Research Article
Volume 1 Issue 1 - 2018
Effect of Banana Peel Extract on Sensory and Bacteriological Quality of Marinated Beef
Dept. Food Hygiene, Faculty of Veterinary Medicine, Suez Canal University, Animal Health Research Institute, Port-said Branch
*Corresponding Author: Ali Meawad Ahmed, Dept. Food Hygiene, Faculty of Veterinary Medicine, Suez Canal University, Animal Health Research Institute, Port-said Branch.
Received: October 31, 2018; Published: November 22, 2018
Abstract
Marination is the process of soaking foods in a seasoned, meat marination using different marinades not only improve the beef sensory quality, but also improve the bacteriological quality. Banana is one of the oldest cultivated and the most popular fruits distributed allover the world. Peoples supposed to consume fruits and discard their peel as waste. The present study was carried out to use the banana peel extracts (BPE) as supplement to traditional marination ingredients of beef for improving their microbial quality and safety. Beef samples were marinated with salt, onion, tomato and 1%, 3% and 5% of banana peel aqueous extracts then stored at 4°C for 4 hours. The sensory parameters of meat before roasting indicated good values with the addition of 1% BPE while were improved by marination with different concentrations of BPE after roasting by panel testing. BPE exhibited antimicrobial activity against Staphylococcus aureus in vitro, it also induced antimicrobial activity against aerobic and enterobacteriaceae with increase the reduction percent by increasing the concentration of banana peel. Salmonella spp. and E. coli was not detected by available laboratory methods in all examined beef samples. The results ensured that the Egyptian banana peels extract have antibacterial effect against aerobic, enterobacteriaceae and S. aureus bacteria. So it can be used as natural preservative for meat and meat products.
Keywords: Beef; Marination; Banana peel extract; Meat sensory quality; Bacteriological quality
Introduction
Beef has been an important part of the human diet throughout human evolution. Beef as part of a healthy, varied diet, provides a rich source of high biological value protein and essential nutrients, some of which are more bioavailable than in alternative food sources (Wyness, 2015).
Meat provides excellent growth media for a variety of microflora some of which are pathogens and other are non-pathogens (Jay., et al. 2005). Microbial growth is one of the mechanisms chargeable for beef spoilage. The main sources of these microorganisms are the skin and the intestinal tract of the slaughter animal. The number of microflora in meat depends on different factors as; preslaughter husbandry practices; age of the slaughtered animal; temperature controls during slaughtering, processing and distribution; handling during slaughtering, evisceration and processing; preservation methods; type of packaging and handling and storage by consumer (Cerveny., et al. 2009).
Beef spoilage leads to significant economic losses for the meat industry. Several investigators allover the world are challenged to reduce these economic losses, the meat industry is looking for prolongation of meat and meat products shelf-life by natural preservation methods and fulfilling the consumers’ demands for high quality, convenience and improved flavor (Pathania., et al. 2010).
Marination is the process of soaking or pickling meat in a seasoned liquid (marinade mixture) for hours before cooking. The objective of marination is improvement the sensory values like tenderness, flavoring, juiciness of meat, and enhancement of microbiological safety of meat. These desirable properties of a good marinated meat are achieved by the interaction of marinade ingredients with meat (Palang, 2004).
The microbial growth and multiplication in non-marinated beef meat is about 0.9 log cfu/cm2 in total viable counts (TVCs) after 24h greater than 9.5 log cfu/cm2 after 8 days of refrigerated storage (Kargitou., et al. 2011). The development of microorganism in non-marinated beef meat usually increases during refrigerated storage. Marination inhibits microbial growth in beef meat and the bacterial development remains below 103cfu/g (Knöchel., et al. 2007).
Banana peel has been traditionally used as medicinal material for the treatment of various ailments such as burns, anaemia, diarrhoea, ulcers, inflammation, diabetes and cough (Pereira and Maraschin, 2015). Banana peel contains high phenohlic content which possesses antimicrobial activity (Morais., et al. 2015).
Banana peel is rich in proteins, fiber, potassium, essential amino acids, and unsaturated fatty acids (González-Montelongo., et al. 2010). Banana peel extract of banana peel could be considered as a good antibacterial agent against different species of spoilage bacteria (Mokbel and Hashinaga, 2005).
Fruits peel has been a valuable source for maintaining human health. The antimicrobial properties of banana peels extracts can be of great significance in therapeutic treatments. Aqueous extracts of fresh yellow banana peel could be considered as a good antibacterial agent against both Gram positive and negative bacteria to replace the synthetic medicines in treatment of diseases caused by these bacteria (Chabuck., et al. 2013).
Therefore, this study was carried out to evaluate the effect of beef marination with the natural marinated substances as salt, onion andtomato together with different concentrations of aqueous banana peel extract on bacteriological quality of beef.
Materials and Methods
Sampling
A total of 22 fresh beef samples were purchased from a local butcher?s shop at Port-Said city, 12 hours post-rigor. Each sample was collected from the striplion cut, (image 1) Rose-beef, weight 1.250 kg + 250g covered by their fascia. All samples put in sterile polyethylene bags and kept in ice-box, then immediately transferred to Animal Health Research Institute, Port-Said branch, for evaluation.
A total of 22 fresh beef samples were purchased from a local butcher?s shop at Port-Said city, 12 hours post-rigor. Each sample was collected from the striplion cut, (image 1) Rose-beef, weight 1.250 kg + 250g covered by their fascia. All samples put in sterile polyethylene bags and kept in ice-box, then immediately transferred to Animal Health Research Institute, Port-Said branch, for evaluation.
Marinades
The marinades mixture consists of salt, onion, tomato and banana were purchased from Port-Said a local markets.
The marinades mixture consists of salt, onion, tomato and banana were purchased from Port-Said a local markets.
Preparation of Aqueous Extract of Banana peel
Bananas washed in running tap water in laboratory, banana peels surface sterilized with 70% alcohol, rinsed with sterile distilled water. Peels were taken off and air-dried for two weeks and ground into powder with an electrical blender (German blender, 600w att) and sieved with a mesh of size 0.5 mm, then stored in clean brown bottles at room temperature until needed for use.
