Research Article
Volume 2 Issue 2 - 2020
Biochemical and Histopathological Studies on the role of Selenium Nanoparticles and Selenium Oxide against Chemically-Induced Diabetes in Male Rats.
1,5Zoology Department, Faculty of Science, Mansoura University.Mansoura.35516. Egypt
2Zoology Department, Faculty of Science, Kaferelshikh University. Kaferelshikh .33516.Egypt.
3Chemistry Department, Faculty of Science, Kaferelshikh University. Kaferelshikh .33516.Egypt.
4Dairly science Department, Faculty of agriculture, Kaferelshikh University. Kaferelshikh .33516. Egypt.
2Zoology Department, Faculty of Science, Kaferelshikh University. Kaferelshikh .33516.Egypt.
3Chemistry Department, Faculty of Science, Kaferelshikh University. Kaferelshikh .33516.Egypt.
4Dairly science Department, Faculty of agriculture, Kaferelshikh University. Kaferelshikh .33516. Egypt.
*Corresponding Author: Maher Amer Ali Amer, Zoology Department, Faculty of Science, Mansoura University.Mansoura.35516. Egypt.
Received: June 29, 2020; Published: July 14, 2020
Abstract
The study was designed to evaluate the effect of the biologically produced selenium nanoparticles (SeNPs) against streptozotocin (STZ) induced diabetic using adult male rats. Sixty mg/kg of STZ was injected in rats to induce diabetes. Animals either treated with SeNPs, selenium oxide (SeO2) or a mixture of SeNPs and SeO2 at a dose level of 0.1 mg Se/kg body weight/day. The results demonstrated that selenium or its nano form has antioxidant and catalytic effect and both were able to prevent the histological injury in pancreatic and liver tissues of rats. This study suggests that Se and SeNPs can ameliorate cellular damage resulting in diabetes and has a protective effect to minimize the risk of diabetic complications which may be attributed to its free radical scavenging effect.
Keywords: Streptozotocinl; Diabetes; Selenium, Selenium nanoparticles; Oxidative stress; Liver toxicity; Antioxidants
Introduction
Diabetes mellitus (DM) is an endocrine metabolic disorder (1). Antioxidants have important roles in biological systems by scavenging free radicals which may lead to oxidative damage of biological molecules such as proteins, lipids, and DNA. DM is increasing in the world as a result of population aging urbanization and obesity, contributing to high mortality and morbidity rates. The study of physiological methods of diabetes becomes important for the emergence of novel therapeutic procedures. DM is a multifactorial disease that associated with many pathological alterations that affect almost every part of the body. Oxidative stress increase is an important contributor in the progression and development of diabetes and its problems. Diabetes commonly happens with production of free radicals or impaired antioxidant (2).
Liver is the key organ involved in detoxifying of free radicals and oxidative stress that occurs in diabetes early stages of. So, means to decrease the oxidative stress formation are great important in the treatment of DM (3). In diabetes, glucose is prone to oxidation resulting in generation of hydrogen peroxide and hydroxyl radical that consider the most reactive and toxic form of free radicals (3). DM associated with the production of reactive oxygen species (ROS) and oxidative stress in presence of hyperglycemia (4). DM reduces ability of tissue to utilize carbohydrates, leading to disturbance in the metabolism of fat and protein (5). Hyperglycaemia is a high amount of glucose circulates in the blood. Hyperglycaemia leads to many long-term complications in the eyes, kidneys, nerves, heart, and blood vessels (6).
Streptozotocin (STZ) leads to deficiency of insulin which consider as a diabetogenic agent (7). Oxidative stress may occur as consequence of abnormal ratios in glucose and lipid metabolism which promote hyperglycemia and dyslipidemia leading to the development of atherosclerosis and cardiovascular complications in diabetic patient (8). Hyperglycemia in diabetes leads to oxidative stress, it has been suggested that the nutritional supplementation of antioxidants might reduce the oxidative stress and protect tissue from ROS damage (9). ROS are capable of reacting with unsaturated lipid and initiating chain reactions of lipid peroxidation in membranes (10), leading to serious pathogenesis such as myocardial infraction (11, 12).
Tumor necrosis factor alpha TNF-α has important role in insulin resistance and pathogenesis of type 2 diabetes (13). It also has important effects on whole body lipid and glucose metabolism (14, 15). Studies on TNF-α mediated insulin resistance in cultured cells as well as in whole organisms have also demonstrated that TNF-α induces insulin resistance, at least in part, through its ability to inhibit intracellular signaling from the insulin receptor (16, 17). Insulin resistance and type 2 diabetes often occur along with other metabolic abnormalities like obesity (18) and hypertension (19).
Later discoveries propose that disabled antioxidant status is included in oxidative stretch related with diabetes (20). Glutathione (GSH) has important functions as a direct free-radical scavenger, co-substrate for glutathione peroxidase activity, co-factor for many enzymes and forms conjugate in endo- and xenobiotic reactions (21). Also, SOD is an antioxidant enzyme that can catalyze the conversion of two superoxides into H2O2 and oxygen. It plays a major role in the defense system against the cytotoxic effects of superoxide radicals (22). β-cells were particularly sensitive to reactive oxygen species due to their low free-radical quenching (antioxidant) enzymes like catalase, glutathione peroxidase, and superoxide dismutase (23, 24).
Selenium is one of the essential trace elements for human and animal’s health. It has functions in balancing of the redox system; improve functions of immune system and acts as anticarcinogenic (25, 26). Selenium has a powerful antioxidant which effect in dysfunctions happened in diabetes (27). Tremendous number of studies was focused on the impact of Se on DM in human and animal models. There is a clear link between certain selenoproteins and glucose metabolism or insulin resistance. The relationship between selenium and type 2 diabetes is undoubtedly complex with possible harmful effect occurring both below and above the physiological range for optimal activity of some or all selenoproteins (28, 29, 30, 31). The aim of the present study is to explore the effect of 2 forms of selenium; selenium oxide and selenium nanoparticles (SeNPs) against streptozotocin (STZ) induced diabetic using adult male rats.
Material and Methods
Chemicals
Selenium (IV) (sodium selenite), selenium oxide and streptozotocin (STZ) were purchased from sigma chemical CO. (Sigma-Aldrich chemie GmbH. Germany).
Selenium (IV) (sodium selenite), selenium oxide and streptozotocin (STZ) were purchased from sigma chemical CO. (Sigma-Aldrich chemie GmbH. Germany).
Green synthesis and recovery of selenium nanoparticles (SeNPs)
Pure SeNPs were prepared using 100 ppm of filter sterilized (Sartorius AG, Germany) sodium selenite, Na2SeO3. 5H2O (Sigma-Aldrich, Switzerland) and pure mixed culture (1:1) of Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) (NCAIM B 02206) and Streptococcus thermophilus (S. thermophilus) (CNCM I-1670) obtained from the National Collection of Agricultural and Industrial Microorganisms, Budapest, Hungary according to the method described by Prokisch and Zommara (32).
Pure SeNPs were prepared using 100 ppm of filter sterilized (Sartorius AG, Germany) sodium selenite, Na2SeO3. 5H2O (Sigma-Aldrich, Switzerland) and pure mixed culture (1:1) of Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) (NCAIM B 02206) and Streptococcus thermophilus (S. thermophilus) (CNCM I-1670) obtained from the National Collection of Agricultural and Industrial Microorganisms, Budapest, Hungary according to the method described by Prokisch and Zommara (32).
