated medium or by consumption of food containing the chemical [3]. In fish, which is often at the higher level of the aquatic food chain, substantial amounts of metals may accumulate in their soft and hard tissues through the effects of bioaccumulation and become toxic when accumulation reaches a substantially high level. HMs accumulate in the gonadal tissues of fish, reaching a concentration of up to 1000?fold higher than in the surrounding water environment and becoming extremely harmful for reproduction [4]. Pollutants enter into fish through a number of routes: via skin, gills, oral consumption of water, food and non-food particles [5]. Once absorbed, pollutants are transported in the blood stream to either a storage point (i.e. bone) or to the liver for transformation and/or storage. The levels of HMs in aquatic ecosystems are now among the highest reported in the world and they are reaching unprecedented levels [6].
Consumption of food containing these contaminants by aquatic organisms may not only affect their productivity and reproduction but it will also affect the health of human being that depends on these organisms as a source of protein [7]. The overall consequences of trace metal contamination in aquatic ecosystems are reduction in biological species richness and diversity and change in species composition [8].
The response to chemical stress can be used as biomarkers or sentinels of environmental conditions [9]. Biomarkers are early responses or measurable biological event due to exposure to pollutants after acute or chronic exposure and the morphological findings has been largely considered in bio monitoring studies. Due to urban, industrial and agricultural activities, freshwater sources are dumped with different kinds of chemicals that affect the inhabiting biota [10]. Thus, in view of the quality of public food supplies, their levels in aquatic environment should be monitored regularly to check water quality and animal health [11].
Among HMs, Cadmium (Cd) is a chemical element with atomic number 48. This soft, bluish-white metal is from group II B that has an atomic weight of 112.41 with specific gravity of 8.65; the ionic form of Cd (Cd2+) is usually combined with ionic forms of oxygen (Cadmium oxide, CdO), Chlorine (Cadmium chloride, CdCl2), or Sulfur (Cadmium sulfate, CdSO4). Cd is considered as one of the most toxic HMs [12] and environmental pollutant toxic to number of tissues [13]. This non-essential HM enters human and animal bodies via different industrial products, environmental pollution and different contaminated foods. Cd occurs naturally in the environment in significant amounts but its release in the recent past is steadily increasing due to human activities causing pollution at considerably toxic level. Thus, the deleterious effects of HMs on aquatic ecosystems necessitate the continuous monitoring of their accumulation in key species, since it affords indication of temporal and spatial extent of the process and impact on organism’s health [14].
There has been an increasing interest in the utilization of fishes as bioindicators of the integrity of aquatic environmental systems [15-17]. Nile Tilapia (Oreochromis niloticus) is an opportunistic omnivorous, consume a variety of feed, living or drying materials, animal or plant and has economic importance in Asian market. It grows well at high densities when good water quality is maintained, but they are also amazingly tolerant of poor or variable water quality. Cd accumulates in sediments and water and finally bioaccumulates in fish and its toxicity affects in fish health and production. The entrance of Cd into the human body and animal food chain due to the anthropogenic activities implies an adverse effect. Elevated rate of Cd in foods may make food security risk. Therefore the present study is undertaken to investigate growth and survivality of Nile Tilapia (O. niloticus) under Cd exposure and to sway the Cd accumulation in gills, liver, gonad, kidney and muscles exposed to different doses of Cd. The results obtain from this study may help policy makers to determine safety levels of HMs in environment as well as in edible fish species.
Materials and Methods
Collection and acclimatization of fingerlings
To investigate the growth and survivality of Nile Tilapia (O. niloticus, Linnaeus) under Cd exposure and levels of Cd accumulation in various organs 300 healthy fingerlings measuring 7-8 cm were purchased from a commercial fish seed hatchery, transported and gently released into rearing tanks with sufficient aeration for acclimatization. Fish were acclimatized for seven days in two 300 liter tanks (each containing 150 fingerlings). During this acclimatization period fingerlings were fed with grinded commercial pellets. After that the fingerlings were transferred to the experimental barrels.
To investigate the growth and survivality of Nile Tilapia (O. niloticus, Linnaeus) under Cd exposure and levels of Cd accumulation in various organs 300 healthy fingerlings measuring 7-8 cm were purchased from a commercial fish seed hatchery, transported and gently released into rearing tanks with sufficient aeration for acclimatization. Fish were acclimatized for seven days in two 300 liter tanks (each containing 150 fingerlings). During this acclimatization period fingerlings were fed with grinded commercial pellets. After that the fingerlings were transferred to the experimental barrels.
Rearing of fingerlings
Nile Tilapia (O. niloticus) fingerlings were acclimatized to the rearing condition and were reared for 12 weeks. Then the fish were weighed individually, selected and distributed into 21 barrels of 100 L size in such a way that biomass of all tanks become similar. The numbers of fishes in each barrel were 10. Commercial fish feed (Arman fish feed Company, Gazipur) were bought from local market. Approximately 10 g fish feed were given daily 2 times in each rearing tank. Uneaten food and the feces were removed after 30 minutes of feeding from all the tanks daily.
Nile Tilapia (O. niloticus) fingerlings were acclimatized to the rearing condition and were reared for 12 weeks. Then the fish were weighed individually, selected and distributed into 21 barrels of 100 L size in such a way that biomass of all tanks become similar. The numbers of fishes in each barrel were 10. Commercial fish feed (Arman fish feed Company, Gazipur) were bought from local market. Approximately 10 g fish feed were given daily 2 times in each rearing tank. Uneaten food and the feces were removed after 30 minutes of feeding from all the tanks daily.
Design of Experiment
The experiment was designed in CRD method [18]. There were six treatments groups T-I, TII, T-III, T-IV, T-V, T-VI where doses were used 0.5 mg/L, 1.0 mg/L, 1.5 mg/L, 2.0 mg/L, 2.5 mg/L, 3.0 mg/L respectively and control with no Cd dose, each having three replications. In each treatment, specific concentration of Cd (Cadmium Metal, Chemsavers, USA) was added daily. Same pH and temperature and other water quality parameters were maintained during the whole experimental period (Table 1). At the end of treatment with Cd, the Cd exposed fish organs were dissected and kept frozen at -40ºC until used.
The experiment was designed in CRD method [18]. There were six treatments groups T-I, TII, T-III, T-IV, T-V, T-VI where doses were used 0.5 mg/L, 1.0 mg/L, 1.5 mg/L, 2.0 mg/L, 2.5 mg/L, 3.0 mg/L respectively and control with no Cd dose, each having three replications. In each treatment, specific concentration of Cd (Cadmium Metal, Chemsavers, USA) was added daily. Same pH and temperature and other water quality parameters were maintained during the whole experimental period (Table 1). At the end of treatment with Cd, the Cd exposed fish organs were dissected and kept frozen at -40ºC until used.