Bananas washed in running tap water in laboratory, banana peels surface sterilized with 70% alcohol, rinsed with sterile distilled water. Peels were taken off and air-dried for two weeks and ground into powder with an electrical blender (German blender, 600w att) and sieved with a mesh of size 0.5 mm, then stored in clean brown bottles at room temperature until needed for use.
Ninety grams of the powdered peels was dispensed in 900 ml of distilled water in a 1L capacity conical flask. The mixture was vigorously stirred with a magnetic stirrer and then allowed to stand for 48h. It was stirred again and filtered through a Whatman filter paper lined funnel into a conical flask. The filtrate was evaporated at 40ºC with a water bath to obtain the solid crude extract (Ehiowemwenguan., et al. 2014).
Marination of the samples
The connective tissues and fat were trimmed off from the beef sample.The beef samples were cut into pieces about 3x3x1 cm3 and then, divided into control and 4 treated groups, each group weighted 200 gram. Marination mixture consisted of salt 17 gm/kg, onion 30 gm/kg, tomato 50 gm/kg and supplemented with 1, 3 or 5% banana peel aqueous extract. The first group (control) beef sample was immersed into sterile distilled water. The second group, beef sample was marinated with marination mixture without banana peel extract. The third, fourth and fifth groups, beef samples were marinated with marination mixture supplemented with 1, 3 and 5% banana peel aqueous extract respectively.
The connective tissues and fat were trimmed off from the beef sample.The beef samples were cut into pieces about 3x3x1 cm3 and then, divided into control and 4 treated groups, each group weighted 200 gram. Marination mixture consisted of salt 17 gm/kg, onion 30 gm/kg, tomato 50 gm/kg and supplemented with 1, 3 or 5% banana peel aqueous extract. The first group (control) beef sample was immersed into sterile distilled water. The second group, beef sample was marinated with marination mixture without banana peel extract. The third, fourth and fifth groups, beef samples were marinated with marination mixture supplemented with 1, 3 and 5% banana peel aqueous extract respectively.
Each group placed into polypropylene containers. The marination mixture was added to cover all the meat slices, followed by agitation to ensure an even distribution of the solid elements of the marinades. All containers had been over-wrapped with a polyethylene cover and stored at 4°C for 4h. The meat pieces were turned over, to ensure uniform marination. All beef groups were divided into two halves, the first half for bacteriological evaluation and the other half for sensory evaluation (Istrati., et al. 2011).
Sensory evaluation before roasting :
Colour and Odour Evaluation :
Eleven members of non-trained panelists were used to evaluate sensory colour and odour characteristics of beef samples, then expressed the evaluation through 5 point headonic scales, 1. very poor; 2. poor; 3. common; 4. good and 5. very good (Szczesniak, 1987).
Colour and Odour Evaluation :
Eleven members of non-trained panelists were used to evaluate sensory colour and odour characteristics of beef samples, then expressed the evaluation through 5 point headonic scales, 1. very poor; 2. poor; 3. common; 4. good and 5. very good (Szczesniak, 1987).
Roasting protocol for beef palatability tests :
The beef samples were put in oven rack in position of the center of the oven then, preheat oven ten minutes or until 160ºC is reached. The oven temperature and samples were recorded using food digital thermometer. All samples were roasted to internal temperature of 60ºC. After that, the roasting pan was removed from oven and ready for sensory evaluation.
The beef samples were put in oven rack in position of the center of the oven then, preheat oven ten minutes or until 160ºC is reached. The oven temperature and samples were recorded using food digital thermometer. All samples were roasted to internal temperature of 60ºC. After that, the roasting pan was removed from oven and ready for sensory evaluation.
Sensory evaluation after roasting :
About 3x3x1 cm3 from each of the beef samples (raw and roasted) were cut into small pieces. The samples were prepared then given to panel members (n = 11) who were not trained in the sensory analysis of meat. Five characteristics point as were given to panelists as following: 1. very poor; 2. poor; 3. common; 4. good and 5. very good. Panelists were considered the above points for evaluation of colour, odour, taste, tenderness and juiciness of the beef samples (Szczesniak, 1987).
About 3x3x1 cm3 from each of the beef samples (raw and roasted) were cut into small pieces. The samples were prepared then given to panel members (n = 11) who were not trained in the sensory analysis of meat. Five characteristics point as were given to panelists as following: 1. very poor; 2. poor; 3. common; 4. good and 5. very good. Panelists were considered the above points for evaluation of colour, odour, taste, tenderness and juiciness of the beef samples (Szczesniak, 1987).
In vitro antimicrobial activity testing using Agar disk diffusion assay according to (Shahid., et al. 2007):
Loop full growths from S. aureus isolates were inoculated into nutrient broth incubated at 37°C for 18 hours. The bacterial suspensions were diluted with normal saline. The turbidity was adjusted and compared with standard tube (McFarland number 0.5) to yield a uniform suspension containing 1.5×108 CFU/ml. Cotton swab was dipped into adjustment suspension and streak the entire Mueller- Hinton agar surface of plates and the plates were left for one 5-15 minutes at room temperature to dry. Then sterile filter paper disc 0.6 cm in diameter was dipped into different concentrations of banana peels extract and placed gently on the Mueller Hinton agar plate that has already been inoculated with the test organism. This was incubated at 37ºc for 24h after which the plates were viewed for the presence or absence of zone of inhibition of the test organisms around the test and control discs. Antibacterial activity was evaluated by measuring the diameter of inhibition zone. Any disc with a difference of 1 mm or more was considered positive for antibacterial activity.
