Research Article
Volume 2 Issue 3 - 2020
Effect of foliar Fertilization on Ananas comosus L. Merr. cv. 'Cayena lisa' Acclimatization
Plant Biotechnology Institute. Marta Abreu Central University of Las Villas. Highway to Camajuaní km 5.5. Santa Clara. Villa Clara. Cuba. CP 54 830
*Corresponding Author: Ortelio Hurtado, Plant Biotechnology Institute. Marta Abreu Central University of Las Villas. Highway to Camajuaní km 5.5. Santa Clara. Villa Clara. Cuba. CP 54 830.
Received: May 06, 2020; Published: June 01, 2020
Abstract
The low survival and slow growth of in vitro pineapple plants (Ananas comosus L. Merr.) in acclimatization stage limit the use of biotechnological techniques for it propagation. The aim of this study was to determine the effect of foliar fertilization in the acclimatization of pineapple plants cv. 'Smooth Cayenne'. Two variants of foliar fertilization were compared. The first, plants were fertilized daily after the last irrigation with a minimum dose increased until three months of culture. The second included the same fertilizer at maximum dose with daily dose foliar applications after the last irrigation 10 days from planting to three months of cultivation. As a control, unfertilized plants were included. Every 20 days to three months of culture height (cm) of plants was measured, the number of leaves per plant was quantified and the length and width of the leaves was measured. It was observed that fertilization had effect under the experimental conditions tested on the plants variables. After 90 days of culture plants obtained in the treatment with daily fertilization at maximun dose (option 2), met the requirements of height, length and width of the leaf for transplantation to field conditions.
Key words: Pineapple; Propagation; Zeolite
Introduction
Pineapple (Ananas comosus L. Merr.) Is the most important species in the Bromeliacea family. The genus Ananas has its center of origin in the Guyanese shield and the Amazon, in an area between 10o in the north and south latitudes and from 55o to 75o west longitude (Leal and Antoni, 1981), it is found naturally south of Brazil, north of Argentina and Paraguay and on the southern edges of the Amazon.
Pineapple is the second tropical crop of world importance after bananas, and contributes more than 20% of the world volume of tropical fruits (Coveca, 2002). Seventy percent of the pineapple produced in the world is consumed as fresh fruit in the country that produces it. The world production of pineapple exceeded 24.79 million tons in 2013 and ranks seventh in world production among fruit trees and fourth in tropical fruits (FAO, 2014).
Interest in this crop has increased, due to its market demand, but producers have difficulty meeting the need for 'child' plants to establish new plantations. The lack of plants is due to the multiplication rate in natural form is very low (Griffith, 1998). It is there where tissue culture techniques have come to play an important role. In this culture, in vitro propagation offers a superior dimension to the producer, allowing the rapid multiplication of a new variety from a few plants. This has been described by several authors, who have used different stories such as: leaf sections (Daquinta, 1998; Dolgov et al., 1998), axillary buds (Almeida et al., 2002), crown buds (Fitchet- Purnell, 1993; Brenes, 2005) and in all cases they managed to regenerate shoots and obtain plants. Furthermore, they have used temporary immersion systems for their in vitro propagation (Escalona et al., 1999; González-Olmedo et al., 2005).
However, the potential use of these procedures is limited by the low survival and slow growth of the plants in the acclimatization phase, which requires six to eight months for the plants to reach 20-30 cm and can be transplanted into the field (Texeira et al., 2001). Although progress has been made in the study of plant physiology under ex vitro conditions (Aragón et al., 2012), there are still no satisfactory results that reduce acclimatization time.
One of the alternatives for reducing cultivation time could be related to the supply of nutrients to plants. In this sense, studies have been carried out on the effect of the application of growth regulators such as gibberellic acid (AG3) with fertilizers (Yanes et al., 2001, the inoculation of bacteria promoting plant growth (González et al., 1997; Baldotto et al., 2010), the type of substrate and culture conditions (Mengesha et al., 2013) that have shown positive results in the experimental conditions tested. Taking into account the above, this work aimed to determine the effect of foliar fertilization in the acclimatization of pineapple plants cv. 'Cayena lisa'.
