Mini Review
Volume 1 Issue 1 - 2019
Diabetes- The Sacchariferous Epidemic
A.B. Diagnostics A-1, Rajouri Garden, New Delhi 110027 India
*Corresponding Author: Anubha Bajaj, A.B. Diagnostics A-1, Rajouri Garden, New Delhi 110027 India.
Received: September 29, 2019; Published: December 06, 2019
DefinitionDiabetes is defined as a chronic disorder where the individual ability to produce and respond to the hormone insulin is impaired with a consequent anomalous metabolism of carbohydrates and enhanced blood glucose levels.
Categorization Contemporary and traditional classification of diabetes is 1) Type 1 diabetes which occurs due to autoimmune mediated destruction of beta cells resulting in an absolute deficiency of insulin. 2) Type 2 diabetes which ensues on account of progressive decimation of insulin secretion from beta cells with a concurrence of insulin resistance. 3) Gestational diabetes mellitus which is commonly discerned in second or third trimester of pregnancy in an absence of overt diabetes prior to gestation. 4) Diabetes appearing secondary to specific factors such as the monogenic diabetes syndrome with neonatal diabetes, maturity onset diabetes of the young (MODY), disorders of exocrine pancreas as cogitated in cystic fibrosis, pancreatitis and diabetes induced with drugs or chemicals such as glucocorticoids, organ transplantation and therapy for autoimmune deficiency syndrome [1,2].
Characteristics Type 1 and 2 variants of diabetes are contemplated as heterogeneous conditions with a variable clinical presentation and disease progression. Type 1 diabetes appearing in children can manifest polyuria and/or polydipsia. An estimated one third subjects can initially represent diabetic ketoacidosis. Type 1 and type 2 diabetes can demonstrate diverse genetic and environmental factors which induce a progressive decimation of beta cell mass and/or function with an ensuing clinical manifestation of hyperglycaemia. Implicated individuals can depict a possible emergence of identical, chronic complications with variable rate of disease progression [1,2].
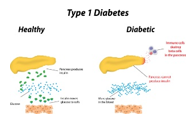
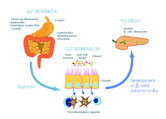
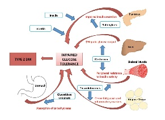
Pathogenesis Type 1 diabetes can display persistence of diverse and concurrent autoantibodies, the presence of which is almost indicative of discernible hyperglycaemia and evolving diabetes. Proportionate disease progression is contingent to several factors such as age at initial detection of antibodies, quantification and specificity of antibodies and particular antibody titre. Blood glucose and haemoglobin A1c levels are typically elevated markedly prior to the clinical onset of diabetes. Thus, detection and categorization of diabetes is feasible and recommended prior to the onset of diabetic ketoacidosis [2,3]. Methodology of beta cell demise and dysfunction are inadequately characterized in type 2 diabetes. However, insufficient insulin secretion from beta cells which is accompanied by frequent and concomitant insulin resistance is cogitated. Type 2 diabetes is associated with defective insulin secretion with concordant inflammation, metabolic stress and incriminating genetic factors [3,4].
Investigations Generally, the fasting plasma glucose (FPG), 2 hr plasma glucose (PG) , oral administration of 75 grams anhydrous glucose for glucose tolerance test (GTT) or an haemoglobin A1c are considered as appropriate for evaluating diabetes. Diagnostic confirmation is unnecessary in specific instances of hyperglycaemic crisis, with the appearance of classic symptoms of hyperglycaemia or a random plasma glucose exceeding > 200mg/dl. Apart from aforesaid special circumstances, a confirmatory second investigation is required. Preferably the initially approbated test should be repeated or a different investigation can be performed in the same time frame with a fresh blood sample for confirmation. Additionally if two different investigative results exceed the designated threshold, the findings are also considered confirmatory of diabetes. Diagnosis of diabetes is contingent to the selected confirmatory test [3,4].
Determination For subjects depicting classic symptoms of diabetes a simple measurement of plasma glucose is deemed satisfactory to diagnose diabetes. Nevertheless, appearance of classic symptoms of hyperglycaemia or a hyperglycaemic crisis or a random plasma glucose beyond > 200mg/dl is considered diagnostic of diabetes and requires no further investigation [1].
Immune mediated variants Insulin dependent diabetes or juvenile onset diabetes arise in an estimated 5% to10% subjects and is commonly due to emergence of cell mediated autoimmunity with consequent decimation of pancreatic beta cells. Biomarkers for assessing autoimmune activity can be employed such as islet cell autoantibodies, autoantibodies to GAD (GAD65), insulin, tyrosine phosphatase IA-2 and IA-2β and ZnT8. Type 1 diabetes typically demonstrates one or more of aforesaid autoimmune markers. Type I diabetes also depicts intense association of histocompatibility antigens (HLA) with cogent linkage to DQA and DQB genes. Aforementioned HLA-DR/DQ genetic alleles can be predisposing or protective alleles. Proportion of decimation of beta cells is variable [4,5]. Immune mediated diabetes is common in childhood and adolescence although can emerge within the 8th or 9th decade. Children and adolescents can display an initial manifestation of diabetic ketoacidosis. Multiple genetic predispositions and concurrent environmental factors are cogent in inducing autoimmune decimation of beta cells. Immune mediated diabetes can be associated with adjunctive immune disorders such as Hashimoto’s thyroiditis, Graves’s disease, Addison’s disease, vitiligo, celiac disease, autoimmune hepatitis, myasthenia gravis and pernicious anaemia [5,6].