Loop full growths from S. aureus isolates were inoculated into nutrient broth incubated at 37°C for 18 hours. The bacterial suspensions were diluted with normal saline. The turbidity was adjusted and compared with standard tube (McFarland number 0.5) to yield a uniform suspension containing 1.5×108 CFU/ml. Cotton swab was dipped into adjustment suspension and streak the entire Mueller- Hinton agar surface of plates and the plates were left for one 5-15 minutes at room temperature to dry. Then sterile filter paper disc 0.6 cm in diameter was dipped into different concentrations of banana peels extract and placed gently on the Mueller Hinton agar plate that has already been inoculated with the test organism. This was incubated at 37ºc for 24h after which the plates were viewed for the presence or absence of zone of inhibition of the test organisms around the test and control discs. Antibacterial activity was evaluated by measuring the diameter of inhibition zone. Any disc with a difference of 1 mm or more was considered positive for antibacterial activity.
Bacteriological analysis
Twenty five grams of individual beef samples were aseptically removed from the polypropylene containers and transferred with 225 ml of sterile 0.1% peptone water to a stomacher bag. Serial decimal dilutions were prepared from the stomacher fluids.
Twenty five grams of individual beef samples were aseptically removed from the polypropylene containers and transferred with 225 ml of sterile 0.1% peptone water to a stomacher bag. Serial decimal dilutions were prepared from the stomacher fluids.
Determination of total aerobic count
The pour technique recommended by ISO (2002a) was applied.
The pour technique recommended by ISO (2002a) was applied.
Determination of Enterobacteriaceae counts
The enterobacteriaceae counts were carried out by pour plate method (ISO 21528-2:2004).
The enterobacteriaceae counts were carried out by pour plate method (ISO 21528-2:2004).
Determination of Staphylococcus aureus count
The procedure recommended by ISO (1999) was applied.
The procedure recommended by ISO (1999) was applied.
Detection of E.coli
The procedure of (APHA, 2002) was applied.
The procedure of (APHA, 2002) was applied.
Detection of Salmonellae
According to the method of ISO (2002 b).
According to the method of ISO (2002 b).
Result and Discussion
Sensory evaluation
Sensory evaluation of meat is a common and very useful tool in quality assessment of processed meat products especially after addition of a new additive. It makes use of the senses to evaluate the general acceptability and quality attributes of the products (Heinz and Hautzinger 2007). The data given in table 1 revealed that marination of meat in group II did not adversely affect the meat quality compared to group I. Addition of 1% BPE to the marination mixture remained both colour and odure values of meat with a good quality, while increasing the concentration of BPE (3% and 5%) adversely affected both colour and odure values of meat.
Sensory evaluation of meat is a common and very useful tool in quality assessment of processed meat products especially after addition of a new additive. It makes use of the senses to evaluate the general acceptability and quality attributes of the products (Heinz and Hautzinger 2007). The data given in table 1 revealed that marination of meat in group II did not adversely affect the meat quality compared to group I. Addition of 1% BPE to the marination mixture remained both colour and odure values of meat with a good quality, while increasing the concentration of BPE (3% and 5%) adversely affected both colour and odure values of meat.
| Sensory Character | I | II | III | IV | V |
| Colour | a4.80 ± 0.13 | ab4.40 ± 0.16 | b3.90 ± 0.23 | c2.90 ± 0.24 | c2.30 ± 0.26 |
| Odour | a4.82 ± 0.12 | a4.80 ± 0.13 | ab4.20 ± 0.21 | bc3.80 ± 0.18 | c3.20 ± 0.25 |
Table 1: Effect of Marination and BPE on meat sensory quality before roasting.
Group I = Control Group II = Marination Only Group III = 1% B.P.E. Group IV = 3% B.P.E. Group V = 5% B.P.E. S.E. = Standard Error of Mean
Mean values with different litter within the same raw are significantly difference (P < 0.05).
Group I = Control Group II = Marination Only Group III = 1% B.P.E. Group IV = 3% B.P.E. Group V = 5% B.P.E. S.E. = Standard Error of Mean
Mean values with different litter within the same raw are significantly difference (P < 0.05).
From the obtained results in table 2 we noticed that after roasting, addition of 1% and 3% of banana peels extract to beef didn't deviate the normal colour of roasted beef and keep it acceptable as untreated beef samples, increasing the concentration level of banana peels extract to 5% leading to adverse effect on the roasted beef colour. We also noticed that marination of meat with or without addition of different concentrations of BPE improved the other meat characters (odour, taste, tenderness and juiciness) compared to control group. Marination is used traditionally to improve flavor and tenderness. An important aspect of marination is the increase of yield of the raw meat; marination improved meat texture including a juicier texture and reduction of water loss during cooking (Xargayó., et al. 2001and Sheard and Tali, 2004)
| Sensory Character | I | II | III | IV | V |
| Colour | a4.82 ± 0.14 | a4.80 ± 0.13 | ab4.20 ± 0.20 | bc3.60 ± 0.16 | c3.00 ± 0.21 |
| Odour | a3.00 ± 0.26 | b4.70 ± 0.15 | b4.30 ± 0.14 | b4.10 ± 0.18 | b4.14 ± 0.23 |
| Taste | a1.40 ± 0.15 | b4.43 ± 0.16 | b4.39 ± 0.17 | b4.30 ± 0.21 | b4.31 ± 0.21 |
| Tenderness | a1.20 ± 0.13 | b3.90 ± 0.18 | b4.10 ± 0.23 | b4.20 0.20 | b4.50 ± 0.17 |
| Juiciness | a1.20 ± 0.13 | b3.80 ± 0.19 | bc4.20 ± 0.20 | bc4.21 ± 0.22 | c4.60 ± 0.16 |
Table 2: Effect of Marination and BPE on meat sensory quality after roasting.
Group I = Control Group II = Marination Only Group III = 1% B.P.E. Group IV = 3% B.P.E. Group V = 5% B.P.E. S.E. = Standard Error of Mean
Mean values with different litter within the same raw are significantly difference (P < 0.05).
Group I = Control Group II = Marination Only Group III = 1% B.P.E. Group IV = 3% B.P.E. Group V = 5% B.P.E. S.E. = Standard Error of Mean
Mean values with different litter within the same raw are significantly difference (P < 0.05).
