Research Article
Volume 6 Issue 1 - 2024
Advances in Hair Loss Treatment and the Use of Stem Cells
1MD, MS, Weill Cornell Medical College of Cornell University
2MD, PhD, Cancer Biology and Genetics Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
3MD, MS, Nexus Alliance Biopharma
4R&D associate, Nexus Alliance Biopharma
5Undergraduate at York University, Toronto, ON
2MD, PhD, Cancer Biology and Genetics Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
3MD, MS, Nexus Alliance Biopharma
4R&D associate, Nexus Alliance Biopharma
5Undergraduate at York University, Toronto, ON
*Corresponding Author: Timothy Allen, MD, PhD, Cancer Biology and Genetics Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
Received: September 19, 2024; Published: September 26, 2024
Abstract
Hair follicle regeneration is vital to treat those suffering from alopecia. To accomplish this, various markers can be used to determine the causes of alopecia and subsequent treatment methods—these range from diagnostic, prognostic, and molecular markers to forms of histopathological examination. Through the insight these methods provide, treatment for hair regrowth can be provided. A key form of treatment is through stem cell therapy. Multiple types of stem cells can be used to promote hair growth through various methods. In tandem with the markers, as mentioned earlier, several clinical trials and case reports have shown successful hair regrowth rates. Though some challenges still exist in the realm of compatibility as well as widespread implementation, further research can push these treatment methods to become an adequate answer to alopecia.
Keywords: Alopecia; Androgenetic Alopecia (AGA); Hair Transplant Surgery; Stem Cell Therapy; Hair Follicle Regeneration; Scalp Biopsy and Gene Expression Profiling (GEP)
Hair loss, or alopecia, affects millions of people worldwide, impacting their self-esteem and quality of life. Traditional treatments, such as medications and hair transplant surgery, have relieved many, but recent advances in stem cell therapy offer promising new avenues for hair restoration. Additionally, integrating biomarkers as diagnostic and prognostic tools has significantly improved the management of hair loss.
Alopecia can be caused in innumerable ways, but the most common cause is androgenetic alopecia (AGA) [1], where the afflicted individual is genetically predisposed to alopecia and has androgen activity (AGA) impacts hair follicle growth [1]. AGA is characterized by follicular miniaturization [1], increased telogen follicles [2], perifollicular fibrosis [3], and minimal to no inflammation. The follicular miniaturization is due to a decrease in the diameter of terminal hair follicles, leading them to resemble vellus hairs (fine, short, non-pigmented). The terminal-to-vellus hair ratio typically decreases from the normal 4:1 to less than 2:1 in cases of AGA [1]. As for the increased telogen follicles, AGA shows an increased proportion of hair follicles in the telogen (resting) phase (where typically, about 5-10% of follicles are in the telogen phase; in cases of AGA this percentage may rise to 15-20% or higher) [2]. Mild perifollicular fibrosis (scar tissue around hair follicles) may be observed in AGA [3]. This is typically a subtle feature and is often more prominent around miniaturized follicles. Finally, AGA generally shows little to no inflammatory infiltrate. If inflammation is present, it is usually minimal and localized to the upper portion of the follicle or the isthmus region [4]. This differs from other cases of alopecia, where more significant perifollicular or diffuse inflammatory infiltrates are noted.
While AGA shares some similarities with other common forms of alopecia, there are key distinguishing factors between them, as outlined below:
- Compared to telogen effluvium: In telogen effluvium, there is an increased number of follicles in the telogen phase, like AGA, but without significant follicular miniaturization. Additionally, the terminal to vellus hair ratio remains normal, and there is no considerable fibrosis or inflammation [5]. Thus, telogen effluvium shows diffuse thinning rather than patterned hair loss in AGA.
- Compared to alopecia areata: Alopecia areata is characterized by a peribulbar ("swarm of bees") lymphocytic infiltrate around the hair follicles, particularly at the anagen (growing) phase [1]. Follicles may show miniaturization, but there is a marked presence of inflammatory cells, which is absent or minimal in AGA. Also, "dystrophic" anagen hairs and exclamation mark hairs (tapered near the scalp) may be observed [1].