Selenium determination
Selenium determination was carried out in 1 g of dry samples as previously described (33). The selenium concentration in the stock SeNPs solution was 320 mg/l with a diameter size of 50-200 nm.
Selenium determination was carried out in 1 g of dry samples as previously described (33). The selenium concentration in the stock SeNPs solution was 320 mg/l with a diameter size of 50-200 nm.
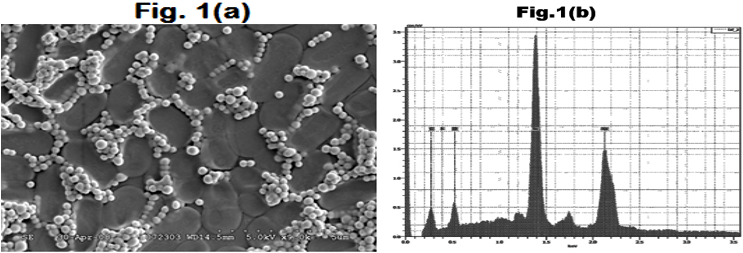
Figure 1a: Scanning electron micrograph of the biologically produced SeNPs
Figure 1b: Plot showing the x-ray fluorescent spectrum of SeNPs.
Figure 1b: Plot showing the x-ray fluorescent spectrum of SeNPs.
Experimental design
Male rats weighing 150-220g were obtained from the animal maintenance unit, Faculty of Science, Kaferelshikh University and acclimated to the laboratory conditions for three weeks. They were maintained at 25ºC and 12 hours light/12 hour dark cycle. The rats were raised on standard commercial rat chow and deionized water ad libitum. The experiment was conducted according to the protocol approved by local ethical committee for in vivo animal experiment. The animals were divided randomly to seven groups with 5 rats in each group and fed on the commercial rat chow for 3 weeks (experiment period). The 1st group had no treatment and served as a control. The 2nd and 3rd groups received a daily oral dose of selenium (SeO2) and SeNPs at a dose of 0.1 mg/kg body weight, respectively. The 4th group received a single dose by Intraperitoneal (IP) injection of 60 mg/kg body weight of STZ as described by Singhal et al. (34). The 5th and 6th groups received a single does by I.P injection of 60 mg/ kg body weight of STZ and received a daily oral dose (0.1 mg\kg body weight) of selenium (SeO2) or SeNPs, respectively. The 7th group received STZ followed by a daily oral dose (0.1 mg\kg body weight) of Selenium (SeO2) and SeNPs combination.
Male rats weighing 150-220g were obtained from the animal maintenance unit, Faculty of Science, Kaferelshikh University and acclimated to the laboratory conditions for three weeks. They were maintained at 25ºC and 12 hours light/12 hour dark cycle. The rats were raised on standard commercial rat chow and deionized water ad libitum. The experiment was conducted according to the protocol approved by local ethical committee for in vivo animal experiment. The animals were divided randomly to seven groups with 5 rats in each group and fed on the commercial rat chow for 3 weeks (experiment period). The 1st group had no treatment and served as a control. The 2nd and 3rd groups received a daily oral dose of selenium (SeO2) and SeNPs at a dose of 0.1 mg/kg body weight, respectively. The 4th group received a single dose by Intraperitoneal (IP) injection of 60 mg/kg body weight of STZ as described by Singhal et al. (34). The 5th and 6th groups received a single does by I.P injection of 60 mg/ kg body weight of STZ and received a daily oral dose (0.1 mg\kg body weight) of selenium (SeO2) or SeNPs, respectively. The 7th group received STZ followed by a daily oral dose (0.1 mg\kg body weight) of Selenium (SeO2) and SeNPs combination.
The diabetic state of rats was confirmed by determination of blood glucose level after seven days of the STZ treatment. At the end of experiment, over-night fasted rats were anaesthetized with 1% isoflurane and their blood was collected in heparin vacutainer's coated tubes, the sera were separated by centrifugation at 4000 rpm for 15 min and kept frozen for biochemical determination. Liver and pancreas were quickly removed, weighted and blotted perfused with cold saline to exclude the blood cells then, blotted on filter paper and stored at - 20ºC for subsequent biochemical analysis. A portion of liver and pancreas tissues were homogenized in ice cold 0.05 mM potassium phosphate buffer (pH 7.4), centrifuged at 6000 rpm for 15 minutes and the supernatant was collected and kept at - 20ºC for further analysis. A portion of liver and pancreas was taken and stored in cooled formal saline for histopathological examinations.
Biochemical analysis
Blood glucose, insulin levels were estimated using kits supplied by Spin react and Abcam rat insulin ELISA kit, respectively. Liver malondialdehyde (MDA) concentration was determined according to the method described by Ohkawa et al. (35). Liver and heart superoxide dismutase (SOD) activity was determined according to the method described by Nishikimi et al. (36). Glutathione (GSH) in liver and heart homogenate was determined according to the method described by Paglia and Valentin (37). Total antioxidant capacity (TAC) in liver homogenate was determined according to the method described by Koracevic et al. (38).
Blood glucose, insulin levels were estimated using kits supplied by Spin react and Abcam rat insulin ELISA kit, respectively. Liver malondialdehyde (MDA) concentration was determined according to the method described by Ohkawa et al. (35). Liver and heart superoxide dismutase (SOD) activity was determined according to the method described by Nishikimi et al. (36). Glutathione (GSH) in liver and heart homogenate was determined according to the method described by Paglia and Valentin (37). Total antioxidant capacity (TAC) in liver homogenate was determined according to the method described by Koracevic et al. (38).
Molecular investigation by real time PCR
Extraction of samples RNA (Liver and pancreas) were done using RNA extraction kits (Thermos Scientific, Fermentase, #K0731) then converted to to cDNA using reverse transcription kits (Thermos Scientific, Fermentase, EP0451). After quantify the concentration of RNA and cDNA automatically by Q5000 (UV-Vis spectrophotometer Q5000\USA) to be sure that the concentrations are pure enough to conduct real time PCR for very pure samples, we run real time PCR with syber green to measure expression of mRNA of target gene in liver and pancreas with B-actin as an internal reference gene according to the protocol (Thermo Scientific, USA, Ko221) and gene specific primers. The web based tool primer3 (http\\www.Genom.wigenome.wi.mit.edu) based on published rat sequences to ensure primer sequence is unique for template sequence we checked similarly to other known sequence with blast (www.ncbi.n1m.gov\blast\Blast.cgi) the final reaction mixture was placed in step one plus real time thermal cycler (Applied Biosystems, Life Technology, USA).
Extraction of samples RNA (Liver and pancreas) were done using RNA extraction kits (Thermos Scientific, Fermentase, #K0731) then converted to to cDNA using reverse transcription kits (Thermos Scientific, Fermentase, EP0451). After quantify the concentration of RNA and cDNA automatically by Q5000 (UV-Vis spectrophotometer Q5000\USA) to be sure that the concentrations are pure enough to conduct real time PCR for very pure samples, we run real time PCR with syber green to measure expression of mRNA of target gene in liver and pancreas with B-actin as an internal reference gene according to the protocol (Thermo Scientific, USA, Ko221) and gene specific primers. The web based tool primer3 (http\\www.Genom.wigenome.wi.mit.edu) based on published rat sequences to ensure primer sequence is unique for template sequence we checked similarly to other known sequence with blast (www.ncbi.n1m.gov\blast\Blast.cgi) the final reaction mixture was placed in step one plus real time thermal cycler (Applied Biosystems, Life Technology, USA).