In vitro Antibacterial activity of banana peels aqueous extract against S. aureus
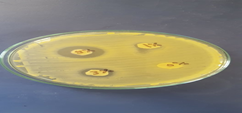
The antibacterial activity of 0, 1%, 3% and 5% banana peels extract against S. aureus were revealed in table 3 and figure 1 Banana peels extract at 1%, 3% and 5% concentration levels had inhibition zone of 0.6, 1.5 and 2 mm respectively.
The antibacterial activity of 0, 1%, 3% and 5% banana peels extract against S. aureus were revealed in table 3 and figure 1 Banana peels extract at 1%, 3% and 5% concentration levels had inhibition zone of 0.6, 1.5 and 2 mm respectively.
This result confirmed that all concentration of banana peels extract had antibacterial effect against S. aureus, this come with agree to Chabuck., et al. (2013); Singh., et al. (2013) and Ehiowemwenguan., et al. (2014).
The antibacterial effect of banana peels extracts were increased as their concentration level increased (figure 1).Banana peels extract had a great antibacterial activity against many pathogens as Staphylococcus aureus, Salmonella typhi and Aeromonas hydrophila (Singh., et al. 2013), also against Bacillus cereus, Salmonella enteritidis, Escherichia coli, Bacillus subtilisand Staphylococcus aureus (Mokbel and Hashinaga, 2005). Banana peelsalso has antibacterial activity against periodontopathogens as Porphyromonasgingivalis (Kapadia.m., et al. 2015).
| Concentration of Banana Peels extract | Diameter of the inhibition zone mm |
| (0%) | 0.0 |
| (1%) | 0.6 |
| (3%) | 1.5 |
| (5%) | 2 |
Table 3: Antibacterial activity of banana peels extract against S. aureus.
Banana peels extract used in old medicine as a natural alternative for killing off a wart; reduces swelling and irritationof a mosquito bite (Kumar., et al. 2012); taken in dysentery and diarrhea and used for treating malignant ulcers (Girish and Satish, 2008) andalso can be used to reduce pain or swelling of injury (Atzingen., et al. 2011). Bioactive compounds in banana peels shown to exert various biological and pharmacological effects (antibacterial, antihypertensive, antidiabetic and anti-inflammatory activities) (Pereira and Maraschin 2015).
The effect of marination on bacteriological quality of beef samples.
The bacteriological evaluation is one of the most important information that indicating the quality and safety of any food article (Libby, 1975).
The bacteriological evaluation is one of the most important information that indicating the quality and safety of any food article (Libby, 1975).
Spices and herbs have been used for centuries by many cultures not only as flavouring agents, but also as food preservatives and prolong the storage life of foods through bacteriostatic or bactericidal activity (Beutchat and Golden, 1989; Cutlre, 1995; Smid and Gorris, 1999; Shan., et al. 2005; Shrinjar and Nemet, 2009; and Velasco and Williams, 2011).
In this study the bacteriological quality of beef samples were shown in table 4. It revealed the existence of aerobic colonies, Enterobacteriaceae and S. aureus, while E. coli and Salmonellae were not detected.
| Group | Aerobic colonies | Enterobacteriaceae | S. aureus | E. coli | Salmonella |
| I | + ve | + ve | + ve | - ve | - ve |
| II | + ve | + ve | + ve | - ve | - ve |
| III | + ve | + ve | + ve | - ve | - ve |
| IV | + ve | + ve | + ve | - ve | - ve |
| V | + ve | + ve | + ve | - ve | - ve |
Table 4: The effect of marination on bacteriological quality of beef samples.
Total Aerobic Bacterial Counts
Beef is regularly contaminated by aerobic bacteria from different sources during processing as hide, floor washings, viscera (intestinal contents), abattoir environment, processing equipment and tools, water, hands, clothing, boots, aprons and tables (Buchanan and Halbrook, 1995; Rahkio and Korkeala, 1997; McEvoy., et al. 2004; Holds., et al. 2007 and Zweifel., et al. 2008).
Beef is regularly contaminated by aerobic bacteria from different sources during processing as hide, floor washings, viscera (intestinal contents), abattoir environment, processing equipment and tools, water, hands, clothing, boots, aprons and tables (Buchanan and Halbrook, 1995; Rahkio and Korkeala, 1997; McEvoy., et al. 2004; Holds., et al. 2007 and Zweifel., et al. 2008).
The total counts of aerobic bacteria per gram of muscular tissue is a good indicator of microbiological safety where, spoilage occur when the microbial population reaches 8 log cfu/g in meat product (Narasimha Rao., et al. 1998). The aerobic plate counts are generally accepted as a criterion for microbial contamination of carcasses and a useful indicator of hygienic conditions of abattoir (Cohen., et al. 2007).
The effect of marination on the mean values of total aerobic counts for groups I, II, III, IV and V were recorded in table 5. The mean values of total aerobic counts were 3x104 + 6.9x103, 2x104 + 4.8x103, 1x104 + 1.8x103, 6.7x103 + 1.4x103 and 4.9x103 + 9.1x102. Group I was the highest total bacterial counts followed by group II, III, IV and V. There was a significant difference (P < 0.05) in means of total bacterial counts between group I and groups III, IV and V, there is no significant difference (P < 0.05) between the uses of different concentrations of banana peels extract.
| Group | Minimum | Maximum | Mean | + *S. E. | **R. P. with group I | ***R. P. with group II |
| I | 3.7 x102 | 9.1x104 | a3x104 | 6.9 x103 | - | - |
| II | 3x102 | 6.7 x104 | ab2.2 x104 | 4.8 x103 | - | - |
| III | 2.8 x102 | 3.7 x104 | bc1x104 | 1.8 x103 | 63% | 49% |
| IV | 2.5x102 | 2.2 x104 | bc6.7x103 | 1.4 x103 | 78% | 69% |
| V | 2x102 | 1.5 x104 | c4.9x103 | 9.1 x102 | 84% | 77% |
Table 5: Effect of marination on total Aerobic count (cfu/g) of beef samples.