- Compared to lichen planopilaris (scarring alopecia): Lichen planopilaris, a type of primary cicatricial alopecia, shows a lymphocytic infiltrate at the level of the isthmus and infundibulum, which is not seen in AGA [4]. Additionally, perifollicular fibrosis and follicular dropout are seen in lichen planopilaris. It often leads to permanent hair loss due to destruction and scarring of the hair follicle [4].
- Compared to frontal fibrosing alopecia (FFA), FFA, a variant of lichen planopilaris, shows perifollicular lymphocytic inflammation, perifollicular fibrosis, and follicular miniaturization4. However, FFA is also characterized by inflammation, which can lead to scarring. The localization of inflammation at the frontal hairline and eyebrows and evidence of scarring help distinguish FFA from AGA [4]. Dense inflammatory infiltrates, more pronounced perifollicular fibrosis, and follicular destruction are distinguishing signs not seen in AGA.
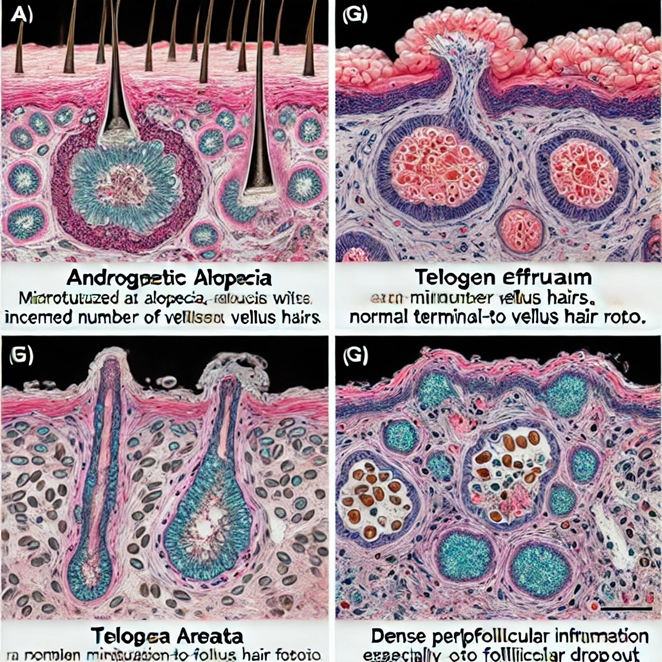
Figure 1: The image above shows the histopathological findings of different types of alopecia, highlighting the specific features that differentiate androgenetic alopecia (AGA) from other hair loss conditions such as telogen effluvium, alopecia areata, and lichen planopilaris. Each section of the image illustrates the unique microscopic characteristics observed in scalp biopsies for these conditions, including follicular miniaturization, inflammation patterns, and fibrosis, which are critical for accurate diagnosis.
In outlining the general types of inflammatory diseases that may lead to hair loss on the scalp, it is essential to investigate the differential diagnoses, consider the clinical indications, and conduct a thorough diagnostic evaluation.
Beyond the impact on one’s psychological well-being, there is also an observed association between AGA and an increased risk of melanoma [1]. Current treatments, including pharmacological options and physical therapies, often prove ineffective over the long term or have significant side effects that limit their use. Consequently, there is a growing need for innovative therapeutic approaches that promote regenerative alternatives to mitigate the effects of dihydrotestosterone (DHT) on the hair follicle microenvironment [1].
Some of the current/emerging clinical treatments include:
Pharmacological Treatments:
The most common drugs used for AGA include:
Pharmacological Treatments:
The most common drugs used for AGA include:
- Minoxidil: A topical vasodilator that stimulates hair growth, though its efficacy varies among individuals. Continuous use is required, and cessation can lead to hair loss recurrence [6].
- Finasteride and dutasteride: Oral 5-alpha reductase inhibitors that reduce DHT levels, thereby slowing hair loss progression. However, these drugs may cause sexual dysfunction and other side effects, necessitating careful patient selection and monitoring [1].
- Topical anti-androgens: Clocortolone, which is currently under investigation for AGA, offers a potentially safer topical approach to blocking DHT at the follicular level [7].
Physical Treatments:
- Low-level laser therapy (LLLT) utilizes red light to stimulate hair follicles and promote growth. Although evidence suggests moderate efficacy, the long-term benefits remain uncertain. The level of LLLT is considered tailored and long-term treatment [8].