Materials and Methods
Vegetal material
In vitro plants of pineapple cv. 'Cayena lisa' (Figure 1), propagated in vitro as described by Daquinta (1998). The plants were in the rooting phase and had a height between 3.0 and 5.0 cm (measured from the base to the insertion of the last leaf), between 6 and 8 active leaves and the presence of roots.
In vitro plants of pineapple cv. 'Cayena lisa' (Figure 1), propagated in vitro as described by Daquinta (1998). The plants were in the rooting phase and had a height between 3.0 and 5.0 cm (measured from the base to the insertion of the last leaf), between 6 and 8 active leaves and the presence of roots.
Growing conditions
The acclimatization phase of the in vitro pineapple plants was carried out in a cultivation house covered by plastic mesh, with which a 70% reduction in solar lighting was achieved. The substrate used was a mixture containing 20% organic matter, 60% zeolite and 20% coconut fiber. Polyethane trays with 70 holes of 56 cm3 capacity each were used. In vitro plants were planted to a depth of 0.5 cm. Phytosanitary control was carried out preventively every three days by applying commercial products at the recommended doses (Triana et al., 1999).
The acclimatization phase of the in vitro pineapple plants was carried out in a cultivation house covered by plastic mesh, with which a 70% reduction in solar lighting was achieved. The substrate used was a mixture containing 20% organic matter, 60% zeolite and 20% coconut fiber. Polyethane trays with 70 holes of 56 cm3 capacity each were used. In vitro plants were planted to a depth of 0.5 cm. Phytosanitary control was carried out preventively every three days by applying commercial products at the recommended doses (Triana et al., 1999).
Irrigation was carried out by micro-sprinkling with a frequency of three daily irrigations and a duration of five minutes each, at 8:00 a.m., 12:00 m and 4:00 p.m. (Triana et al., 1999). With this frequency, a relative humidity was guaranteed, which ranged from 70 to 90%.
Fertilization effect
Two variants were compared to determine the effect of foliar fertilization on the acclimatization of plants in vitro (Table 1). The first consisted of starting foliar fertilization after 15 days of planting with a minimum dose that increased every 15 days until reaching the maximum dosage after three months of cultivation. The plants were fertilized daily after the last irrigation (Triana et al., 1999). The second included the same fertilizers at the maximum dose with daily foliar applications after the last irrigation from 10 days of planting to three months of cultivation. Unfertilized plants were included as control. Each treatment consisted of 45 trays with 3,150 plants each for a total of 9,450 plants. The experiment was repeated three times in the duration of the investigation. Every 20 days until three months of culture, the height (cm) was measured (from the base to the insertion of the last leaf), the number of leaves per plant was quantified, and the length and width of the last leaf emitted were measured.
Two variants were compared to determine the effect of foliar fertilization on the acclimatization of plants in vitro (Table 1). The first consisted of starting foliar fertilization after 15 days of planting with a minimum dose that increased every 15 days until reaching the maximum dosage after three months of cultivation. The plants were fertilized daily after the last irrigation (Triana et al., 1999). The second included the same fertilizers at the maximum dose with daily foliar applications after the last irrigation from 10 days of planting to three months of cultivation. Unfertilized plants were included as control. Each treatment consisted of 45 trays with 3,150 plants each for a total of 9,450 plants. The experiment was repeated three times in the duration of the investigation. Every 20 days until three months of culture, the height (cm) was measured (from the base to the insertion of the last leaf), the number of leaves per plant was quantified, and the length and width of the last leaf emitted were measured.

Table 1: Fertilization variants used in the acclimatization of in vitro plants of pineapple cv. ‘Cayenne Lisa’
Statistic analysis
The data were processed using the SPSS program for Windows version 18. Analysis of variance was performed and the means were compared using the Duncan test (p <0.05) after checking the assumptions of normality and homogeneity of variances.
The data were processed using the SPSS program for Windows version 18. Analysis of variance was performed and the means were compared using the Duncan test (p <0.05) after checking the assumptions of normality and homogeneity of variances.
Results and Discussion
Fertilization was observed to have an effect, under the experimental conditions described above, on the variables evaluated for pineapple plants produced in vitro.