Histological studies
Pancreas and liver were washed in saline and fixed in 10% neutral buffered formalin for 24h. Ascending grades of ethyl alcohol were then used to dehydrate the samples, and Xylene was used as the clearing agent. The samples embedded in wax at 60ºC and blocked for cutting into 4 µm thick section). Haematoxylin and Eosin routine stain was used to study the morphological and pathological changes in all studied groups (Bancroft and Stevens 1996). Immunohistochemical staining of insulin paraffin sections were mounted on coated slides and stained for insulin detection in insulin producing cells, de-paraffinzed slides and bring to distilled water, then incubated in citrate buffer solution (pH 6.0) at 100ºC. The slides were rinsed with large amount of H2O2 and washed by phosphate-buffered saline (PBS, pH 7.4). The slides then incubated with primary antibody (monoclonal insulin antibody) obtained from Cell Signaling (USA) at 4ºC for overnight using a humid chamber. The 2nd day the slides incubated with peroxidase- conjugated secondary antibodies for one hour at room temperature. The slides were incubated in media containing Avidin–Biotin Complex (ABC) for 30min. The slides washed and stained with 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB) as chromogen using kits obtained from RXD systems, Inc. (USA). Then the slides were washed, immersed in hematoxylin as a counterstain, dehydrated, mounted by rinse and covered by cover slips for demonstrating under the light microscopy (Montgomery et. al. 2012).
Pancreas and liver were washed in saline and fixed in 10% neutral buffered formalin for 24h. Ascending grades of ethyl alcohol were then used to dehydrate the samples, and Xylene was used as the clearing agent. The samples embedded in wax at 60ºC and blocked for cutting into 4 µm thick section). Haematoxylin and Eosin routine stain was used to study the morphological and pathological changes in all studied groups (Bancroft and Stevens 1996). Immunohistochemical staining of insulin paraffin sections were mounted on coated slides and stained for insulin detection in insulin producing cells, de-paraffinzed slides and bring to distilled water, then incubated in citrate buffer solution (pH 6.0) at 100ºC. The slides were rinsed with large amount of H2O2 and washed by phosphate-buffered saline (PBS, pH 7.4). The slides then incubated with primary antibody (monoclonal insulin antibody) obtained from Cell Signaling (USA) at 4ºC for overnight using a humid chamber. The 2nd day the slides incubated with peroxidase- conjugated secondary antibodies for one hour at room temperature. The slides were incubated in media containing Avidin–Biotin Complex (ABC) for 30min. The slides washed and stained with 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB) as chromogen using kits obtained from RXD systems, Inc. (USA). Then the slides were washed, immersed in hematoxylin as a counterstain, dehydrated, mounted by rinse and covered by cover slips for demonstrating under the light microscopy (Montgomery et. al. 2012).
Statistical analysis
All data were expressed as mean ± standard error (SE). The statistical significance was evaluated by one way analysis of variance (ANOVA). Values were considered statistically significant when P value of less than 0.05 (P<0.05).
All data were expressed as mean ± standard error (SE). The statistical significance was evaluated by one way analysis of variance (ANOVA). Values were considered statistically significant when P value of less than 0.05 (P<0.05).
Results
Rat's growth and body weight gain
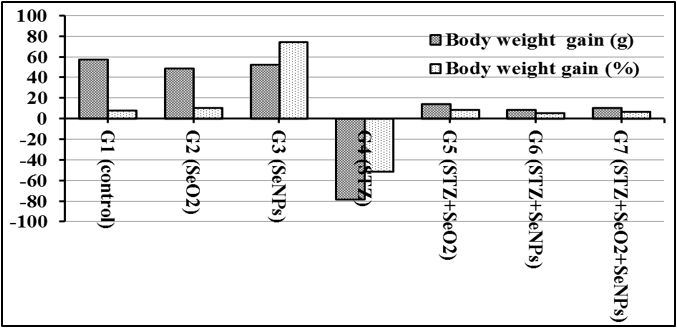
Figure 2 shows body weight gain (g) and body weight gain (%) of rats treated with SeO2, SeNPs, streptozotocin (STZ), STZ along with SeO2, SeNPs or SeO2 + SeNPs. The illustrated data clearly show that the weight of the STZ rats (Group 4) significantly decrease com¬pared to the control (G 1) and the rats treated with SeO2 (G 2) or SeNPs (G 3). Although the rats treated with STZ along with Se resulted in pronounced low body weight gain compared to control (G 1), the Se treatments had protective effect on rat’s body weight loss (G 5, 6, 7).
Figure 2 shows body weight gain (g) and body weight gain (%) of rats treated with SeO2, SeNPs, streptozotocin (STZ), STZ along with SeO2, SeNPs or SeO2 + SeNPs. The illustrated data clearly show that the weight of the STZ rats (Group 4) significantly decrease com¬pared to the control (G 1) and the rats treated with SeO2 (G 2) or SeNPs (G 3). Although the rats treated with STZ along with Se resulted in pronounced low body weight gain compared to control (G 1), the Se treatments had protective effect on rat’s body weight loss (G 5, 6, 7).
Biochemical analysis
Blood level of glucose, insulin and Homa IRI
Blood level of glucose, insulin and Homa IRI
| Animal groups | Glucose | Insulin | Homa-IRI |
| G1 (control) | 72.0 ± 1.38a | 6.90 ± 0.11a | 6.20 ± 0.12a |
| G2 (STZ control) | 265 ± 3.22b | 3.90 ± 0.15b | 3.24 ± 0.14b |
| G3 (SeO2) | 74.0 ± 1.18a | 6.50 ± 0.17c | 6.31 ± 0.17a |
| G4 (SeNPs) | 76.8 ± 1.02a | 6.36 ± 0.12c | 6.16 ± 0.12a |
| G5 (STZ+SeO2) | 113 ± 3.44c | 5.44 ± 0.08d | 5.16 ± 0.08c |
| G6 (STZ+SeNPs) | 136 ± 2.21d | 5.04 ± 0.11e | 4.70 ± 0.10d |
| G7 (STZ+SeO2+SeNPs) | 108 ± 1.82c | 5.60 ± 0.1d | 5.33 ± 0.14c |
a,bdata within column with unlike superscript letters are significantly different at P < 0.05.
Data are mean ± SE for 5 replicates.
Insulin resistance by homeostasis model assessment (HOMA)
The insulin resistance index (IRI) was derived using the homeostasis model assessment (HOMA) as follows: HOMA-IRI= fasting insulin (μIU? ml) × fasting glucose (mg/dl) ? (22.5 x 18)
Table 1: Shows the effect of various treatments on glucose, insulin and Homa-IRI values.
Data are mean ± SE for 5 replicates.
Insulin resistance by homeostasis model assessment (HOMA)
The insulin resistance index (IRI) was derived using the homeostasis model assessment (HOMA) as follows: HOMA-IRI= fasting insulin (μIU? ml) × fasting glucose (mg/dl) ? (22.5 x 18)
Table 1: Shows the effect of various treatments on glucose, insulin and Homa-IRI values.