Group I= Control, Group II = Marination Only, Group III = 1% B.P.E., Group IV = 3% B.P.E., Group V = 5% B.P.E.
Mean values with different litter are significantly difference (P<0.05). , *S. E. standard error **R. P. with group I = Reduction Percent based on result of group I, *** R. P. with group II = Reduction Percent based on result of group II., B.P.E.=Banana Peels Extract.
Group I= Control, Group II = Marination Only, Group III = 1% B.P.E., Group IV = 3% B.P.E., Group V = 5% B.P.E.
Mean values with different litter are significantly difference (P<0.05). , *S. E. standard error **R. P. with group I = Reduction Percent based on result of group I, *** R. P. with group II = Reduction Percent based on result of group II., B.P.E.=Banana Peels Extract.
However, it is usually difficult to make comparisons between surveys because of differences inobjectives, sampling protocols, laboratory procedures. However, the recorded results in the present study are nearly higher than those reported bySumner., et al. (2003); Hutchion., et al. (2005); Zweifel., et al. (2005) and Nouichi and Hamdi (2009).
The mean of total aerobic count of group I (control, raw beef without marination) was higher than previous studies (Roberts., et al. 1984 and Hemmat., et al. 2013). This higher recorded total aerobic counts value in this study reflect the hygienic measures that applied during slaughter, dressing, evisceration,handling of carcasses, cutting and distribution of beef (Upadhyaya., et al. 2012). Treatment of beef with banana peels extract were effective in decreasing the total aerobic counts due to their antibacterial activity against Gram positive and negative bacteria these results agree with Aldean., et al. (2010); Sumathy and Sumathy (2011); Chabuck., et al. (2013) and Ehiowemwenguan., et al. (2014).
Enterobacteriaceae counts
Enterobacteriaceae have an epidemiological importance as some of their members are pathogenic and may cause serious infections and food poisoning outbreaks to human being. The presence of enterobacteriaceae in large numbersin food indicates improper hygienic measures, fecal contamination, dirty equipment or unhygienic handling (PHLS 2002; Gill and Landers 2004).
Enterobacteriaceae have an epidemiological importance as some of their members are pathogenic and may cause serious infections and food poisoning outbreaks to human being. The presence of enterobacteriaceae in large numbersin food indicates improper hygienic measures, fecal contamination, dirty equipment or unhygienic handling (PHLS 2002; Gill and Landers 2004).
The effect of marination on the mean of enterobacteriaceaecounts for groups I, II, III, IV and V were 6.7x102 +1.5x102, 5x102 + 1.2x102, 4x102+5.1x102, 2.9x102+ 6.3x101 and 2.2x102+4.9x101 respectively (table 6).
| Group | Minimum | Maximum | Mean | + S. E. | R. P.with group I | R. P.with group II |
| I | 8x101 | 2.6x103 | a6.7x102 | 1.5x102 | - | - |
| II | 6.8x101 | 2.1x103 | ab5x102 | 1.2x102 | - | - |
| III | 5.3x101 | 1.8x103 | ab4x102 | 5.1x101 | 39% | 19% |
| IV | 3x101 | 1.1x103 | ab2.9x102 | 6.3x101 | 57% | 43% |
| V | 1.5x101 | 9.1x102 | b2.2x102 | 4.9x101 | 67% | 57% |
Table 6: Effect marination on total Enterobacteriaceae count (cfu/g) of beef samples.
Group I= Control, Group II = Marination Only, Group III = 1% B.P.E., Group IV = 3% B.P.E., Group V = 5% B.P.E.
Group I= Control, Group II = Marination Only, Group III = 1% B.P.E., Group IV = 3% B.P.E., Group V = 5% B.P.E.
Group I was the highest total enterobacteriaceae counts followed by group II, III, IV then V. There was a significant difference (P < 0.05)in means of total enterobacteriaceae counts between group I and group V, addition of banana peels extract to beef samples groups (III, IV and V) lead to decrease the number of enterobacteriaceae counts. The extract of banana peels have antibacterial effect against several members of enterobacteriaceae family as recorded by Ehiowemwenguan., et al. (2014) and El-Zawawy (2015).Increasing the concentration of banana peels extract leading to decrease in enterobacteriaceae counts.
The mean of total enterobacteriaceae counts of group I (control, raw beef without marination) was lower than this reported by Raji (2006); Crowley., et al. (2005) and Hemmat., et al. (2013). It is may due to proper hygienic measures followed in abattoir and handling of beef leading to decrease fecal contamination so the enterobacteriaceae counts also decrease. Enterobacteriaceaeis an indicator for fecal contamination of the carcass (Murray., et al. 2001; Schaffner and Smith 2004; Zweifel., et al. 2005; Paterson 2006 and Paulsen, 2011).
Staphylococcus aureus
In this study table 7showed the effect of marination on total S. aureus count of the examined beef samples. The mean for groups I, II, III, IV and V were 4.9x101 + 0.18x101, 4x101 + 0.19x101, 3.1x101 + 0.18x101, 2.3x101 + 0.17x101 and 1.9x101 + 0.16x101 respectively. There was a significant difference (P < 0.05) in means of total S. aureus counts between all groups except between 3% and 5% banana peels extract. Existence of food-borne pathogens as S. aureus in beef samples is agreed with the recorded result bySharma and Chattopadhyay (2015).