Monogenic diabetes syndrome Monogenic dysfunctions can engender aberrant beta cell function as elucidated with neonatal diabetes and maturity-onset diabetes of the young (MODY). Aforesaid diabetes syndrome account for an estimated <5 % instances of diabetes.
Neonatal Diabetes Instances of diabetes occurring below < 6 months of age are nomenclated as congenital or neonatal diabetes. Majority (80% to 85%) of subjects demonstrate an underlying monogenic factor of disease emergence. Neonatal diabetes is an exceptional occurrence beyond 6 months of age. Paradoxically, auto immune mediated type 1 diabetes is infrequent prior to 6 months of age [1]. Neonatal diabetes can manifest as a transient or permanent disorder. Transient variant of diabetes arises on account of genetic overexpression of chromosome 6q24, is reoccurring in around 50% instances and can be managed with alternative agents rather than insulin administration. Permanent variant of neonatal diabetes with an autosomal dominant pattern of inheritance occurs due to genomic mutations encoding Kir6.2 subunit (KCNJ11) and SUR1 subunit (ABCC8) of the β-cell K(atp) channel. Mutation of insulin gene also frequently induce permanent neonatal diabetes [1].
Maturity-onset diabetes of the young (MODY) Hyperglycaemia characteristically initiated at an early age of below <25 years is the hall mark of MODY. An autosomal dominant mode of disease inheritance is cogitated accompanied by genetic anomalies within a minimal of thirteen genes emerging on various chromosomes. MODY typically demonstrates impaired insulin secretion with minimal or absent deficiency of insulin function (with an absence of concurrent obesity). Frequently cogitated variants include GCK-MODY (MODY2), HNF1A-MODY(MODY3) and HNF4A-MODY (MODY1) (1). MODY can be contemplated as a diagnosis in individuals with atypical diabetes or the implication of multiple family members with diabetes depicting non-characteristic features of type1 or type 2 diabetes. Genetic assay for MODY can be decided by assessing biomarkers such as urinary C-peptide/creatinine ratio and antibody screening. An accurate diagnosis of monogenic diabetes is crucial to obtain in order to prevent the administration of suboptimal or harmful therapeutic agents and delay in diagnoses within family members [5,6]. Monogenic diabetes should be contemplated in subjects with a) Diabetes appearing within first 6 months of life and demonstrating INS or ABCC8 mutations. b) Diabetes devoid of typical features of type 1 or 2 variants such as non-reactivity for diabetes associated autoantibodies, non-obese persons, absence of adjunctive metabolic factors or particularly strong family history of diabetes. d) Stable, mild fasting hyperglycaemia betwixt 100 mg/dl to 150 mg/dl and a consistent haemoglobin A1c betwixt 5.6% to 7.6%, especially when detected in non-obese persons. MODY as a diagnosis remains elusive and difficult to define. Detection of autoantibodies for type 1 diabetes precludes additional investigation for monogenic diabetes [1].
| •FPG >126mg/dl or 7.0 mmol/L. Fasting is defined as no caloric intake for at least 8 hours. OR • 2 hour PG ≥ 200mg/dl(11.1 mmol/L) during OGTT as per WHO standards with 75g anhydrous glucose load OR •Haemoglobin A1c ≥ 6.5% OR •A patient in hyperglycaemic crisis, classic symptoms of hyperglycaemia, random plasma glucose of ≥ 200mg/dl (11.1 mmol/L) |
Table A: Criterion for diagnosis of diabetes [1].
|
Table B: Assessing Diabetes and Prediabetes in Asymptomatic Adults [1].
| Criterion •Overweight (> 85% percentile for age and sex, weight for height and >120% of ideal weight for height (A). Additional risk factors • Maternal history of diabetes or GDM during gestation (A) •Family history of type 2 diabetes mellitus in first or second degree relative (A) • Race/ Ethnicity (A) • Signs of insulin resistance or conditions associated with insulin resistance (acanthosis nigricans, hypertension, dyslipidaemia, polycystic ovary syndrome and small for gestational age birth weight (B). |
Table C: Risk assessment of Type 2 Diabetes or Prediabetes in Asymptomatic Children/Adolescents ( < 18 years) [1].
References
- American Diabetes Association. (2018). “Classification and Diagnosis of Diabetes- Standards of Medical Care in Diabetes - 2018”. Diabetes. 41(Suppl 1): S13-S27.
- Skyler JS, Bakris GL., et al. (2017). “Differentiation of diabetes by pathophysiology, natural history and prognosis”. Diabetes. 66: 241-255.
- Insel RA, Dunne JA., et al. (2015). “Staging pre-symptomatic type 1 diabetes: a scientific statement of JDRF, Endocrine Society and American Diabetes Association”. Diabetes care. 38: 1964-1974.
- Bergenstal RM, Gal RL., et al. (2017). “T1D exchange racial differences study group; racial differences in the relationship of glucose concentration and haemoglobin Aic”. Ann Intern Med. 167: 95-102.
- Welsh KJ, Kirkman MS., et al. (2016). “Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions”. Diabetes care. 29: 1299-1306.
- Naylor RN, John PM., et al. (2014). “Cost-effectiveness of MODY genetic testing ; translating genomic advances into practical health applications”. Diabetes Care. 37: 202- 209.
- Image1 Courtesy : American Diabetes Association 8)Image 2 Courtesy : Wikidoc.com 9)Image 3 Courtesy : Research Gate 10)Image 4 Courtesy : Paediatrics Getaway.
Citation: Anubha Bajaj. (2019). Diabetes- The Sacchariferous Epidemic. Journal of Biotechnology and Immunology 1(1).
Copyright: © 2019 Anubha Bajaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.