Level of MDA, SOD, GSH, TAC values
| Animal groups | MDA | SOD | GSH | TAC |
| Animal groups | 33.00 ± 0.91a | 15.93 ± 1.28ad | 32.00 ± 1.45ad | 119.08 ± 6.91ac |
| G1 (control) | 63.32 ± 0.86b | 5.90 ± 0.60b | 12.82 ±1.78b | 65.90±5.58b |
| G2 (STZ control) | 32.20 ± 0.82a | 17.61 ± 1.33a | 34.25±2.67a | 130.50±7.83a |
| G3 (SeO2) | 32.86 ± 0.67a | 17.20 ± 0.75a | 30.38±2.04ad | 121.90±8.29ac |
| G4 (SeNPs) | 40.70 ± 0.74c | 11.16 ± 1.44c | 26.82 ± 1.59cd | 107.36±6.27c |
| G5 (STZ+SeO2) | 49.78 ± 0.87d | 12.70 ± 1.49cd | 21.67 ± 1.31c | 101.90±4.82c |
| G6 (STZ+SeNPs) | 40.20 ±0.81c | 14.40 ±0.97ac | 28.03 ±1.23d | 112.30±5.17ac |
Data are mean ± SE for 5 replicates.
a,bdata within column with unlike superscript letters are significantly different at P <0.05.
Table 2: Effect of various treatments on MDA, SOD, GSH, TAC concentrations in rat's liver.
a,bdata within column with unlike superscript letters are significantly different at P <0.05.
Table 2: Effect of various treatments on MDA, SOD, GSH, TAC concentrations in rat's liver.
| Animal groups | TNF -α gene | |
| Pancreas | Liver | |
| G1 (control) | 1.0 ± 0.02a | 1.02 ± 0.01a |
| G2 (STZ control) | 17.15 ± 0.12b | 14.52 ± 0.23b |
| G3 (SeO2) | 0.91 ± 0.02a | 0.96 ± 0.04a |
| G4 (SeNPs) | 0.89 ± 0.06a | 0.97 ± 0.01a |
| G5 (STZ+SeO2) | 12.82 ± 0.24c | 10.48 ± 0.06c |
| G6 (STZ+SeNPs) | 7.26 ± 0.10d | 6.73 ± 054d |
| G7 (STZ+SeO2+SeNPs) | 3.39 ± 0.05e | 2.48 ± 1.09e |
Data are mean ± SE for 5 replicates.
a,bdata within column with unlike superscript letters are significantly different at P <0.05.
Table 3: Effect of various treatments on rat’s pancreas and liver TNF-α value.
a,bdata within column with unlike superscript letters are significantly different at P <0.05.
Table 3: Effect of various treatments on rat’s pancreas and liver TNF-α value.
Blood glucose and insulin
Glucose in diabetic rats significantly increased compared with control rats (p <0.001) after using of selenium, its nanoparticles and combination of it. Significant decrease in blood glucose compared with normal control (table and figure).blood insulin in diabetic group decreased significantly compared with control group after administration selenium, selenium nanoparticles and its combination led to a significant increase in this index respectively when use selenium and its nanoparticles show no significant changes in blood insulin compared with normal control in group 3, 4.
Glucose in diabetic rats significantly increased compared with control rats (p <0.001) after using of selenium, its nanoparticles and combination of it. Significant decrease in blood glucose compared with normal control (table and figure).blood insulin in diabetic group decreased significantly compared with control group after administration selenium, selenium nanoparticles and its combination led to a significant increase in this index respectively when use selenium and its nanoparticles show no significant changes in blood insulin compared with normal control in group 3, 4.
Oxidative stress biomarkers
A significant increase in MDA in diabetic liver rats compared to control liver rats compared to diabetic liver rats. No significant changes were found in MDA level when using selenium in group 3, selenium nanoparticles group 4 compared to normal control group.
A significant increase in MDA in diabetic liver rats compared to control liver rats compared to diabetic liver rats. No significant changes were found in MDA level when using selenium in group 3, selenium nanoparticles group 4 compared to normal control group.
A significant decrease in SOD in diabetic liver rats compared to control liver rats at after administration (se , its nanoparticles) and their combination led to increasing in liver sod level respectively compared to diabetic liver rats.in group 3, group 4 no significant changes in SOD level compared to normal control. A significant decreasing in diabetic liver TAC level compared to TAC liver of normal control. A significant increasing in TAC level after administration of combination selenium, its nanoparticles and SeO2, its nanoparticles respectively compared to diabetic group insignificant changes in TAC liver level in group 3 and 4 compared to normal control. a significant decreasing in diabetic liver GSH level compared to GSH liver of normal control but returned increasing after administration of combined selenium and its nanoparticles respectively.no change in group 3,4. A significant increasing in TNF- α liver level in diabetic rats compared to normal control after administration (Se , its nanoparticles) and their combination led to decreasing in TNF-α comparing to diabetic rats. A significant increasing in TNF- α pancreas level in diabetic rats compared to normal control after administration (se, its nanoparticles) and their combination led to decreasing in TNF-α comparing to diabetic pancreas
Histopathological Findings: Pancreatic tissues
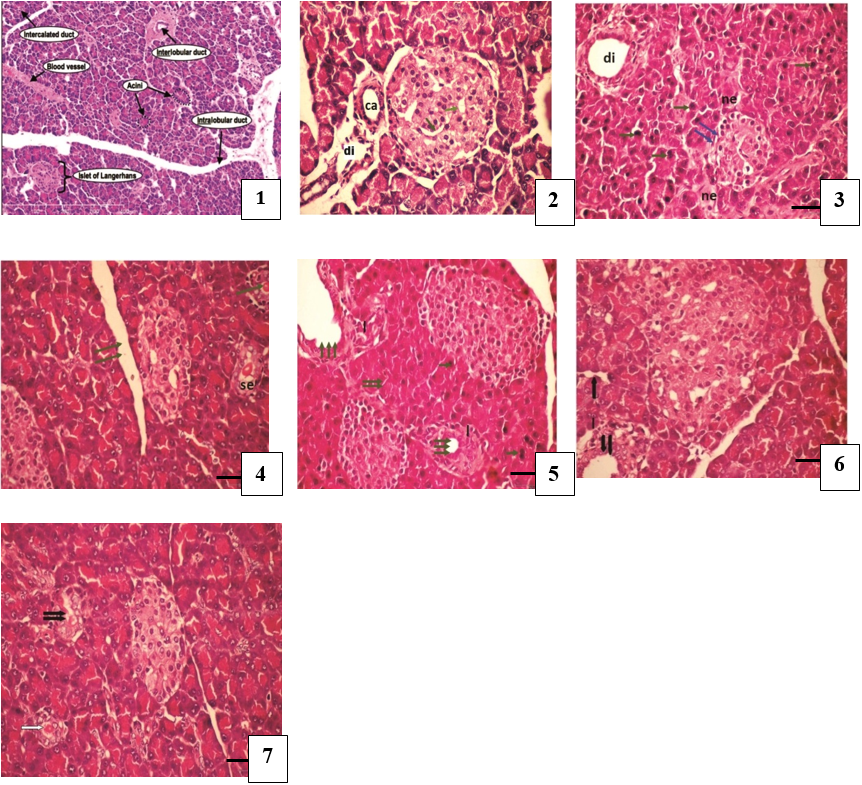
Figure 3: Pancreatic tissues histopathological slides of STZ induced diabetic rat's treated with SeO2 or SeNPs. (H&E stain (Bar = 50µm)).