In this study table 7showed the effect of marination on total S. aureus count of the examined beef samples. The mean for groups I, II, III, IV and V were 4.9x101 + 0.18x101, 4x101 + 0.19x101, 3.1x101 + 0.18x101, 2.3x101 + 0.17x101 and 1.9x101 + 0.16x101 respectively. There was a significant difference (P < 0.05) in means of total S. aureus counts between all groups except between 3% and 5% banana peels extract. Existence of food-borne pathogens as S. aureus in beef samples is agreed with the recorded result bySharma and Chattopadhyay (2015).
| Group | Minimum | Maximum | Mean | + S. E. |
| I | 3.5x101 | 6.0x101 | a4.9x101 | 0.18x101 |
| II | 2.9x101 | 5.0x101 | b4.0x101 | 0.19x101 |
| III | 2.0x101 | 4.1x101 | c3.1x101 | 0.18 x101 |
| IV | 1.2x101 | 3.2x101 | d2.3x101 | 0.17 x101 |
| V | 1.0x101 | 2.8x101 | de1.9x101 | 0.16 x101 |
Table 7: Effect of marination on total Staphylococcus aureus count (cfu/g) of beef samples.
Group I= Control, Group II = Marination Only, Group III = 1% B.P.E., Group IV = 3% B.P.E., Group V = 5% B.P.E. Mean values with different litter are significantly difference (P < 0.05). , *S. E. standard error **R. P. with group I = Reduction Percent based on result of group I, ***R. P. with group II = Reduction Percent based on result of group II., B.P.E.=Banana Peels Extract.
Group I= Control, Group II = Marination Only, Group III = 1% B.P.E., Group IV = 3% B.P.E., Group V = 5% B.P.E. Mean values with different litter are significantly difference (P < 0.05). , *S. E. standard error **R. P. with group I = Reduction Percent based on result of group I, ***R. P. with group II = Reduction Percent based on result of group II., B.P.E.=Banana Peels Extract.
The means of total S. aureus counts of group I in this study is lower than this reported by Hemmat., et al. (2013). The highest number of S. aureus in beef indicates the presence of cross-contamination, which usually related to human skin, hair, discharge, hand and improper personal hygiene of employees during handling and processing (CSA, 2011).
This result confirmed that banana peels extract had significant antibacterial activity against S. aureus, this agree withRojas., et al. (2006); Fagbemi., et al. (2009); Ighodaroro., et al. (2009) and Sumathy and Sumathy (2011).
Conclusion
From the achieved results in the present study, it can be concluded that: The marination of beef for a period of time not less than four hours with salt, onion and tomato improve the sensory quality beef and possess antibacterial activity against aerobic colonies, enterobacteriaceae and S. aureus. Application of Egyptian banana (Musa acuminate) peels extract in beef marination with concentrations 1%, 3% and 5% exhibited a variable antibacterial effect against aerobic colonies, enterobacteriaceae and S. aureus.
References
- Aldean, A.A. Al-Jumaily, E.F. and Al-Safar, M.A. (2010). The Effect of Banana Skin on the Bacterial Infections of the Chronic Gingivitis Patients. African Journal of Paediatric Surgery 7(1). 145-149.
- APHA (2002). Compendium of Methods for the Microbiological Examination of Foods. 4th Ed., APHA Technical Committee on Microbiological Methods for Foods. Washington DC, USA.
- Atzingen, D.A. Gragnani, A. Veiga, D.F. Abla, L.E. Mendonça, A.R. Paula, C.A. Juliano, Y. Correa, J.C. Faria, M.R. and Ferreira, L.M. (2011). Gel from unripe Musa sapientum peel to repair surgical wounds in rats. Acta Cirurgica Brasileira 26(5). 379–382.
- Beuchat, L.R. and Golden, D.A. (1989) . Antimicrobials occurring naturally in foods. Food Technology. 43(1) . 134 -142.
- Buchanan, L.R. and Halbrook, B. (1995). Data needed to develop Microbial Food Safety Systems for Slaughter, Processing, and Distribution. An economic Research Service report. Tracking Foodborne Pathogens from Farm to Table. United States Department of Agriculture Conference Proceedings January 9-10, 1995. Washington, DC, USA . 71-80.
- Cerveny, J. Meyer, J.D. and Hall, P.A. (2009) . Microbiological Spoilage of Meat and Poultry Products In . Compendium of The Microbiological Spoilage of Foods and Beverages. Food Microbiology and Food Safety, W.H. Sperber and M.P. Doyle (Eds.). Springer Science and Business Media, NY, 69-868.
- Chabuck, G.Z. Al-Charrakh, H.A. Hindi, K.N. and Hindi, K.S. (2013) . Antimicrobial Effect of Aqueous Banana Peel Extract, Iraq. Pharmaceutical Sciences 1. 73-75.
- Cohen, N. Ennaji, H. Bouchrif, B. Hassar, M. and Karib, H. (2007). Comparative study of microbiological quality of raw poultry meat at various seasons and for different slaughtering processes in Casablanca (Morocco). The Journal of Applied Poultry Research 16(4) . 502–508.
- Crowley, H. Cagney, C. Sheridan, J.J. Anderson, W. McDowell, D.A. Blair, I.S. Bishop, R.H. and Duffy, G. (2005). Enterobacteriaceae in beef products from retail outlets in the Republic of Ireland and comparison of the presence and counts of E. coli O157 .H7 in these products. Food Microbiology 22(5). 409–414.
- CSA (Central Statistical Authority of Ethiopia) (2011). The 2010 Population and Housing Census of Ethiopia. 2011.
- Cutler, H.G. (1995). Natural product flavor compounds as potential antimicrobials, Insecticides, and medicinal. Agro-Food-Industry Hi - Tech 6. 19–2.
- Ehiowemwenguan, G. Emoghene, A.O. and Inetianbor, J.E. (2014). Antibacterial and phytochemical analysis of Banana fruitpeel. IOSR J of Pharmacy 4(8). 18-25.
- El-Zawawy A.N. (2015). Antioxidant, Antitumor, Antimicrobial Studies and Quantitative Phytochemical Estimation of Ethanolic Extracts of Selected Fruit Peels. International Journal of Current Microbiology and Applied Sciences 4(5). 298-309.
- Fagbemi Josephine, F. Ugoji Esther Adenipekun Tayo Adelowotan and Omotoyin (2009). Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), lemon grass (Cymbopogoncitratus S.) and turmeric (Curcuma longa L.) on pathogens. African Journal of Biotechnology 8(7). 1176-1182.