Growth Performance of Nile Tilapia (O. niloticus)
Growth of Nile Tilapia (O. niloticus) in length (cm) and weight (g) was measured and the following parameters were used to evaluate growth performance at different treatments: 1. Length gain (cm) = Mean final length- Mean initial length 2. Weight gain (g) = Mean final weight- Mean initial weight
Growth of Nile Tilapia (O. niloticus) in length (cm) and weight (g) was measured and the following parameters were used to evaluate growth performance at different treatments: 1. Length gain (cm) = Mean final length- Mean initial length 2. Weight gain (g) = Mean final weight- Mean initial weight
Bioaccumulation of Cd in Nile Tilapia (O. niloticus)
Cd accumulation in various parts of Nile Tilapia (O. niloticus) was estimated at the end of the experiment. Fish were stored in a cooler packed with ice block in order to maintain the freshness and later transported to the laboratory for dissection of the organs after removing the scales and washing thoroughly. The laboratory apparatus were washed with concentrated soap and rinsed with tap water then distilled water, then acid–washed in 1M Hydrochloric acid (HCl) for 24 hours and rinsed in distilled water to ensure that all traces of cleaning reagent were removed prior to use. The gills, muscle, liver, kidneys and gonads were removed on a dissection board using stainless dissecting equipment which had been swabbed in 70% alcohol to prevent any possible contamination of the samples.
Cd accumulation in various parts of Nile Tilapia (O. niloticus) was estimated at the end of the experiment. Fish were stored in a cooler packed with ice block in order to maintain the freshness and later transported to the laboratory for dissection of the organs after removing the scales and washing thoroughly. The laboratory apparatus were washed with concentrated soap and rinsed with tap water then distilled water, then acid–washed in 1M Hydrochloric acid (HCl) for 24 hours and rinsed in distilled water to ensure that all traces of cleaning reagent were removed prior to use. The gills, muscle, liver, kidneys and gonads were removed on a dissection board using stainless dissecting equipment which had been swabbed in 70% alcohol to prevent any possible contamination of the samples.
Cd accumulations in various organs were analyzed by a two-step process: dry-ashing and acid digestion. Firstly, the samples were placed in acid-washed crucibles and oven-dried to constant weight at 120°C [19]. After, the dried samples were placed in the furnace at 500-600°C for another 12-14 hours until the samples turned into a white ash mineral. Samples were put in dry labeled plastic containers and stored in desiccator until digestion. For the acid digestion second step, the ashed powder samples (10g) were dissolved in concentrated nitric acid or in HNO3/H2O2 with appropriate dilution (the samples being diluted to 25 mL with deionized water). HMs in the acid-resolved samples were determined using an atomic absorption spectrophotometer (AAS, Spectra 220 FS, Varian, Palo Alto, CA, USA). Blank digestion was also performed to quantify possible contamination during sample preparation and analysis. The standard solution was prepared before analysis of the current work. Cd accumulation in various parts of fish body was estimated as follows: Gills = Mean final accumulation in gills, Kidney = Mean final accumulation in kidney,Gonad= Mean final accumulation in gonad, Liver = Mean final accumulation in liver, Muscle = Mean final accumulation in muscle. The actual concentration was calculated on the basis of total amount of the sample taken and expressed in mg/kg.
Statistical Analysis
The data was expressed as means± Standard Deviation (SD). For the statistical analysis of data Microsoft Excel was used and “Statistics 10” software was used for “ANOVA” in 5% level of significance, P < 0.05. Data were expressed as mean ± standard error of mean.
The data was expressed as means± Standard Deviation (SD). For the statistical analysis of data Microsoft Excel was used and “Statistics 10” software was used for “ANOVA” in 5% level of significance, P < 0.05. Data were expressed as mean ± standard error of mean.
Results and Discussion
Growth of Nile Tilapia (O. niloticus) under Cd exposure
The pH, temperature (ºC) and other rearing conditions were maintained the same in all the treatments during the whole experimental period (Table 1). The growth of Nile Tilapia (O. niloticus) in term of length was hampered due to exposure of Cd. In the present study, the highest length (27.92 ± 3.2 cm) was found in control where there was no Cd exposure (Table 2), moreover in T-VI the length gain was found the lowest (16.14 ± 1.2 cm) where the Cd exposure was the highest. The length gain was found to decrease gradually with the gain of Cd concentration. Cd exposure may lead to the results of some pathophysiological damages and metabolic changes which affect growth rate reduction in fish [20].
The pH, temperature (ºC) and other rearing conditions were maintained the same in all the treatments during the whole experimental period (Table 1). The growth of Nile Tilapia (O. niloticus) in term of length was hampered due to exposure of Cd. In the present study, the highest length (27.92 ± 3.2 cm) was found in control where there was no Cd exposure (Table 2), moreover in T-VI the length gain was found the lowest (16.14 ± 1.2 cm) where the Cd exposure was the highest. The length gain was found to decrease gradually with the gain of Cd concentration. Cd exposure may lead to the results of some pathophysiological damages and metabolic changes which affect growth rate reduction in fish [20].
| Parameters | Value |
| Temperature (°C) | 28 ± 2 |
| pH | 7.3 – 7.6 |
| Dissolved oxygen (mg/l) | 6.8-7.5 |
| Salinity (mg/l) | 0.3- 0.35 |
| Alkalinity as CaCO3 (mg/l) | 162-168 |
Table 1: Water quality parameters used in the experiment.
| Treatments | Mean initial length (cm) | Mean final length (cm) | Mean length gain (cm) |
| Control | 7 ±.029 | 27.92 ± 3.2 | 20.92 ± 3.249 |
| T-I | 7 ±.037 | 20.64 ± 2.8 | 13.64 ± 2.837 |
| T-II | 7 ±.043 | 18.57 ± 2.3 | 11.57 ± 2.343 |
| T-III | 7 ±.023 | 17.64 ± 2.1 | 10.64 ± 2.123 |
| T-IV | 7 ±.047 | 17.14 ± 1.5 | 10.14 ± 1.547 |
| T-V | 7 ±.022 | 16.57 ± 1.4 | 9.57 ± 1.422 |
| T-VI | 7 ±.022 | 16.14 ± 1.2 | 9.14 ± 1.222 |
The mean numbers were calculated as mean ± standard deviation
Table 2: Length gain of fish in different treatments.
Table 2: Length gain of fish in different treatments.