The effect of Se on rat's pancreatic tissues is shown in Figure. 2 Normal histological architecture is seen in the pancreatic islet of the control group (Figure 2-1) which shows the endocrine portion of the pancreas form of many small cluster of cells called islet of Langerhans, the islet are seen as pale staining groups of cell embedded in a darker staining exocrine tissue consist of the acini, which are appear shaped of short tubular groups of pancreatic cells at the tip of interlobular ducts, the acinus cell has a big basal spherical nucleus, the islet cells are well differentiated into edge small dark nuclei and mostly pale nuclei with large cytoplasm (Figure 2-2).
In untreated diabetic rats degenerative and necrotic changes were consistently found, such a shrunken or reduced islets of Langerhans surrounded by hyprechromatic epithelial cells of acini. The nucleus of the necrotic cells indicated marginal hyperchromasia; the median blood vessels (arrows) between the islet cells were also seen. (Figure 2-3) the reduction of islet of Langerhans size (double arrows), surrounded by hyperchromatic epithelial cells of acini (arrow) as well as necrotic cells (ne) and dilated interlobular duct (di) (Figure 2-4) ) in the SeNPs treated group, however, the majority of the cells of islets of Langerhans were protected, increase size and number of the islets, the cells of islets are rounded nuclei with large cytoplasm and the blood vessels were invasive between them, presence of secretion in the interlobular duct (se) . Figure (2-5) shows the pancreases of diabetic rat treated with Se revealing proliferating islet cells. The section shows also eosinophilic active cells with hyperchormatic nuclei, other with necrotic cells and marked dilation of interlobular duct surrounded by infiltrating lymphocyte cells were still observed. Figures (2-6) and (2-7) show a case of diabetic rat pancreas treated with SeNPs + SeO2 revealing enlarged size of islets of Langerhans with proliferating islet cells and well differentiates and recovering of exocrine pancreas. The acini cells appeared with rounded basal nuclei and mild defatted intercalated duct.
Histology of liver
Figure (3) shows the effect of Se on rat's pancreatic tissues. The control rat liver, showing normal hepatocytes having round nuclei with prominent nucleolus and smoothly cytoplasm, hepatic central vein and mild dilated hepatic sinusoids attached with Kupffer’s cells and few necrotic cells was present (Figure 3-1). Control rat liver show hepatic central vein (cv), normal hepatocytes have rounded nuclei with prominent nucleolus and smoothly cytoplasm. Mild dilation of the hepatic sinusoids (s) attached with Kupffer's cell (k). Also, few necrotic cells (ne) were present. (Figure3-2) In case of diabetic rat liver administrated STZ, showing a focal area of proliferating and infiltrating lymphocytes at portal tract including portal artery, large hemorrhage, two dilated bile duct and most hepatocytes were necrotic rat liver administrated STZ,. In case of diabetic rat, liver shows area of infiltrating lymphocyte formed a nodule at the central vein surrounded by small proliferating hepatocytes and some necrotic one (Figure 3-3) Diabetic rat liver shows area of infiltrating lymphocyte (L) formed a nodule at the central vein surrounded by small proliferating hepatocytes and some necrotic one(ne). Figure (3-4) shows another case of diabetic rat liver treated with STZ revealing hemorrhage large dilated central vein surrounded by group of a apoptotic hepatocytes spread among necrotic. (Figure 3-5). In case of diabetic rat, liver treated with SeO2 shows reduction of the infiltrating lymphocytes with wide portal tract and sinusoid and reduction of the necrotic hepatocytes (Figure 3-6). Normal hepatocytes in diabetic rat liver treated with SeNPs showing mild dilatation of portal tract with few in filtrating lymphocytes regenerative hepatocytes were taken their architecture. The presence of two normal bile ducts (bd).
Figure (3) shows the effect of Se on rat's pancreatic tissues. The control rat liver, showing normal hepatocytes having round nuclei with prominent nucleolus and smoothly cytoplasm, hepatic central vein and mild dilated hepatic sinusoids attached with Kupffer’s cells and few necrotic cells was present (Figure 3-1). Control rat liver show hepatic central vein (cv), normal hepatocytes have rounded nuclei with prominent nucleolus and smoothly cytoplasm. Mild dilation of the hepatic sinusoids (s) attached with Kupffer's cell (k). Also, few necrotic cells (ne) were present. (Figure3-2) In case of diabetic rat liver administrated STZ, showing a focal area of proliferating and infiltrating lymphocytes at portal tract including portal artery, large hemorrhage, two dilated bile duct and most hepatocytes were necrotic rat liver administrated STZ,. In case of diabetic rat, liver shows area of infiltrating lymphocyte formed a nodule at the central vein surrounded by small proliferating hepatocytes and some necrotic one (Figure 3-3) Diabetic rat liver shows area of infiltrating lymphocyte (L) formed a nodule at the central vein surrounded by small proliferating hepatocytes and some necrotic one(ne). Figure (3-4) shows another case of diabetic rat liver treated with STZ revealing hemorrhage large dilated central vein surrounded by group of a apoptotic hepatocytes spread among necrotic. (Figure 3-5). In case of diabetic rat, liver treated with SeO2 shows reduction of the infiltrating lymphocytes with wide portal tract and sinusoid and reduction of the necrotic hepatocytes (Figure 3-6). Normal hepatocytes in diabetic rat liver treated with SeNPs showing mild dilatation of portal tract with few in filtrating lymphocytes regenerative hepatocytes were taken their architecture. The presence of two normal bile ducts (bd).
Figure (3-7) shows diabetic rat liver treated with SeNPs + SeO2, revealing mild dilation of the hemorrhage portal tract, few infiltrating lymphocytes, few necrotic hepatocyte and well appearance of the hepatocytes with rounded nuclei and activated cytoplasm.

Figure 4: Liver tissues histopathological slides of STZ induced diabetic rat's treated with SeO2 or SeNPs. (H&E stain (Bar = 50µm).