- Gill, C.O. and Landers, C. (2004). Proximate sources of bacteria on boneless loins prepared from routinely processed and detained carcasses at a pork packing plant. International Journal of Food Microbiology 97(2). 171- 178.
- Girish, H.V. and Satish, S. (2008). Antibacterial activity of important medicinal plants on human pathogenic bacteria – a comparative analysis. World Application of Science J 5(3). 267-271.
- González-Montelongo, R.M. Lobo, G. and González, M. (2010) . Antioxidant activity in banana peel extracts . Testing extraction conditions and relatedbioactive compounds. Food Chemistry 119 .1030-1039.
- Hemmat, M.I. Amin, R. Saleh, A.O. and El-Shafay, M.S. (2013) . Quality of beef and edible offal at abattoir level. Benha Veterinary Medical JOURNAL 25(2). 254?263.
- Heinz, G. and Hautzinger, P. (2007). Meat Processing Technology . For Small-to Medium-Scale Producers. RAP Publication, ISBN . 978-974-7946-99-4.
- Holds, G. Pointon, A. Lorimer, M. Kiermeier, A. Raven, G. and Sumner, J. (2007). Microbial profiles of carcasses and minced meat from Kangaroos processed in South Australia. International Journal of Food Microbiology 123(1-2). 88-92.
- Hutchison, M.L. Walters, L.D. Avery, S.M. Reid, C.A. Wilson, D. and Howell, M. (2005). A comparison of wet–dry swabbing and excision sampling methods for microbiological testing of bovine, porcine, and ovine carcasses at red meat slaughterhouses. Journal of Food Protection 68(10). 2155–2162.
- Ighodaroro, O.M. Mairiga, J.P. and Adeyi, A.O. (2009). Reducing and Antiproxidant profiles of flavonoids in Ocimumgratissimum. International Journal of Chemical Science 2 85-9.
- ISO International Organisation for standardization (1999) . International Organisation for standardization. No.6888-1. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coagulase positive staphylococci.
- ISO International Organisation for standardization (2002a). International Organisation for standardization. No.4833. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of microorganisms. Colony count technique at 30ºC.
- ISO International Organisation for standardization (2002b). International Organization for standardization. No.6579. Microbiology of food and animal feeding stuffs – Horizontal method for the detection of Salmonella spp.
- ISO International Organisation for standardization 21528-2 .2004) . Microbiology of food and animal feeding stuffs – Horizontal methods for the detection an enumeration of Enterobacteriaceae – Part 2 . Colony-count method.
- Istrati, D. Constantin, O. Ionescu, A. Vizireanu, C. and Dinic?, R. (2011) . Study of the combined effect of spices and marination on beef meat vacuum packaged. The Annals of the University Dunarea de Jos of Galati. Fascicle VI – Food Technology 35(2). 75-85.
- Jay, J.M. Loessner,M.J. and Golden,D.A.(2005). Modern Food Microbiology, 7th Edn., Springer Science and Business Media. NY 63-101. ISBN . 0387231803
- Kapadia, P.S. Pushpa, S. Pudakalkatti and Shivanaikar, S. (2015). Detection of antimicrobial activity of banana peel (Musa paradisiacaL.) on Porphyromonasgingivalis and Aggregatibacter actinomycetemcomitans. An in vitro study. Contemporary Clinical Dentistry 6(4). 496–499.
- Kargiotou, C. Katsanidis, E. Rhoades, J. Kontominas, M. and Koutsoumanis, K. (2011). Efficacies of soy sauce and wine base marinades for controlling spoilage of raw beef. Food Microbiology 28(1). 158-163.
- Knöchel, S. Mach, N. and Karlsson, A. (2007). Microbial and physical changes in marinated beef of high and normal pH during storage in different atmospheres. In. 53rd International Congress of Meat Science and Technology 1. 37-38.
- Kumar, S. Bhowmik, D. Duraivel, S. and Umadevi, M. (2012). Traditional and Medicinal Uses of Banana. Journal of Pharmacognosy and Phytochemistry 1(3). 51–63.
- Libby, J.A. (1975) . Meat hygiene.4th Ed., LFA and Febiger, Philadelphia.
- McEvoy, J.M. Sheridan, J.J. Blair, I.S. and McDowell, D.A. (2004). Microbial contamination on beef in relation to hygiene assessment based on criteria used in EU Decision 2001/471/EC. International Journal of Food Microbiology 92(2). 217–225.
- Mokbel, M.S. and Hashinaga, F. (2005). Antibacterial and Antioxidant Activities of Banana (Musa, AAA cv. Cavendish) Fruits Peel. American Journal of Biochemistry and Biotechnology 1(3). 125-131.
- Morais, D.R. Rotta, E.M. Sargi, S.C. Schmidt, E.M. Bonafe, E.G. Eberlin, M.N. and Visentainer, J.V. (2015). Antioxidant activity, phenolics and UPLC-ESI-MS of extractsfrom different tropical fruits parts and processed peels. Food Research International Journal. 77(3). 392–399.
- Murray, K.A. Gilmour, A. and Madden, R.H. (2001). Microbiological quality of chilled beef carcasses in Northern Ireland . a baseline study. Journal of Food Protection 64(4). 498–502.
- Narasimha RAO, D., NAIR, K.K.S. and SAKHARE, P.Z. (1998). Meat microbiology and spoilage in tropical countries. The Microbiology of Meat and Poultry (A. Davies and R. Board, eds.) 220-265, Blackie Academic & Professional, London.
- Nouichi, S. and Hamdi, T.M. (2009). Superficial Bacterial Contamination of Ovine and Bovine Carcasses at El-Harrach Slaughterhouse (Algeria).European J. of Scientific Research 38(3). 474-485.
- Palang, Y.E. (2004). The role of ingredients and processing conditions on marinade penetration, retention and color defects in cooked marinated chicken breast meat. J. of Food Science 1-147.