The growth of Nile Tilapia (O. niloticus) in term of weight gain was also hampered due to exposure of Cd. The highest weight gain was found in control whereas the lowest weight gain was observed at highest concentration of Cd exposure (table 3). Weight gain was found to increase gradually with the decrease of Cd concentration. Exposure duration evidently affected sensitivity of fish larvae and influence the weight gain in Heteropneutes fossilis [21]. Study showed that the age of the fish, a potentially confounding factor when studying Cd bioaccumulation because Cd concentrations in liver and kidney increase with the age and affect fish growth [22,23]. Thus it is unlikely that poor growth is the result of energetic deficiency brought on by the need to mobilize and eliminate toxicant [24].
| Treatments | Mean initial weight (g) | Mean final weight (g) | Mean weight gain (g) |
| Control | 10 ± 0.21 | 288.65 ± 4.7 | 278.65 ± 4.91 |
| T-I | 10 ± 0.31 | 163.6 ± 4.3 | 153.6 ± 4.61 |
| T-II | 10 ± 0.27 | 142.5 ± 3.2 | 132.5 ± 3.47 |
| T-III | 10 ± 0.23 | 132.5 ± 3.1 | 122.5 ± 3.33 |
| T-IV | 10 ± 0.33 | 123.6 ± 2.7 | 113.6 ± 3.03 |
| T-V | 10 ± 0.21 | 112.5 ± 2.5 | 102.5 ± 2.71 |
| T-VI | 10 ± 0.25 | 102.5 ± 2.4 | 92.5 ± 2.65 |
The mean numbers were calculated as mean ± standard deviation
Table 3: Weight gain of fish in different treatments.
Table 3: Weight gain of fish in different treatments.
Mortality rate of Nile Tilapia (O. niloticus) under Cd exposure
Nile Tilapia (O. niloticus) died due to Cd exposure in different concentrations. All treatment barrels got 100 % mortality by the third week but the time needed to reach 100 % mortality varied among different treatments. 100 % mortality was observed in day 21, 19,18,18,17 and 16 for T-I, T-II, T-III, T-IV, T-V and T-VI respectively (Table 4). No mortality was observed in the control because there was no contamination of Cd. Generally, the higher metal concentration in the environment, the more may be taken up and accumulated by fish. Quickest mortality was found in T-VI, showed the adverse effect of higher Cd exposure. Cd is poorly regulated by organisms, thereby increasing the likelihood that whole-body residues will increase with increasing exposure concentration [25] which affects the survivality of experimental organisms. Effects of long-term exposure can include larval mortality and temporary reduction in growth [26].
Nile Tilapia (O. niloticus) died due to Cd exposure in different concentrations. All treatment barrels got 100 % mortality by the third week but the time needed to reach 100 % mortality varied among different treatments. 100 % mortality was observed in day 21, 19,18,18,17 and 16 for T-I, T-II, T-III, T-IV, T-V and T-VI respectively (Table 4). No mortality was observed in the control because there was no contamination of Cd. Generally, the higher metal concentration in the environment, the more may be taken up and accumulated by fish. Quickest mortality was found in T-VI, showed the adverse effect of higher Cd exposure. Cd is poorly regulated by organisms, thereby increasing the likelihood that whole-body residues will increase with increasing exposure concentration [25] which affects the survivality of experimental organisms. Effects of long-term exposure can include larval mortality and temporary reduction in growth [26].
| Treatments | First week | Second week | Third week | Total |
| Control | 00 | 00 | 00 | 00 |
| T-I | 03 | 06 | 18 | 30 |
| T-II | 06 | 06 | 21 | 30 |
| T-III | 09 | 09 | 12 | 30 |
| T-IV | 04 | 08 | 18 | 30 |
| T-V | 09 | 06 | 15 | 30 |
| T-VI | 03 | 12 | 15 | 30 |
Table 4: Number of fish died in different treatments.
Accumulation of Cd in Gills of Nile Tilapia (O. niloticus) under Cd exposure
In Gills of Nile Tilapia (O. niloticus),lowest accumulation of Cd was found (0.041 ± 0.0029) mg/kg in T-I and highest Cd accumulation was obtained (0.315 ± 0.0043) mg/kg in T-VI (Figure 1). Gills are in direct contact with the water and if the water is contaminated this will be the main route for the contaminants to enter the fish body. Then with the blood flow, HMs can reach other internal organs and tissues. Ahmed., et al. reported that Ayre (Sperata aor) collected from river Dhaleshwari contains 0.307 mg/kg Cd in gills [27]. In the present study, the Cd concentration in gills of Nile Tilapia (O. niloticus) in all treatments were lower during first and second week than that of the findings of Ahmed., et al. but it was found high in third week in T-VI than the findings of Ahmed., et al. [27]. Metal ions are usually absorbed through passive diffusion or carrier mediated transport over the gills while metals associated with organic materials are ingested and absorbed by endocytosis through intestine. It has been suggested that Cd ions enter the chloride cells in the gills through calcium channels [28]. Gills are also reported to act as storehouse of Cd in experimental studies [29, 30]. Wong., et al. studied morphological and biochemical changes in the gills of Tilapia (O. mossambicus) after experimental Cd exposure [31]. In scanning electron microscopic studies, they found an augmentation of microbridges in pavement cells and an increase in the apical membrane of chloride cells. They further reported chloride cells as a prime target of Cd toxicity, resulting into fish hypocalcemia.
In Gills of Nile Tilapia (O. niloticus),lowest accumulation of Cd was found (0.041 ± 0.0029) mg/kg in T-I and highest Cd accumulation was obtained (0.315 ± 0.0043) mg/kg in T-VI (Figure 1). Gills are in direct contact with the water and if the water is contaminated this will be the main route for the contaminants to enter the fish body. Then with the blood flow, HMs can reach other internal organs and tissues. Ahmed., et al. reported that Ayre (Sperata aor) collected from river Dhaleshwari contains 0.307 mg/kg Cd in gills [27]. In the present study, the Cd concentration in gills of Nile Tilapia (O. niloticus) in all treatments were lower during first and second week than that of the findings of Ahmed., et al. but it was found high in third week in T-VI than the findings of Ahmed., et al. [27]. Metal ions are usually absorbed through passive diffusion or carrier mediated transport over the gills while metals associated with organic materials are ingested and absorbed by endocytosis through intestine. It has been suggested that Cd ions enter the chloride cells in the gills through calcium channels [28]. Gills are also reported to act as storehouse of Cd in experimental studies [29, 30]. Wong., et al. studied morphological and biochemical changes in the gills of Tilapia (O. mossambicus) after experimental Cd exposure [31]. In scanning electron microscopic studies, they found an augmentation of microbridges in pavement cells and an increase in the apical membrane of chloride cells. They further reported chloride cells as a prime target of Cd toxicity, resulting into fish hypocalcemia.