Immunohistological findings
Figure (4) shows immunostaining section of STZ induced diabetic rat's pancreas treated with SeO3 or SeNPs. Figure (4-1) shows a section of the rat pancreas used as a negative control of the immunostain where no interaction was seen due to the absence of the 1ry insulin polycolonal antibody. Paraffin section of the control rat pancreas shows brown granules present in the cytoplasm of β-cells of the islet cells due to the interaction of AB- AG of 1ry insulin polycolonal antibody indicated to the B cell with moderate expression and a negative blue color cell indicated to the alpha cell (Figure 4-2). In diabetic rat pancreas the section shows brown granules of islet cells due to the interaction of AB-Ag of lry insulin polycolonal antibody indicating insulin expression with moderate expression of the reduction of β-cell numbers and negative blue color cell indicated to the alpha cell at the periphery of small islets. (Figure 4-3) In diabetic rat pancreas treated with SeNPs show brown granules of islet cells due to the interaction of AB-Ag of 1ry insulin polycolonal antibody, an increased insulin expression and increased number of islet of Langerhans, proliferation of alpha cells was seen as a blue color cell at the periphery of islet. Interestingly, SeNPs treatment caused marked increase in the number of insulin immunopositive cells and appearance of single β-cell was spread extra the islet have diffuse pale brown color between the exocrine acini. Figure (4-4) shows pancreas of diabetic rat treated with SeO2 showing brown granules of islet cells due to the interaction of AB-Ag of 1ry insulin polycolonal antibody with decreased of insulin expression while an increased number of proliferating β-cell and alpha cells was seen as blue color cell at periphery of islet. Also, there are numerous of single β-cell was spread extra the islet have a diffuse pale brown color between the exocrine acini. Figure(4-5) the rat pancreas treated with Se show brown granules of islet cells due to the interaction of AB-Ag of 1ry insulin polycolonal antibody, a decreased of insulin expression while an increased number of proliferating β-cells and α-cells was seen as a blue color cell at the periphery of islet. Also, invasive of α-cells between the β-cells was seen in enlarged islet
Figure (4) shows immunostaining section of STZ induced diabetic rat's pancreas treated with SeO3 or SeNPs. Figure (4-1) shows a section of the rat pancreas used as a negative control of the immunostain where no interaction was seen due to the absence of the 1ry insulin polycolonal antibody. Paraffin section of the control rat pancreas shows brown granules present in the cytoplasm of β-cells of the islet cells due to the interaction of AB- AG of 1ry insulin polycolonal antibody indicated to the B cell with moderate expression and a negative blue color cell indicated to the alpha cell (Figure 4-2). In diabetic rat pancreas the section shows brown granules of islet cells due to the interaction of AB-Ag of lry insulin polycolonal antibody indicating insulin expression with moderate expression of the reduction of β-cell numbers and negative blue color cell indicated to the alpha cell at the periphery of small islets. (Figure 4-3) In diabetic rat pancreas treated with SeNPs show brown granules of islet cells due to the interaction of AB-Ag of 1ry insulin polycolonal antibody, an increased insulin expression and increased number of islet of Langerhans, proliferation of alpha cells was seen as a blue color cell at the periphery of islet. Interestingly, SeNPs treatment caused marked increase in the number of insulin immunopositive cells and appearance of single β-cell was spread extra the islet have diffuse pale brown color between the exocrine acini. Figure (4-4) shows pancreas of diabetic rat treated with SeO2 showing brown granules of islet cells due to the interaction of AB-Ag of 1ry insulin polycolonal antibody with decreased of insulin expression while an increased number of proliferating β-cell and alpha cells was seen as blue color cell at periphery of islet. Also, there are numerous of single β-cell was spread extra the islet have a diffuse pale brown color between the exocrine acini. Figure(4-5) the rat pancreas treated with Se show brown granules of islet cells due to the interaction of AB-Ag of 1ry insulin polycolonal antibody, a decreased of insulin expression while an increased number of proliferating β-cells and α-cells was seen as a blue color cell at the periphery of islet. Also, invasive of α-cells between the β-cells was seen in enlarged islet
Also, invasive of alpha cell between β-cells was seen in enlarged islet. In case of diabetic rats pancreas treated with SeNPs and SeO2 showing interaction of AB-Ag of 1ry insulin polycolonal antibody, an increase of insulin expression and increased number of islet of Langerhans. Also, reduction of alpha cells was seen as a blue color cells at the periphery of islets (Figure 4-6).
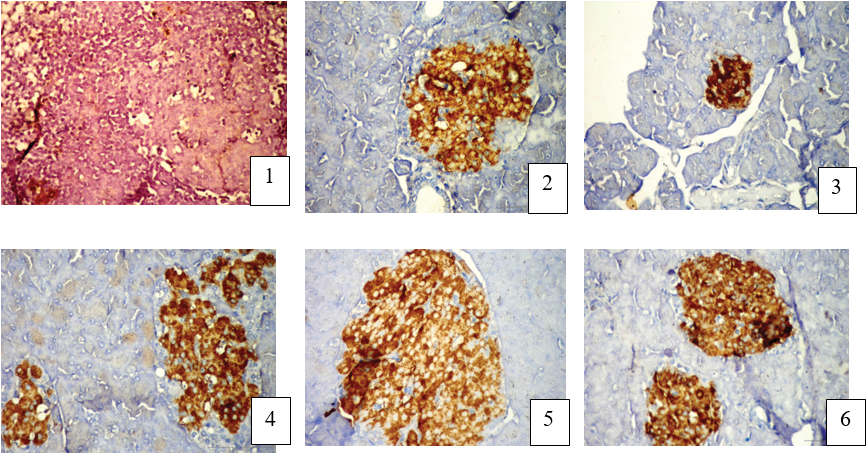
Figure 5: Immunohistological slides of STZ induced diabetic rat's pancreatic tissues treated with SeO2 or SeNPs(DAB stain (Bar = 50µm).
Discussion
The effect of selenium oxide and selenium nano particles on blood parameters was studied during the experimental period. There is increasing evidence of therapeutic effect of SeO2 and SeNPs against diabetic disease. The present study demonstrate anti-diabetic action of selenium and its nanoparticles with upgrading of metabolic risk factors , oxidative stress and inflammatory cytokine . SeO2 nanoparticles structure support a role as antioxidant or a biological free radical scavenger. Oxidative stress referred to as a reactive oxygen species (ROS)/antioxidant imbalance, occurs when the net amount of ROS exceeds the antioxidant capacity. Oxidative stress happen due to elevation in generation of ROS, followed by antioxidant systems depression. Production of ROS are derived from various oxidation pathways that leading to cellular deregulation. superoxide anion (O·?2), hydrogen peroxide (H2O2) and hydroxyl radical (HO.), which results in the production of other free radicals for example, nitric oxide (NO) and peroxinitrite (ONOO.), also called reactive nitrogen species (RNS) (34,35). (36,37). The study showed that hyperglycemia increases the production of hydroxyl radical in the liver of diabetic rats (38). Additionally, oxidative stress due to hyperglycemia and inflammation result in diabetic nephropathy (39). selenium benefit in diabetes as result of the SeO2 is characterized by mono-disperse particles that are single crystals with few twin boundaries that responsible for the free radical scavenging(40). Furthermore, Se nanoparticles stimulate SOD activity (41,42). On the other side, Se is an essential trace element possessing chemopreventive, cardioprotective, antiproliferative effects (43) and colitis dysfunctions (44, 45). Our data showed a significant rise in oxidative stress and a reduction in antioxidant enzymes. Moreover, ROS have avital role in the hepatic glucose production and regulation. The anti-diabetic drugs that act through inhibition of hepatic gluconeogenesis produce concurrent antioxidant effects beneficial in the treatment of diabetes. Atioxidant potential decrease and complications of diabetes increase which include blindness, cardiovascular disease, nephropathy, and nerve damage (46).