- Micro-needling involves creating micro-injuries to stimulate wound healing pathways, enhance the absorption of topical agents, and potentially increase hair growth [7].
Regenerative Therapies:
- Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF): These autologous blood-derived therapies contain growth factors that promote hair follicle regeneration. Early studies show promising results, but more research is needed to establish standardized protocols [9]. Platelet-rich plasma (PRP) therapy for alopecia, or hair loss, involves injecting a concentration of a patient's platelets into the scalp to stimulate hair growth [10]. The efficacy of PRP in treating alopecia is primarily attributed to the growth factors released by the platelets, which promote tissue repair, regeneration, and new hair follicle development [11].
The main growth factors commonly found in PRP that are believed to play a role in hair regrowth include:
Key Growth Factors in PRP for Alopecia: Platelet-derived growth Factor (PDGF) Promotes blood vessel formation, cell proliferation, and tissue regeneration. It helps increase the supply of nutrients and oxygen to hair follicles, encouraging hair growth [12].
Vascular Endothelial Growth Factor (VEGF): Stimulates the formation of new blood vessels (angiogenesis) around hair follicles, improving blood circulation and nutrient delivery, which is vital for hair growth and follicle survival [13].
Epidermal Growth Factor (EGF): Promotes cell growth and differentiation, repairing damaged hair follicles and supporting new hair production [14].
Transforming Growth Factor Beta (TGF-β): Involved in cell differentiation, proliferation, and regulation of the hair growth cycle. While TGF-β can have both inhibitory and stimulatory effects on hair follicles, in the context of PRP therapy, its controlled release is believed to help transition hair follicles from the dormant (telogen) phase to the growth (anagen) phase [14].
Insulin-Like Growth Factor 1 (IGF-1): Supportscell growth and survival, reduces hair follicle miniaturization, and extends the duration of the anagen phase, leading to thicker and longer hair growth [15].
Keratinocyte Growth Factor (KGF): Stimulates the growth of keratinocytes, the predominant cells in the epidermis, which play a crucial role in forming and maintaining hair follicles [16].
Fibroblast Growth Factor (FGF): Involved in the proliferation of dermal papilla cells (cells at the base of the hair follicle) and the elongation of hair shafts [17].
These growth factors work synergistically to enhance hair follicle activity, promote new hair growth, and improve the overall health and thickness of the hair. No specific commercial products contain all the individual growth factors used in PRP (Platelet-Rich Plasma) therapy, as PRP itself is a biological product derived from the patient’s blood. There are commercially available kits designed to facilitate the preparation of PRP, which inherently contains these growth factors. The exact concentration and composition of growth factors in PRP can vary depending on the individual patient and the preparation method [10].
PRP Kits and Systems
Several companies provide PRP preparation systems or kits that help isolate and concentrate platelets from a patient's blood. These kits do not specifically list individual growth factors but are designed to produce PRP that contains a mixture of growth factors, including those mentioned earlier (PDGF, VEGF, EGF, etc.).
Several companies provide PRP preparation systems or kits that help isolate and concentrate platelets from a patient's blood. These kits do not specifically list individual growth factors but are designed to produce PRP that contains a mixture of growth factors, including those mentioned earlier (PDGF, VEGF, EGF, etc.).
Here are some commonly used PRP preparation systems:
Regen PRP Kit (Regen Lab)
A closed, sterile system uses a single-spin centrifuge to prepare PRP. It is one of the most popular systems for producing PRP with a standardized concentration of platelets and growth factors [18].
Regen PRP Kit (Regen Lab)
A closed, sterile system uses a single-spin centrifuge to prepare PRP. It is one of the most popular systems for producing PRP with a standardized concentration of platelets and growth factors [18].
Arthrex Angel System
A more sophisticated device that uses a three-sensor technology to control the concentration of platelets, leukocytes, and red blood cells. The system allows customization of the PRP content, which may help target specific therapeutic outcomes [19].
A more sophisticated device that uses a three-sensor technology to control the concentration of platelets, leukocytes, and red blood cells. The system allows customization of the PRP content, which may help target specific therapeutic outcomes [19].