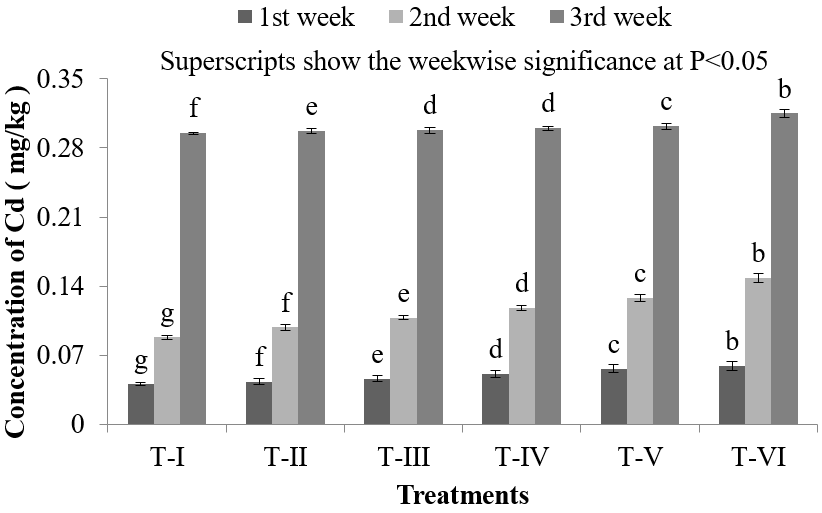
Figure 1: Concentration of Cd in gills of O. niloticus during the experimental period at different treatments.
Accumulation of Cd in Muscles of Nile Tilapia (O. niloticus) under Cd exposure
Body HMs level is related to its waterborne concentration only if HMs is taken up by the fish from water. In the muscles of Nile Tilapia (O. niloticus), Cd accumulation was found lowest in T-I (0.004 ± 0.0021) mg/kg and highest in T-VI (0.179 ± 0.0048) mg/kg (Figure 2). When 3.0 mg/L of Cd dose was exposed into water, the Cd accumulation in the muscles of Nile Tilapia (O. niloticus) was found (0.007 ± 0.004) mg/kg, (0.067 ± 0.0049) mg/kg and (0.179 ± 0.0048) mg/kg in first, second and third week respectively (Figure 2). Muscles are the main site for POPs (persistent organic pollutants such as pesticides, PAHs, PCBs etc.) accumulation and Cd accumulation found lowest in muscles of Nile Tilapia (O. niloticus) because muscles do not actively accumulate HMs and seems to have a very fast decontamination rate. Bioaccumulation of Cd in flesh of Ayre fish (S. aor) collected from river Dhaleshwari was found 0.17 mg/kg [27] which was higher than 0.1 mg/kg, the FAO approved level (Table 5). However, the concentration of Cd found in the present study in the muscle of Nile Tilapia (O. niloticus)was lowerthan S. aor during first and second weeks of the experiment, but the concentration was obtained higher at the end of third week in treatment T-IV, T-V, T-VI. The mean total Cd content as determined by the analysis of the edible pats of the fishery products shall not exceed 0.05 ppm mg/kg of fresh weight (Table 5) [32-36]. This average limit is, however, increased to 0.1 mg/kg of fresh weight for edible parts of the some species including: Dicologoglossa mneata (Wedge sale), Anguilla anguilla (Eel), Truchurus trachurus (Horse Mackerel or Scad), Mugil labrosus labrosus (grey mullet, DipZodus vulgaris (Common two-banded seabream) and Sardina pilchardus (European pilchard or sardine) [32].
Body HMs level is related to its waterborne concentration only if HMs is taken up by the fish from water. In the muscles of Nile Tilapia (O. niloticus), Cd accumulation was found lowest in T-I (0.004 ± 0.0021) mg/kg and highest in T-VI (0.179 ± 0.0048) mg/kg (Figure 2). When 3.0 mg/L of Cd dose was exposed into water, the Cd accumulation in the muscles of Nile Tilapia (O. niloticus) was found (0.007 ± 0.004) mg/kg, (0.067 ± 0.0049) mg/kg and (0.179 ± 0.0048) mg/kg in first, second and third week respectively (Figure 2). Muscles are the main site for POPs (persistent organic pollutants such as pesticides, PAHs, PCBs etc.) accumulation and Cd accumulation found lowest in muscles of Nile Tilapia (O. niloticus) because muscles do not actively accumulate HMs and seems to have a very fast decontamination rate. Bioaccumulation of Cd in flesh of Ayre fish (S. aor) collected from river Dhaleshwari was found 0.17 mg/kg [27] which was higher than 0.1 mg/kg, the FAO approved level (Table 5). However, the concentration of Cd found in the present study in the muscle of Nile Tilapia (O. niloticus)was lowerthan S. aor during first and second weeks of the experiment, but the concentration was obtained higher at the end of third week in treatment T-IV, T-V, T-VI. The mean total Cd content as determined by the analysis of the edible pats of the fishery products shall not exceed 0.05 ppm mg/kg of fresh weight (Table 5) [32-36]. This average limit is, however, increased to 0.1 mg/kg of fresh weight for edible parts of the some species including: Dicologoglossa mneata (Wedge sale), Anguilla anguilla (Eel), Truchurus trachurus (Horse Mackerel or Scad), Mugil labrosus labrosus (grey mullet, DipZodus vulgaris (Common two-banded seabream) and Sardina pilchardus (European pilchard or sardine) [32].
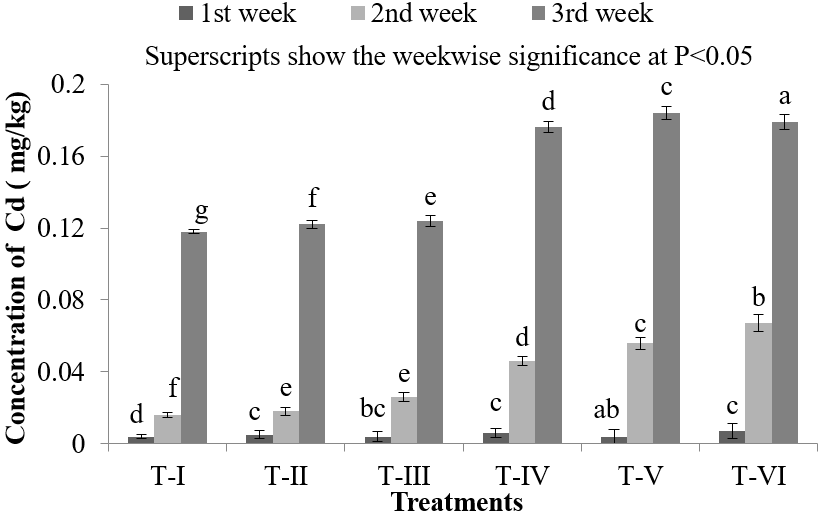
Figure 2: Concentration of Cd in muscles of O. niloticus during the experimental period at different treatments.