Lipids are reported as one of the primary targets of ROS. Hydroperoxides have toxic effects on cells both directly and through degradation to highly toxicity may also react with transition metals like iron or copper to form stable aldehydes, such as malondialdehyde (MDA), that damage cell membranes (47) Increased level of MDA in diabetics suggests that peroxidative injury may be involved in the development of diabetic complications. The increase in lipid peroxidation is also an indication of decline in defense mechanisms of enzymatic and nonenzymatic antioxidants (48). GSH detoxify foreign radicals, act as coenzyme in several enzymatic reactions, and also prevent tissue damage (49). As a consequence of increased oxidative status, GSH showed the frequent alteration in its concentration. Plasma GSH/GSSG showed a significant decrease in type 2 diabetes as compared to normal (50). the antioxidant potential of SeO2 nanoparticles and selenium might be a mechanism for their In fact, Our results showed that Nano- selenium administration increased the liver antioxidant activity and defense mechanism through increasing GSH concentration, decreases MDA concentration, increase catalase and SOD activity as compared to control and selenium groups Moreover, Nano- selenium administration caused a significant increase TAC as compared to control and selenium groups while no changes have been seen in group 3,4 compared to control group. One of the most accepted hypotheses of Se antioxidant activity is the ability of Se to keep the glutathione in the reduced form where glutathione has the ability to detoxify the ROS. The action of Se is achieved by the selenium-containing enzyme GSH-Px protects cells against ROS all this results agreed with a (51). Our current study show that administration of Selenium in the nano size (Nano Se) possess a more potent antioxidant activity as the GSH content and TAC ,the ability of the nanoparticles to offer several pharmacokinetic advantages, such as specific drug delivery, high metabolic stability, high membrane permeability, improved bioavailability, improved bioavailability, and long duration of action. The physicochemical properties of nanoparticles, such as size, surface charge, and hydrophobicity, affect their mucosal absorption characteristics, and smaller nanoparticles show higher trans cellular uptake than do larger ones (52). Insulin regulates metabolism by controlling the uptake and consumption of glucose in organs such as the liver and kidney and in adipose tissue and skeletal muscle. This regulation is achieved by controlling the actions of various metabolic enzymes. Increased oxidative stress as well as reduction in antioxidant capacity could be related to the complications in patients with diabetes such as oxidative DNA damage and insulin resistance (53).
Studies on the biological activities of selenium and its nano-forms revealed that hollow spherical nanoparticles of selenium have strong antioxidant properties (54). Similar studies declared that nano-selenium has the ability to act as an antioxidant with reduced risk of ordinary selenium toxicity (55).
To provide more effective Se dosing regimens, nanoparticle formulations have been developed. The concept is that the Se nanoparticles provide a slow release of Se ions, there by reducing acute toxicity. A few studies have demonstrated lower toxic potency of Se nanoparticles than of dissolved ionic Se species. This suggests that to some extent, Se from nanoparticles is less bioavailable (56) In addition, nanoparticles have a very large surface to volume ratio and are known to bind to e.g., proteins, and reactions may be catalyzed by the nano particle surface (57).
The chronic hyperglycemia can directly promote an inflammatory state, where the increase in cytokines can lead to destruction of the pancreatic β cells and mal- function of the endocrine pancreas in both type 1 and type 2 diabetes (58). Commonly, type 1 and type 2 DM are considered inflammatory processes (58) as there is a significant increase in IL-6, IL-18, IL-1 and TNF-α in the blood of patients with this disease (60). TNF-a also has important effects on whole body lipid and glucose metabolism (61). And TNF-α have been defined as an autocatalytic mechanism that can lead to programmed cell death (apoptosis) (62).the diabetic state induces an increase of TNF-α and of its receptor TNF-R1 in the liver (63). The onset of diabetes is accompanied by development of major biochemical and functional abnormalities in the liver, including alterations in carbohydrate, lipid, and protein metabolism, and changes in antioxidant status (64).
Conclusion
The present work indicates that DM-induced severe biochemical and histochemical changes in the liver and pancreas and SeNPs have a protective effect and minimize the risk of diabetic complications. This protection may be due to the free radical scavenging effect of this metal effect. selenium in its ordinary or nano form have antioxidant and catalytic effect beyond their activity on detoxifying free radicals by remaining active in tissues for extended periods through moving spontaneously between the oxidized and reduced state and their ability on. However, further studies are needed to get a deep insight into the hypoglycemic activity of SeNPs and/or the possible toxicity increases after SeNPs are administered for a long time before starting any clinical trials.
References
- Rahimi R, Nikfar S, Larijani B, Abdollahi M. (2005). A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59: 365-373
- Di Naso FC, Simões Dias A, Porawski M, Marroni NA. (2011). Exogenous superoxide dismutase: action on liver oxidative stress in animals with streptozotocin - induced diabetes. Exp Diabetes Res 2011: 754132
- Kajbaf F, Mojtahedzadeh M, Abdollahi M. (2007). Mechanism's underlying stress-induced hyperglycemia in critically ill patients. Therapy 4: 97-106
- Coskun O, Kanter M, Korkmaz A, Oter S. (2005). Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 51: 117-23.
- Je HD, Shin CY, Park HS, Huh IH, Sohn UD. (2001). The comparison of vitamin C and vitamin E on the protein oxidation of diabetic rats. J. Auton. Pharm. 21:231-36.
- Szkudeslski T. (2001). The Mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 50: 536-46.
- Bray TM. (2000). Dietary Antioxidants and Assessment of Oxidative Stress. Nutr. 16: 578-80.
- Coskun O, Kanter M, Korkmaz A, Oter S. (2005). Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 51: 117
- Slater T. (1984). Free-radical mechanism in tissue injury. Biochem J 222: 1-15.
- Salvemini D, Cuzzocrea S. (2003). Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med 31: S29–S38.
- Loeper J, Goy J, Rozenstajin L. (1991). Lipid peroxidation and protective enzymes during myocardial infarction. Clin Chim Acta 196: 119-126.
- Fujii H, Shimizu M, Ino H, Yamaguchi M, Terai H, Mabuchi H, and Michishita I, Genda A. (2002). Oxidative stress correlates with left ventricular volume after acute myocardial infarction. Jpn Heart J. 43: 203-209.
- Elias D, Prigozin H, Polak N, Rapoport M, Lohse A W, Cohen I R. (1994). Autoimmune diabetes induced by the b –cell toxin STZ. Diabetes 43: 992-8
- El-Bayoumy, K. (2001). The protective role of selenium on genetic damage and on cancer. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 475: 123-139.
- Ayaz M, Celik HA, Aydin HH, Turan B. (2006). Sodium selenite protects against diabetes-induced alterations in the antioxidant defense system of the liver. Diabetes Metab Res Rev 22: 295-299.
- Wang, H., Zhang, J., Yu, H. (2007). Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radical Biology and Medicine 42: 1524-1533.
- Jozanov-Stankov O, Demajo M, Djujic I, Mandic M. (1998). Selenium intake as a modulator of responsiveness to oxidative stress. J Environ Pathol Toxicol Oncol. 17(34): 251–257.
- Jia X, Liu Q, Zou S, Xu X, Zhang L. (2015). Construction of selenium nanoparticles/beta-glucan composites for enhancement of the antitumor activity. Carbohydrate Polym. 117: 434–442.
- Sunil C, Agastian P, Kumarappan C, Ignacimuthu S. (2012). In vitro antioxidant, antidiabetic and antilipidemic activities of Symplocos cochinchinensis (Lour.) S. Moore bark. Food Chem Toxicol. 50(5): 1547–1553.
- Lenzen S. (2008). The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 51(2): 216–226.
- Elias D, Prigozin H, Polak N, Rapoport M, Lohse A W, Cohen I R. (1994). Autoimmune diabetes induced by the b –cell toxin STZ. Diabetes 43: 992-8.
- Maritim, A., Sanders, R. & Watkins III, J. (2003). Diabetes, oxidative stress, and antioxidants: a review. Journal of Biochemical and Molecular Toxicology, 17(1): 24-38.
- Caldwell, R.B., El-Remessy, A.E.B. & Caldwell, R.W. (2008). Oxidative stress in diabetic retinopathy. Diabetic Retinopathy 217-242.
- Conget, I. (2002). Diagnosis, classification and cathogenesis of diabetes mellitus. Revista Española de Cardiología, 55: 528-535.
- Laakso, M. (2010). Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care, 33(2): 442-449.
- Tiedge, M., Lortz, S., Drinkgern, J. & Lenzen, S. (1997). Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes, 46(11): 1733-1742.