In the case of height, already at 30 days of culture (Figure 2) significant differences were observed between the treatments, which were maintained until 90 days. The highest values were obtained in the daily fertilization variant with the maximum fertilizer dose. In this treatment 90 days after cultivation (Figure 3), as an average, the height of the plants was doubled compared to the treatment with progressive fertilization.
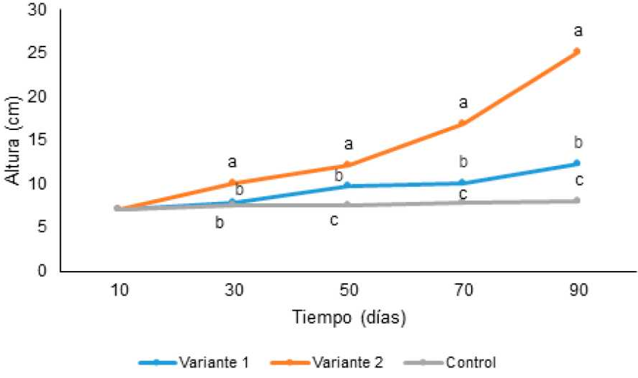
Different letters at each time indicate significant differences between treatments according to the Duncan test (p <0.05) .n = 3150 plants
Figure 2: Height of plants of Ananas comosus L. cv. ‘Cayena lisa’ in acclimatization phase undergoing different fertilization variants.
Figure 2: Height of plants of Ananas comosus L. cv. ‘Cayena lisa’ in acclimatization phase undergoing different fertilization variants.
Taking into account that Triana et al. (1999) defined that pineapple plants with height> 25 cm could be transplanted to the field, after 90 days of cultivation, the plants fertilized daily with the maximum dose (variant 2) (Figure 3) met this requirement unlike the rest of treatments. Variant 1 of foliar fertilization every 15 days and with progressive doses was applied prior to this work in the IBP and to reach these values the plants required six months of cultivation. These results indicated that the management of the nutritional conditions of the plants in the acclimatization phase, under the experimental conditions tested, allows to shorten the cultivation time, which has been defined by several researchers between six and eight months (González et al. , 1997; Texeira et al., 2001).
Regarding the number of leaves, as well as their characteristics (length and width), the highest values, as well as for the height, were observed in the plants with daily fertilization with the maximum dose (variant 2) with significant differences with respect to the remaining treatments (Figures 3 and 4). It is highlighted the fact that with this variant the plants showed increases in the number of leaves from 30 days, while with variant 1 of fertilization (progressive increase in the concentration applied every 15 days) 50 days were required to obtain these results , which shows that the nutritional needs of these plants, under the experimental conditions tested, were higher. The composition of the substrate used had a higher proportion of zeolite and coconut fiber than organic matter, and therefore the nutritional needs must be covered mainly by the foliar route.

Figure 3: Plants of Ananas comosus L. cv. ‘Cayena lisa’ after 90 days in acclimatization phase with daily foliar fertilization.
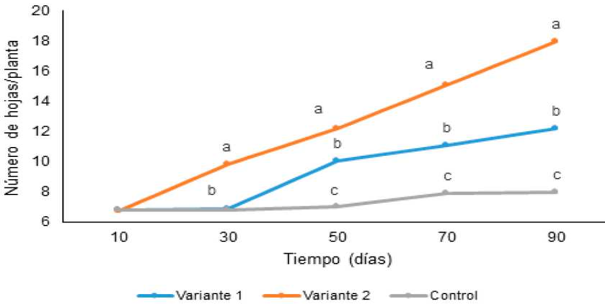
Different letters at each time indicate significant differences between treatments according to the Duncan test (p <0.05) .n = 3150 plants
Figure 4: Number of leaves of Ananas comosus L. cv. ‘Cayena lisa’ in acclimatization phase undergoing different fertilization variants.
Figure 4: Number of leaves of Ananas comosus L. cv. ‘Cayena lisa’ in acclimatization phase undergoing different fertilization variants.