Accumulation of Cd in Liver of Nile Tilapia (O. niloticus) under Cd exposure
Like other organs, the lowest Cd concentration in liver of Nile Tilapia (O. niloticus) was found (1.038 ± 0.012) mg/kg in T-I and the highest Cd concentration was found (1.743 ± 0.049) mg/kg in T-VI (Figure 3). Liver is the main site for toxicant bioaccumulation, storage and detoxification that’s why the concentration of Cd was found (1.038 ± 0.012) mg/kg, (1.213 ± 0.022) mg/kg and (1.523 ± 0.024) mg/kg in first, second and third week respectively, When the Cd dose was 0.50 mg/L, liver accumulates high concentrations of HMs, irrespectively of the uptake route. The liver is considered a good monitor of water pollution with HMs since their concentrations accumulated in this organ are often proportional to those present in the environment [37]. HMs levels in the liver rapidly increase during exposure, and remain high for a long time of depuration, when other organs are already cleared. Cd accumulates in liver of fishes in high concentrations [38]. It also induces various pathological changes in liver tissues including engorgement of blood vessels, congestion, vacuolar degeneration of hepatocytes, necrosis of pancreatic cells and fatty changes in the peripancreatic hepatocytes [39].
Like other organs, the lowest Cd concentration in liver of Nile Tilapia (O. niloticus) was found (1.038 ± 0.012) mg/kg in T-I and the highest Cd concentration was found (1.743 ± 0.049) mg/kg in T-VI (Figure 3). Liver is the main site for toxicant bioaccumulation, storage and detoxification that’s why the concentration of Cd was found (1.038 ± 0.012) mg/kg, (1.213 ± 0.022) mg/kg and (1.523 ± 0.024) mg/kg in first, second and third week respectively, When the Cd dose was 0.50 mg/L, liver accumulates high concentrations of HMs, irrespectively of the uptake route. The liver is considered a good monitor of water pollution with HMs since their concentrations accumulated in this organ are often proportional to those present in the environment [37]. HMs levels in the liver rapidly increase during exposure, and remain high for a long time of depuration, when other organs are already cleared. Cd accumulates in liver of fishes in high concentrations [38]. It also induces various pathological changes in liver tissues including engorgement of blood vessels, congestion, vacuolar degeneration of hepatocytes, necrosis of pancreatic cells and fatty changes in the peripancreatic hepatocytes [39].
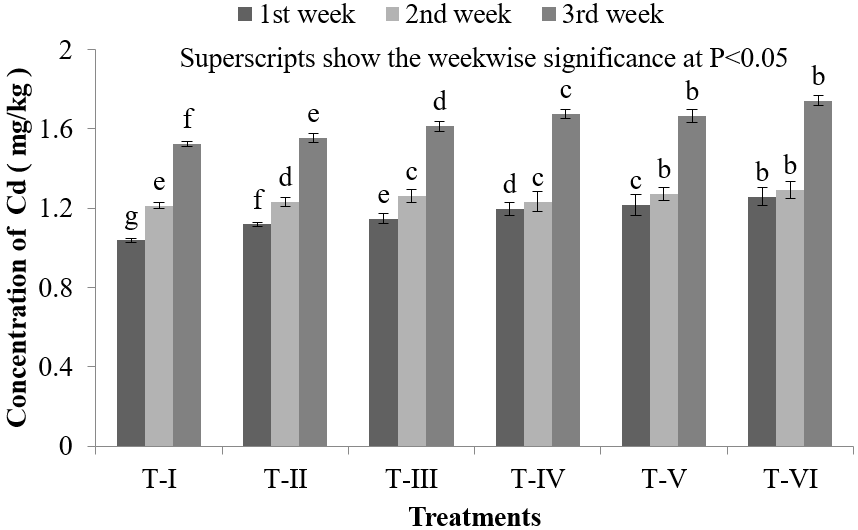
Figure 3: Concentration of Cd in liver of O. niloticus during the experimental period at different treatments.
Accumulation of Cd in kidney of Nile Tilapia (O. niloticus) under Cd exposure
In the present investigation, the lowest Cd accumulation in the kidney of Nile Tilapia (O. niloticus) was found in T-I (0.1 ± 0.0026) mg/kg and the highest in T-VI (0.383 ± 0.0041) mg/kg (Figure 4). HMs concentrations in the kidney rise slower than in liver, and usually reach slightly lower values, except Cd and Zinc that show very high affinity to kidneys [40]. This is in agreement with the Environmental Health Criteria of Cd (Table 5) which reported that Cd is accumulated in the body in various tissues, but the main sites of accumulation in aquatic organisms are the kidney and liver. The maximum permissible limit for Cd is 3.33 μg/g [Table 5]. However the concentration of Cd accumulated in kidney of Nile Tilapia (O. niloticus)in any of treatments was lower than that of the standard limit. Kidney is the principle target organ of Cd toxicity and chronic Cd exposure in almost all animal species is characterized by varying degree of renal damage [41,42].
In the present investigation, the lowest Cd accumulation in the kidney of Nile Tilapia (O. niloticus) was found in T-I (0.1 ± 0.0026) mg/kg and the highest in T-VI (0.383 ± 0.0041) mg/kg (Figure 4). HMs concentrations in the kidney rise slower than in liver, and usually reach slightly lower values, except Cd and Zinc that show very high affinity to kidneys [40]. This is in agreement with the Environmental Health Criteria of Cd (Table 5) which reported that Cd is accumulated in the body in various tissues, but the main sites of accumulation in aquatic organisms are the kidney and liver. The maximum permissible limit for Cd is 3.33 μg/g [Table 5]. However the concentration of Cd accumulated in kidney of Nile Tilapia (O. niloticus)in any of treatments was lower than that of the standard limit. Kidney is the principle target organ of Cd toxicity and chronic Cd exposure in almost all animal species is characterized by varying degree of renal damage [41,42].

Figure 4: Concentration of Cd in kidney of O. niloticus during the experimental period at different treatments.
| International standards | HMs in fish muscles (μg/g dry. wt.) | |
| Cd | Reference | |
| FAO | 0.17 | 32 |
| FAO/WHO limit | 1.67 | 33 |
| WHO | 3.33 | 34 |
| European community | 0.17 | 35 |
| England | 0.67 | 36 |
Table 5: Maximum permissible limit of HMs in fish muscle (μg/g dry. wt.) according to the international standards.