- Dokken, B.B. (2008). The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectrum, 21(3): 160-165.
- Beutler, B., and A. Cerami. (1989). The biology of cachectin/TNF-a primary mediator of the host response. Annu. Rev. Immunol. 7: 625-655.
- Grunfeld, C., and K. R. Feingold. (1991). The metabolic effects of tumor
- Feinstein, R., H. Kanety, M. Z. Papa, B. Lunenfeld, and A. Karasik. (1993). Tumor necrosis factor-a suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 268: 26055-26058.
- Hotamisligil, G. S., D. L. Murray, L. N. Choy, and B. M. Spiegelman (1994). TNF-a inhibits signaling from insulin receptor. Proc. Natl. Acado Sci. USA. 91: 4854-4858.
- Prokisch, J. and Zommara, M. A. (2011). Process for producing elemental selenium nanospheres. US8003071 USA patent
- Kar, T. and Misra, A. K. (1999). Therapeutic properties of whey used as fermented drink. Revista de Microbiologia, 30: 163-169.
- Prokisch, J., Szeles, E., Kovacs, B., Daroczy, L. and Zommara, M. (2008). Formation of metal selenium nanospheres in bacteria: is it a possible detoxification mechanism? Cereal Res. Commu., 36 (2S): 947-950.
- Prokisch, J., Szegvári, I., Széles, É., Kovács B. and Gy?ri, Z (2006). Normalization method for evaluation of metal contamination of soil. Cereal Res. Commu., 34: 263-266.
- Fridovich, I. (1995). Superoxide radical and superoxide dismutases. Annual Review of Biochemistry, 64, 97-112.
- Selemidis, S., Sobey, C.G., et al. (2008). NADPH oxidases in the vasculature: Molecular features, roles in dis- ease and pharmacological inhibition. Pharmacology & Therapeutics, 120: 254-291.
- Ohkuwa, T., Sato, Y., et al. (1995). Hydroxyl radical for- mation in diabetic rats induced by streptozotocin. Life Sciences, 56, 1789-1798.
- Winiarska, K., Drozak, J., et al. (2004). Diabetes-induced changes in glucose synthesis, intracellular glutathione status and hydroxyl free radical generation in rabbit kidney-cortex tubules. Molecular and Cellular Biochemistry, 261: 91-98.
- Frances, D.E., Ronco, M.T., et al. (2010). Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: New insights into the insulin effect. Jour- nal of Endocrinology, 205: 187-200.
- Elmarakby, A.A. and Sullivan, J.C. (2012). Relationship between oxidative stress and inflammatory cytokines in
- Zhang F, Chan SW, Spanier JF, Apak F, Jin Q, Robinson RD, Herman IP. (2002). Cerium oxide nanoparticles: size-selective formation and structure analysis. Appl Phys Lett 80: 127-129.
- Korsvik C, Patil S, Seal S, Self WT. (2007). Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb) 10: 1056-1058
- Celik HA, Aydin HH, Deveci R, Terzioglu E, Karacali S, Saydam G, Akarca U, Batur Y. (2004). Biochemical and morphological characteristics of selenite-induced apoptosis in human hepatoma Hep G2 cells. Biol Trace Elem Res 99: 27-40
- Abdollahi M, Rahmat-Jirdeh N, Soltaninejad K. (2001). Protection by selenium of lead-acetate-induced alterations on rat submandibular gland function. Hum Exp Toxicol 20: 28-33
- Ayaz M, Celik HA, Aydin HH, Turan B. (2006). Sodium selenite protects against diabetes-induced alterations in the antioxidant defense system of the liver. Diabetes Metab Res Rev 22: 295-299
- Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. (2011). Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods 21: 200-208.
- J. Styskal, H. van Remmen, A. Richardson, and A. B. Salmon. (2012). “Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models?” Free Radical Biology and Medicine, vol. 52, no. 1, pp. 46–58.
- B. Halliwell and S. Chirico, “Lipid peroxidation: its mechanism, measurement, and significance,” American Journal of Clinical N
- R. R. Saddala, L.Thopireddy,N. Ganapathi, and S. R. Kesireddy, (2013). “Regulation of cardiac oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with aqueous extract of Pimpinella tirupatiensis tuberous root,” Experimental and Toxicologic Pathology, vol. 65, no. 1-2, pp. 15–19, Nutrition, vol. 57, supplement 5, pp. 715S–724S, 1993.
- V. Calabrese, C. Cornelius, V. Leso et al., (2012). “Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes,” Biochimica et Biophysica Acta, vol. 1822, no. 5, pp. 729-736.
- C. J. Tsai, C. J. Hsieh, S. C. Tung, M. C. Kuo, and F. C. Shen. (2012). “Acute blood glucose fluctuations can decrease blood glutathione and adiponectin levels in patients with type2diabetes,” Diabetes Research and Clinical Practice, vol. 98, no. 2, pp. 257–263.
- Köhrle J. and Gärtner R. (2009): Selenium and thyroid. Best Pract Res Clin Endocrinol Metab. 23(6): 815–827.
- Petros, R. and DeSimone, J.M. (2010): Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov., 9(8): 615−627.
- M. Lodovicia, L. Giovannellia, V. Pitozzia, E. Bigaglia, G. Bardinib, and C. M. Rotellab. (2008). “Oxidative DNA damage and plasma antioxidant capacity in type 2 diabetic patients with good and poor glycaemic control,” Mutation Research, vol. 638, no. 1-2,pp. 98–102.
- Gao X, Zhang J. and Zhang L. (2002). Hollow sphere selenium nanoparticles: their in-vitro anti-hydroxyl radical effect. Adv Mater.14 (4): 290–293.
- Wang H, Zhang J. and Yu H. (2007). Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 42(10):1524–1533.
- (Zhang et al., 2001; Zhang et al., (2005). Jia, Li & Chen, 2005; Benko et al., 2012).
- Klein J. (2007). Probing the interactions of proteins and nanoparticles. Proceedings of the National Academy of Sciences of the United States of America 104: 2029-2030.
- Ahrens, B. (2011). Antibodies in metabolic diseases. New Biotechnology, 28, 530-537.
- Esposito, K., Nappo, F., et al. (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation, 106: 2067-2072.
- Foss, N.T., Foss-Freitas, M.C., et al. (2007). Impaired cytokine production by peripheral blood mononuclear cells in type 1 diabetic patients. Diabetes & Metabolism, 33, 439-443.
- Beutler, B., and A. Cerami. (1989). The biology of cachectin/TNF-a primary mediator of the host response. Annu. Rev. Immunol. 7: 625-655.
- Jones, B.E., Lo, C.R., et al. (2000). Hepatocytes sensitized to tumor necrosis factor-alpha cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. The Journal of Biological Chemistry, 275: 705-712.
- Ingaramo, P.I., Ronco, M.T., et al. (2011). Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Molecular Immunology, 48: 1397-1407.
- Saxena, A.K., Srivastava, P., et al. (1993). Impaired anti- oxidant status in diabetic rat liver. Effect of vanadate. Biochemical Pharmacology, 45, 539-542.
Citation: Maher Amer Ali Amer., et al. (2020). Biochemical and Histopathological Studies on the role of Selenium Nanoparticles and Selenium Oxide against Chemically-Induced Diabetes in Male Rats. Archives of Nutrition and Public Health 2(2).
Copyright: © 2020 Maher Amer Ali Amer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.