- Paterson, D.L. (2006). Resistance in gram-negative bacteria . Enterobacteriaceae. The American Journal of Medicine 119 (6 Suppl 1). 520-528.
- Pathania, A. Mckee, S.R. Bilgili, S.F. and Singh,M. (2010). Antimicrobial activity of commercial marinades against multiple strains of Salmonella spp. Int. J. Food Microbiol 139(3). 214- 217.
- Paulsen, P. (2011). Hygiene and microbiology of meat from wild game . An Austrian view. In Game Meat ... P. Paulsen, A. Bauer and M. Vodnansky, 19–37. Wageningen . Wageningen Academic Publishers. Paulsen, P., J.
- Pereira, A. and Maraschin, M. (2015). Banana (Musa spp) from peel to pulp . Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. of Ethnopharmacolocy 160. 149–163.
- PHLS (2002). Standard Operating Procedure—Enumeration of Enterobacteriaceae by the Colony Count Technique, F23. P. 4 Technical Service Division, PHLS HQ.
- Rahkio, T.M. and Korkeala, H.J. (1997). Airborne bacteria and carcass contamination in slaughterhouses. Journal of Food Protection 60(1). 38-42.
- Raji, A.I. (2006). Bacteriological Quality of Dried Sliced Beef (Kilishi) Sold In Ilorin Metropolis. J. of Applied Sciences and Environmental Management 10(1). 97-100.
- Roberts, T.A. Hudson, W.R. Whelehan, D.P. Simonsen, B. Olygaard, K. Labots, H. Snijders, J.A. van Hoof, J. Debevere, J. Dempster, J.F. Devereux, J. Leistner, L. Gehra, H. Gledel, J. and Fournaud, J. (1984). Number and distribution of bacteria on some beef carcasses at selected abattoirs in some member states of the European communities. Meat Science 11(3). 191-205.
- Rojas, J. Ochoa, J. and Monoz, J. (2006). Screening for antimicrobial activity of ten medicinal plants used in colombian folkloric medicine . a possible alternative in treatment of non-nosocomial infection. B.M.C. Alternative Medicine 2006 6(2).
- Schaffner, W. and Smith, S. (2004). Indicator organisms, pp. 773-773. In Encyclopaedia of Meat Sciences, Elsevier, Academic Press, Amsterdam.
- Shahid, A. Siddique, M. Reham, S. Hameed, S. and Hussain, A. (2007). Evaluation of a microbiological growth inhibition assay as a screening test for the presence of antibiotic residues in poultry meat. Amer. J. Food Technol. 2(5). 457-461.
- Shan, B. Cai, Z. Sun, M. and Corke, H. (2005). Antioxidant capacity of 26 spice extracts and Charactization of their phenolic constituents. J. of Agri. and Food Chem. 53(20). 7749 -7759.
- Sharma, K.P. and Chattopadhyay, U.K. (2015). Assessment of Microbial load of raw meat Samples sold in the Open Markets of city of Kolkata. IOSR J. of Agriculture and Veterinary Science 8(3). 24-27.
- Sheard, R. and Tali, A. (2004). Injection of salt, tripolyphosphate and bicarbonate marinade solutions to improve the yield and tenderness of cooked pork loin. Meat Sci. 68(2). 305-311.
- Shrinjar, M.M. and Nemet, N.T. (2009). Antimicrobial effects of spices and herbs essential oils. Biblid . 1450-7188, 40, 195-209.
- Singh, Ch. Kathiresan, K. Boopathy, N. Anandhan, S. and Govindan, T. (2013). Evaluation of microbial potential of different colored banana peels. Int. J. of Preclinical and Pharmaceutical Research 4(2). 62-64.
- Smid, J. and Gorris , M. (1999). Natural antimicrobials for food preservation. In . Rahman, M.S. (Ed), Handbook of food preservation. Marcel Dekker, New york. 285-308.
- Sumathy, N.H. and Sumathy, J.H. (2011). Antibacterial and Antifungal Activity of Musa Fruit Peels against Skin and Gastrointestinal Tract Diseases. Herbal Tech Industry. Short communication 2011 9-11.
- Sumner, J. Petrenas, E. Dean, P. Dowsett, P. West G. RinieWiering, R. and Raven G. (2003). Microbial contamination on beef and sheep carcasses in South Australia. Int. J. of Food Microbiology 81(3). 255–260.
- Szczesniak, S.A. (1987). Correlating sensory with instrumental texture measurements—an overview of recent developments 1. J. of Texture Studies 18(1). 1-15.
- Upadhyaya, M. Poosaran, N. and Fries, R. (2012). Prevalence and predictors of Salmonella spp. in retail meat shops in Kathmandu. J. Agri. Sci. Technol. 2. 1094-1106.
- Velasco, V. and Williams, P. (2011). Improving meat quality through natural antioxidants. Chilean J. of Agricultural Research 71(2). 313-322.
- Wyness, L. (2015). The future of animal products in the human diet . health and environmental concerns. Nutrition Society 75. 227–232
- Xargayó, M., J. Lagares, E. Fernandez, D. Ruiz, and D. Borrell. (2001). Fresh meat spray marinating . The influence of spray injection on the quality of marinated products.
- Zweifel, C. Baltzer, R. and Stephan, R. (2005). Microbiological contamination of cattle and pig carcasses at five abattoirs determined by swab sampling in accordance with EU. Decision 2001/471/EC. Meat Science 69(3). 559-566.
- Zweifel, C. Fischer, R. and Stephan, R. (2008). Microbiological contamination of pig and cattle carcasses in different small-scale Swiss abattoir. Meat Science 78(3). 225-231.
Citation: Ali Meawad Ahmed Taghreed Ahmed Hafez and Mona Mohammed Eissawy. (2018). Effect of Banana Peel Extract on Sensory and Bacteriological Quality of Marinated Beef. Archives of Nutrition and Public Health 1(1).
Copyright: © 2018 Ali Meawad Ahmed Taghreed Ahmed Hafez and Mona Mohammed Eissawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.