Accumulation of Cd in Gonad of Nile Tilapia (O. niloticus) under Cd exposure
In fish, HMs are taken up through different organs because of the affinity of such organs for the accumulation of HMs. In this process, many HMs are concentrated at different levels in different organs of the body [43]. Other organs like intestine and gonads of fishes also appear susceptible for ill effects of Cd toxicity [44, 45]. In the collected samples of gonads, the lowest accumulation of Cd was found in T-I (0.286 ± 0.0021) mg/kg and highest (0.371 ± 0.0041) mg/kg was found in T-VI in the third week of Cd exposure (Figure 5). In the first week, concentration of Cd was found (0.044 ± 0.0027) mg/kg, which was found to increase gradually in the second and third week of Cd exposure where the Cd dose was 0.50 mg/L (Figure 5). In contrast when the amount of Cd was 3.0 mg/L the concentration of Cd was found much higher in all the samples (Figure 5). As the doses increases, Cd accumulation was found to accumulate at increased level because of the higher exposure of Cd in the environment. Reproduction process and early life stages of fish are the most sensitive for elevated Cd level [46]. HMs may insert their deleterious effects on fish reproduction and gamete development via disruption of the endocrine system and the inhibition of hormone production, such as disruption of hypothalamic-pituitary system [47]. Exposure to various HMs decreases the fecundity of fish populations, either indirectly via accumulation in the reproductive organs or directly by acting on sperm and ova [48]. In the present study Cd accumulation was found higher which may eventually effects fecundity and reproduction of Nile Tilapia (O. niloticus) which may ultimately threats species extinction.
In fish, HMs are taken up through different organs because of the affinity of such organs for the accumulation of HMs. In this process, many HMs are concentrated at different levels in different organs of the body [43]. Other organs like intestine and gonads of fishes also appear susceptible for ill effects of Cd toxicity [44, 45]. In the collected samples of gonads, the lowest accumulation of Cd was found in T-I (0.286 ± 0.0021) mg/kg and highest (0.371 ± 0.0041) mg/kg was found in T-VI in the third week of Cd exposure (Figure 5). In the first week, concentration of Cd was found (0.044 ± 0.0027) mg/kg, which was found to increase gradually in the second and third week of Cd exposure where the Cd dose was 0.50 mg/L (Figure 5). In contrast when the amount of Cd was 3.0 mg/L the concentration of Cd was found much higher in all the samples (Figure 5). As the doses increases, Cd accumulation was found to accumulate at increased level because of the higher exposure of Cd in the environment. Reproduction process and early life stages of fish are the most sensitive for elevated Cd level [46]. HMs may insert their deleterious effects on fish reproduction and gamete development via disruption of the endocrine system and the inhibition of hormone production, such as disruption of hypothalamic-pituitary system [47]. Exposure to various HMs decreases the fecundity of fish populations, either indirectly via accumulation in the reproductive organs or directly by acting on sperm and ova [48]. In the present study Cd accumulation was found higher which may eventually effects fecundity and reproduction of Nile Tilapia (O. niloticus) which may ultimately threats species extinction.
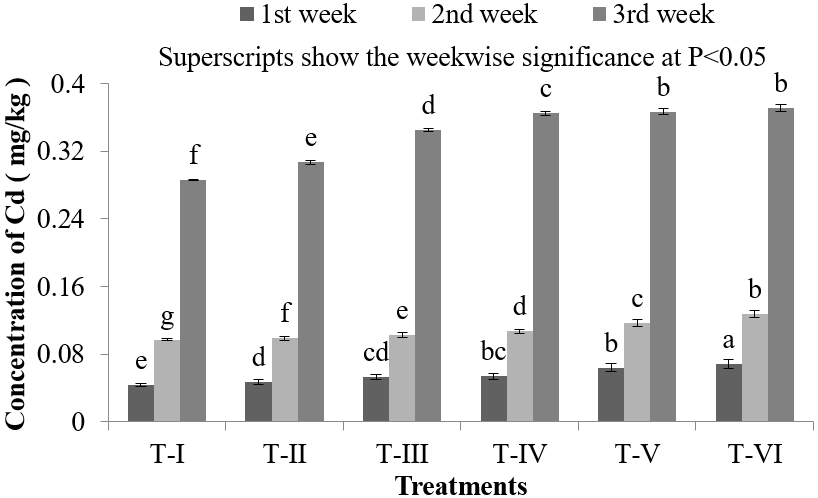
Figure 5: Concentration of Cd in liver of O. niloticus during the experimental period at different treatments.
Conclusion
HMs entering the aquatic ecosystem can be deposited in aquatic organisms through the effects of bioaccumulation and become toxic when accumulation reaches a substantially high level. Generally, accumulation depends on HMs concentration, time of exposure, way of metal uptake, environmental conditions (water temperature, pH, hardness, salinity), and intrinsic factors (fish age, feeding habits) [46]. Various HMs show different affinity to fish tissues and most of them accumulate mainly in liver, kidney and gills. Fish muscles, comparing to the other tissues, usually contain the lowest levels of metals. The present investigation was done to study Cd accumulation in Nile Tilapia (O. niloticus) under Cd exposure for 3 weeks to observe the growth and survivality and level of Cd accumulation in various organs. Significant accumulation of Cd in the organs of Nile Tilapia (O. niloticus) was observed. Several studies show that tissue-specific Cd accumulation in fish with chronic exposure [46] but different tissues show different capacity for accumulating HMs. The atomic absorption data showed the order of Cd contamination was: liver > kidney > gonad > gills > muscles. Liver is the main site for toxicant bioaccumulation, storage and detoxification showed greater accumulation of Cd accumulation and lowest concentration of Cd was found in muscle as muscle do not actively accumulate HMs. Muscle is the main edible part of fish and the highest concentration of Cd was found in muscle was 0.184 ± 0.0048 mg/kg and lowest was 0.004 ± 0.0021 mg/kg whereas the recommended value of Cd is 0.08 mg/kg [Table 5]. Accumulation tendency of Cd by the muscle tissue which consists of mainly the edible parts of a fish is comparatively low according to the studies carried out by many authors [49-56].
Cd is primarily in the Liver and Kidney but it may reach high concentrations in the gill, alimentary canal and muscles as well [46]. Further studies have confirmed these findings in different fish such as O. niloticus, Cyprinus carpio and Labeo umbratus, Tilapia nilotica, Tilapia zilli, Clarias anguillaris, Protoptenus, Eutropius niloticus and Synodentis budgetti [49-56]. Rauf et al. observed minimum concentrations in the liver (4.26 ± 1.57 and 6.23 ± 1.14 μg/g) that was higher than gills (1.10 ± 0.53 and 1.46 ± 0.52 μg/g) for Cd and Chromium, respectively in Catla catla, Labeo rohita [54]. Very minor concentration of Cd was found in control which was statistically insignificant. This accumulation might be occurred due to presence of Cd in fish feed. This experiment showed that long exposure in Cd affect fish by accumulating Cd in various organs. It may have adverse effects as ultimate consumer of fish is human. With regards to the objectives of this research and the results obtained, this current study has provides useful information and a baseline for future along with continuous studies on the Cd accumulation in commercially important fish species This is potentially essential as aquaculture grows rapidly especially for Nile Tilapia (O. niloticus), and it may affect human health if consumed HMs accumulated fish for longer period of time.