Yanes et al. (2001), obtained in the acclimatization phase significant increases in the length of the leaves of pineapple plants of cv. 'Cayena lisa' that were produced in vitro, by applying in addition to a foliar fertilizer, gibberellic acid (AG3). These authors with 200.0 mg l-1 of AG3 reached up to 16.2 cm. However, in the present study, a leaf length for this same cultivar greater than 17 cm was obtained only with the application of fertilizers. Similarly, Villalobos et al. (2012) reported that with foliar fertilization with a mixture of 16.0 g NPK and 1.0 g of Multimicro Combi (Haifa Chemicals Ltd.) in 16 liters of water, applied every 10 days, at 30 days of cultivation, leaf D of the plants it reached an average of 9.66 cm in length and 9.24 cm in width.
In the same way that in the case of height, already after 90 days of cultivation, the plants obtained in the treatment with daily fertilization with the maximum dose (variant 2), fulfilled the requirements of leaf length for transplanting under conditions of field and practically doubled in numerical values, the results of variant 1. After 90 days of cultivation, an average value of 2.5 cm of leaf width was reached, higher than that reported by Yanes et al. (2001) (1.5 cm leaf width) when applying only foliar fertilizations. The plants that were not subject to foliar fertilization (control) showed slow growth with the lowest values in the variables evaluated with respect to the rest of the treatments after 30 days of cultivation.
Several factors influence the growth of pineapple plants in the acclimatization phase, among which lighting, substrate, irrigation regime and fertilization stand out (Brenes, 2005; González-Olmedo et al., 2005; Mengesha et al., 2013). On the above, Aragón et al. (2012) showed that the optional metabolism C3/CAM is characteristic of pineapple plants in the first two months of cultivation depending on environmental conditions. Therefore, it is necessary to deepen studies that allow modifying the procedures in the acclimatization phase to decrease the cultivation time with greater plant growth. Although there have been advances in this regard, the scientific results are not always comparable since the experimental conditions are different. The results of this study confirm the need to provide plants with nutrients during the acclimatization phase that guarantee these purposes and open new questions for the design of cultivation strategies with biological and economic efficiency.
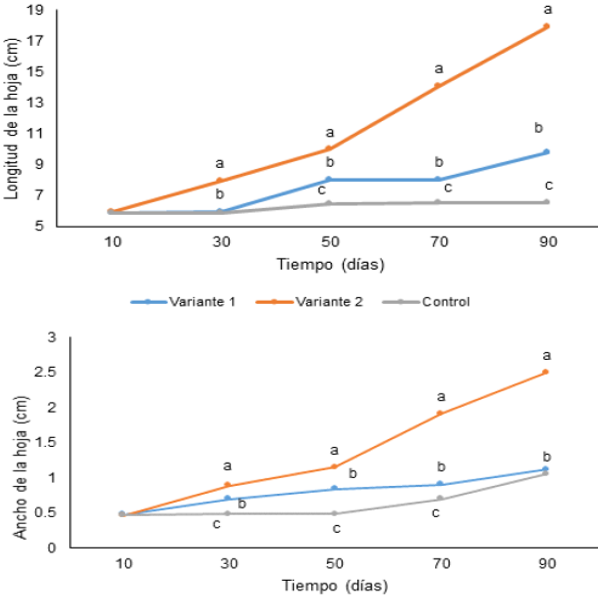
Different letters at each time indicate significant differences between treatments according to the Duncan test (p <0.05) .n = 3150 plants
Figure 5: Characteristics of the leaves of Ananas comosus L. cv. ‘Cayena lisa’ in acclimatization phase undergoing different fertilization variants. A- leaf length, B-leaf width.
Figure 5: Characteristics of the leaves of Ananas comosus L. cv. ‘Cayena lisa’ in acclimatization phase undergoing different fertilization variants. A- leaf length, B-leaf width.
Conclusions
The frequency and dose of fertilizers applied to pineapple plants cv. 'Cayena lisa' in the acclimatization phase influences its growth and the length of the cultivation period.