References
- Arif, T., Azam, M., Siddiqui, K., Ali, A., Choi, I and Mohd, Q., Haq, R. (2015). Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. International journal of molecular sciences.
- Bechan, S., Singh, S., and Nikhat J.S. (2014). Biomedical implications of heavy metals induced imbalances in redox systems. BioMed Research International Volume 2014, Article ID 640754, 26 pages
- UNEP (2010). Final review of scientific information on cadmium. Chemicals Branch, DTIE, United Nations Environment Programme.
- Kime, D. E., Ebrahimi, M., Nysten, K., Roelants, I., Moore, H. D. M. and Ollevier, F. (1996). Use of computer assisted sperm analysis (casa) for monitoring the effects of pollution on sperm quality of fish; application to effects of heavy metals. Aquat. Toxicol., 36(1): 223-237.
- Karadede, H., Oymak, S.A. and Unlu, E. (2004). Heavy metals in mullet, Liza abu and catfish, Silurus triostegus, from the Ataturk Dam Lake (Euphrates), Turkey. Environ. Int., 30: 183-188.
- Adham, K.G. (2001). Metallic micro-pollutants in the harvest of Oreochromis niloticus (Linnaeus, 1757) from polluted waters: Wildlife and Human Concerns, J. Biol. Sci., 4 (12): 1550-1558.
- Davies, O.A, Allison, M.E, Uyi, H.S. (2006). Bioaccumulation of heavy metals in water, sediment and periwinkle (Tympanotonus fuscatus var radula) from the Elechi Creek, Niger Delta. African J. Biotechnol., 5(10): 968-973.
- Javed, M. (2005). Heavy Metal Contamination of Freshwater Fish and Bed Sediments in the River Ravi Stretch and Related Tributaries. Pakistan J. Biolog. Sci., 8(10): 1337-1341.
- Philips, D.J.H. and Rainbow, P.S. (1994). Biomonitoring of Trace Aquatic Contaminants. 2nd Edn., Chapman and Hall, London, UK.
- Abdel-Moneim, A.M., Al-Kahtani, M.A. and Elmenshawy, O.M. (2012). Histopathological biomarkers in gills and liver of (Oreochromis niloticus) from polluted wetland environments, Saudi Arabia. Chemosphere, 88: 1028-1035.
- Palaniappan, P.L.R.M. and Karthikeyan S. (2009). Bioaccumulation and depuration of chromium in the selected organs and whole body tissues of freshwater fish Cirrhinus mrigala individually and in binary solutions with nickel. J. Environ. Sci., 21(2): 229-236.
- Okocha, R.C. and Adedeji, O.B. (2011). Overview of cadmium toxicity in fish. J. AppliedSci.Res., 7:1195-1207.
- Klaassen, C.D., Liu, J. and Choudhuri, S. (1999). Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol., 39: 267-274.
- Kotze, P, du Preez, H.H, Van Vuren, J.H.J. (1999). Bioaccumulation of Cu and Zn in Oreochromis mossambicus and Clarius gariepinus, from the Olifants River, Mpumalanga, South Africa. Water SA., 25(1): 99-110.
- Adams, S.M., (2002). Biological indicators of aquatic ecosystem stress. American Fisheries Society, Bethesda, MD., USA., pp: 644.
- Akueshi, E.U., Oriegie, E., Ocheakiti, N., and Okunsebor, S. (2003). Levels of some heavy metals in fish from mining lakes on the Jos Plateau, Nigeria. Afr. J. Nat. Sci., 6:82-86.
- Jenkins, J.A. (2004). Fish bioindicators of ecosystem condition at the Calcasieu Estuary, Louisiana. Open-File Report 2004-1323, National Wetlands Research Center, USGS, Lafayette, pp: 47.
- Salkind, N. J. (2010). Encyclopedia of research design Thousand Oaks, CA: SAGE Publications, Inc. doi: 10.4135/9781412961288
- Muisa, N. (2010). Impacts of alum residues from Morton Jaffray water works on water quality and fish, Harare, Zimbabwe. J. Environ. Sci., 4: 30
- Hansen, J.A., Welsh, P.G., Lipton, J., and Suedkamp, M.J. (2002). The effects of long-term cadmium exposure on the growth and survival of juvenile bull trout (Salvelinus confleuentus). Aquat. Toxicol., 58: 165-174.
- Puvaneswari, S. and Karuppasamy, R. (2007). Accumilation of cadmium and its effects on the survival and growth of larvae of Heteropneustes fossilis (Bloch, 1974). J of Fish and Aquatic Sc. 2(1): 27-37.
- Ciardullo S, Aureli F, Coni E, Guandalini E, Iosi F, Raggi A, Rufo G, and Cubadda F. (2008). Bioaccumulation potential of dietary arsenic, cadmium, lead, mercury and selenium in organs and tissues of Rainbow trout (Oncorhyncus mykiss) as a function of fish growth. J. Agric. Food Chem., 56: 2442-2451. 77.
- Farkas A, Salánki J, Specziár A. (2003). Age-and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res., 37: 959-964.
- Giguere, A., Campbell, P.G.C., Hare, L., McDonald, D.G. and Rasmussen, J.B. (2004). Influence of lake chemistry and fish age on cadmium, copper and zinc concentrations in various organs of indigenous yellow perch (Perca flavescens). Can. J. Fish Aquat. Sci., 61: 1702-1716.
- Adams, W.J., Blust, R., Borgmann, U., Brix, K.V., and DeForest, D.K. (2011). Utility of tissue residues for predicting effects of metals on aquatic organisms. Integr. Environ. Assess. Manage., 7: 75-98.
- AMAP. (1998). AMAP assessment report: Arctic pollution issues. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway.
- Ahmed, T. A., Mandal, S., Chowdhury, D.A., Rayhan, A., Tareq, M. and Rahman M. (2012). Bioaccumulation of some heavy metals in ayre fish (Sperata aor hamilton, 1822), sediment and water of dhaleshwari river in dry season, Bangladesh J. Zool., 40 (1):147-153
- Olsson, P., Kling, P., and Hogstrand, C. (1998). Mechanism of heavy metal accumulation and toxicity in fish. Metal Metabolism in Aquatic Environments, Langston, W.J. and M.J. Bebianno (Eds.). Chapman and Hall, London, UK., ISBN-13: 978-0412803703, pp: 321-341.
- Fafioye, OO, Adebisi, AA and Fagade, SO (2004). Toxicity of Parkia biglobosa and Raphia vinifera extracts on Clarias gariepinus juveniles. African J. Biotechnol. 3(11): 627-630.
- Ramesh, F and Nagaranjan, K (2007). Histopathological changes in gills of Clarias batrachus treated with sago effluent. J. Exp. Zool. 10(1): 169-171.
- Wong, C.K.C., Cheung, R.Y.H. and Wong, M.H. (2000). Heavy metal concentrations in green-lipped mussels collected from Tolo Harbour and markets in Hong Kong and Shenzhen. Environ. Pollut., 109: 165-171.
- FAO (1983) Compilation of legal limits for hazardous substances in fish and fishery Products. FAO Fishery Circular No. 464, Food and Agriculture Organization, 5-100.
- FAO/WHO (1989) Evaluation of certain food additives and the contaminants Mercury, Lead and Cadmium. WHO Technical Report Series No. 505.
- Mokhtar, M. (2009) Assessment Level of Heavy Metals in Penaeus monodon and Oreochromis spp. in Selected Aquaculture Ponds of High Densities Development Area. European Journal of Scientific Research, 30, 348-360.
- EC (European Community) (2005) Commission Regulation No. 78. Official Journal of the European Union, L16/43- L16/45.
- MAFF (Ministry of Agriculture, Fisheries and Food) (1997) Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea. In: Aquatic Environment Monitoring Report No. 52, Center for Environment, Fisheries and Aquaculture Science, Lowestoft.
- Dural, M., Goksu, M.Z.L. and Ozak, A.A. (2007). Investigation of heavy metal levels in economically important fish species captured from the Tuzla Lagoon. Food Chem., 102: 415-421.
- Rangsayatorn N, Kruatrachue M, Pokethitiyook P, Upatham ES, Lanza GR and Singhakaew S. (2004). Ultrastructural changes in various organs of the fish Puntius gonionotus fed cadmium-enriched cyanobacteria. Environ. Toxicol. 19(6): 585-93.
- Dangre, AJ, Manning, S., and Brouwer (2010). Effects of cadmium on hypoxia-induced expression of hemoglobin and erythropoietin in larval sheepshead minnow, Cyprinodon variegates. Aquatic Toxicol. 99(2):168-175.
- Jezierska B., Witeska M. (2006) The Metal uptake and accumulation in fish living in polluted waters. In: Twardowska I., Allen H.E., Häggblom M.M., Stefaniak S. (eds) Soil and Water Pollution Monitoring, Protection and Remediation. NATO Science Series, vol 69. Springer, Dordrecht
- Kumar P, Prasad Y, Patra AK, Ranjan R, Patra RC, Swarup D and Singh SP (2009). Ascorbic acid, garlic extract and taurine alleviate cadmium-induced oxidative stress in freshwater catfish (Clarias batrachus). The Sci. Total Environ.. 407: 5024–5030.
- Vesey, DA. (2010). Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Letters. 198(1):13-19.
- Bervoets, L., Blust, R. and Verheyen, R. (2001). Accumulation of metals in the tissues of three spined stickelback (Gasterosteus aculeatus) from natural fresh waters. Ecotoxicol. Environ. Saf., 48: 117-127.
- Kumar P, Prasad Y, Patra AK and Swarup D (2007). Levels of Cadmium and Lead in tissues of freshwater fish (Clarias batrachus L.) and chicken in western UP (India). Bull. Environ. Contamin. Toxicol. 79: 396-400.
- Singh, AP, Singh, AK and Singh, JPN (2007). Cadmium induced changes on the secretion of branchial mucous cells of peppered loach, Lepdocephalichthys guntea. J. Exp. Zool.. 10(1): 65-68.
- Perera, P.A.C.T., Suranga, P., Kodithuwakku, T.V., Sundarabarathy and Edirisinghe, U. (2015). Bioaccumulation of Cadmium in Freshwater Fish: An Environmental Perspective. Insight Technology. DOI: 10.5567/ECOLOGY-IK.2015.1.12
- Ebrahimi, M., Taherianfard, M. (2011). The effects of heavy metals exposure on reproductive systems of cyprinid fish from Kor River. Iranian Journal of Fisheries Sciences. 10(1) 13-24 2011
- Rurangwa, E., Roelants, I., (1998). The minimum acceptable spermatozoa to egg ratio for artificial insemination and the effects of heavy metal pollutants on sperm motility and fertilization ability in the African catfish (Clarias gariepinus, Burchell 1822). Journal of Fish Biology, 53, 402–13.
- Akan, J.C., Abdulrahman, FI., Sodipo, OA., and Akandu, PI. (2009). Bioaccumulation of some heavy metals of six fresh water fishes caught from Lake Chad in Doron Buhari, Maiduguri, Borno State, Nigeria. J. Applied Sci. Environ. Sanitat., 4: 103-114.
- Ambedkar, G. and Muniyan, M (2012). Analysis of heavy metals in water, sediments and selected freshwater fish collected from Gadilam river, Tamilnadu, India. Int. J. Toxicol. Applied Pharmacol., 2: 25-30.
- Eneji, I.S., Sha'Ato, R and Annune, PA. (2011). Bioaccumulation of heavy metals in fish (Tilapia zilli and Clarias gariepinus) organs from River Benue, North-Central Nigeria. Pak. J. Anal. Environ. Chem., 12: 25-31.
- Gwaski, P.A., Hati, S.S., Ndahi, N.P. and Ogugbuaja, VO. (2013). Emergent pollution bio-monitoring triad (target species-tissuesanalyse) for the aquatic environment of Chad lake. Adv. Applied Sci. Res., 4: 181-189.
- Malik, N., Biswas, A.K., Qureshi, T.A., Borana, K. and Virha, R. (2010). Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ. Monit. Assess., 160: 267-276.
- Rauf, A., Javed, M., and Ubaidullah, M. (2009. Heavy metal levels in three major carps (Catla catla, Labeo rohita and Cirrhina mrigala) from the river Ravi, Pakistan. Pak. Vet. J., 29: 24-26.
- Taweel, A., M. Shuhaimi-Othman, K. and Ahmad, A.K. (2011). Heavy metals concentration in different organs of tilapia fish (Oreochromis niloticus) from selected areas of Bangi, Selangor, Malaysia. Afr. J. Biotechnol., 10: 11562-11566.
- Uwem, G.U., Emile, A.F., Udo, I.J. and Bassey, A.A. (2013). Bioaccumulation of heavy metal in three fresh water fishes caught from cross river system. Eur. J. Exp. Biol., 3: 576-582.
Citation: Zinia Rahman, Istiyak Ahmad and Ibrahim Rashid. (2018). Effects of Cadmium Exposure on Growth and Survival and Accumulation in Various Organs of Nile Tilapia (Oreochromis niloticus, Linnaeus). Journal of Agriculture and Aquaculture 1(1).
Copyright: © 2018 Zinia Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