References
- Almeida WAB, Santana GS, Rodriguez APM, Costa MA. (2002). Optimization of a protocol for the micropropagation of pineapples. Rev. Bras. Fruit 2: 296-300
- Aragón C, Carvalho L, González J, Escalona M, Amäncio S. (2012). The physiology of ex vitro pineapple (Ananas comosus L. Merr. Var. MD-2) as CAM or C3 is regulated by the environmental conditions. Plant Cell Rep 31: 757-769.
- Baldotto L E B, M A Baldotto, L P Canellas, R Bressan-Smith, FL Olivares (2010). Growth promotion of pineapple 'Vitória' by humic acids and Burkholderia spp. during acclimatization. Brazilian Journal of Science of Solo 34 (5): 1593-1600
- Brenes S. (2005). Vegetative and productive characterization of cultivar MD-2 (Ananas comosus) under the climatic conditions of Turrialba. Electronic Journal of the Regional Offices of the University of Costa Rica 6 (11): 27-34.
- Coveca C. (2002). Commission from Veracruz for agricultural commercialization. Government of Veracruz department, Veracruz, Mexico
- Daquinta M. (1998). In vitro propagation of pineapple (Ananas comosus (L) Merr) Thesis presented as an option to the scientific degree of Doctor of Agricultural Sciences. University of Ciego de Ávila. Ciego de Avila.
- Dolgov SV, Shushkova TV, Firsov AP. (1998). Pineapple (Ananas comosus Mess.) Regeneration from leaf explants. Hort Act. 461: 439-444
- Escalona M, Lorenzo JC, González B, Daquinta M, González J, Desjardins Y, Borroto CG. (1999). Pineapple (Ananas comosus (L.) Merr.) Micropropagation in temporary immersion system. Plant Cell Report 18 (9): 743-748.
- FAO. (2014). Statistical database. Tropical foods commodity notes. [Online] At: www.fao.org. Retrieved on January 23, 2015
- Fitchet-Purnell M. (1993). Maximum utilization of pineapple crowns for micro propagation. Hort Act. 334: 325-330
- González R, Domínguez Q, Expósito LA, González JL, Hidalgo M. (1997). Effectiveness of eight Azotobacter strains in the adaptation of pineapple (Ananas comosus L. Merr.) Vitroplants, cv. Cayenne Lisa. Acta Horticulturae 425: 277-281
- González-Olmedo JL, Fundora Z, Molina L, Abdulnour J, Desjardins Y, Escalona M. (2005). New contributions to propagation of pineapple (Ananas comosus L. Merr.) In temporary immersion bioreactors. In Vitro Cellular and Developmental Biology-Plant 41 (1): 87-90.
- Griffith LP. (1998). Tropical Foliage Plants. A Grower's Guide. Ball Publishing Batavia. Illinois.
Leal F, Antoni M (1981) Description and key of the varieties cultivated in Venezuela. Magazine Faculty of Agronomy (Maracay) 29: 51-79 - Mengesha Ayelign, Biruk Ayenew, Tewodros Tadesse. (2013). Acclimatization of in vitro propagated pineapple (Ananas comosuss L., var. Smooth cayenne) plantlets to ex vitro condition in Ethiopia. American Journal of Plant Sciences 4: 317-323.
- Teixeira J B, Cruz A R R, Ferreira F R, Cabral J R. (2001). Biotechnology applied to seedling production: production of pineapple plantlets. Science and Biotechnology Development 3: 42-47
- Triana R, Pérez Z, García L. (1999). Instructions for the cultivation of pineapple phase IV. IBP. Santa Clara.
- Villalobo Ariel, González Justo, Santos Ramón, Rodríguez Romelio. (2002). Morpho-physiological changes in pineapple plantlets (Ananas comosus L. Merr.) During acclimatization. Science. agrotec. Lavras 36 (6): 624-630.
- Yanes E, González O, Rodríguez R. (Use of gibberellins and foliar fertilization during the acclimatization of pineapple vitroplants Cayena Lisa cv. 'Serrana'. Biotechnol
Citation: Ortelio Hurtado. (2020). Effect of foliar Fertilization on Ananas comosus L. Merr. cv. ‘Cayena lisa’ Acclimatization. Journal of Biotechnology and Immunology 2(3).
Copyright: © 2020 Ortelio Hurtado. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