EmCyte Pure PRP
It offers a highly concentrated PRP preparation focused on minimizing contamination by red blood cells and neutrophils, which is believed to optimize the growth factors' regenerative potential [20].
It offers a highly concentrated PRP preparation focused on minimizing contamination by red blood cells and neutrophils, which is believed to optimize the growth factors' regenerative potential [20].
Dr. PRP Kit
Designed for easy use in clinical settings, this kit employs a double-spin method to achieve a higher concentration of platelets, which inherently includes the necessary growth factors for stimulating hair growth [20].
Designed for easy use in clinical settings, this kit employs a double-spin method to achieve a higher concentration of platelets, which inherently includes the necessary growth factors for stimulating hair growth [20].
Harvest PRP System (Terumo BCT)
It uses a dual-spin system to concentrate platelets and growth factors while also providing flexibility in the concentration levels, catering to various medical needs, including hair restoration [20].
It uses a dual-spin system to concentrate platelets and growth factors while also providing flexibility in the concentration levels, catering to various medical needs, including hair restoration [20].
Growth Factor-Enriched Topicals
Some commercially available topical products are enriched with growth factors and may be used alongside PRP therapy for alopecia. However, these are not the same as PRP injections and may contain synthetic or recombinant growth factors.
Some commercially available topical products are enriched with growth factors and may be used alongside PRP therapy for alopecia. However, these are not the same as PRP injections and may contain synthetic or recombinant growth factors.
AQ Skin Solutions Advanced Hair Complex+: Contains synthetic growth factors, including VEGF and KGF, designed to mimic the natural growth factors found in PRP)
Revita Hair Stimulating Shampoo and Conditioner: Enriched with growth factors and peptides to promote scalp health and hair growth).
While these kits and products help prepare and enhance PRP therapy, it is important to consult with a healthcare professional to choose the most appropriate system or product for specific therapeutic goals. The effectiveness of these systems and products can vary depending on individual patient factors and the type of alopecia being treated [21].
- Hair follicle stem cells and mesenchymal stem cell-based therapies involve using stem cells or their derivatives, such as conditioned medium (CM) or exosomes, to regenerate hair follicles. Clinical trials are underway to determine the safety, efficacy, and best delivery methods for these cutting-edge treatments [22].
Nanotechnology and 3D Bioprinting:
- Nano biomaterials and bioinks are being developed to mimic the hair follicle microenvironment, supporting tissue regeneration and cellular therapy. 3D bioprinting offers a novel approach to creating complex structures and enhancing the delivery of cells, growth factors, or drugs to target sites [23].
There are several investigational new drugs (INDs) currently in the pipeline for androgenetic alopecia (AGA). These include novel treatments targeting the androgen receptor pathway and other molecular mechanisms implicated in hair loss, such as:
- KX-826 by Kintor Pharma is an androgen receptor (AR) antagonist and a first-in-class topical drug for AGA. The drug has completed a Phase II clinical trial, showing a good efficacy and safety profile. The U.S. Food and Drug Administration (FDA) has approved continuing Phase II trials in the United States [24].
- Clascoterone by Cassiopea is another promising drug. It is currently in a Phase III trial for male AGA and a Phase II trial for female AGA. Clascoterone is a topical androgen receptor inhibitor that blocks dihydrotestosterone (DHT) from binding to receptors in scalp hair follicles, potentially reducing hair loss [7].
- These emerging therapies reflect a trend toward more targeted treatments for AGA, focusing on the cellular and molecular causes of hair loss. With these drugs still in clinical trials, there is a concerted effort to address the limitations of current therapies, such as minoxidil and finasteride, which are not effective for all patients and have side effects that limit long-term use [6].
Additionally, hair transplant surgery has evolved significantly over the past few decades. Techniques such as Follicular Unit Transplantation (FUT) and Follicular Unit Extraction (FUE) have become the gold standards, offering natural-looking results with minimal scarring [25].
Despite these advancements, the demand for more effective and less invasive treatments continues to drive research in this field. Stem cell therapy has emerged as a revolutionary approach in regenerative medicine for hair restoration. The ability of stem cells to differentiate into various cell types, including hair follicle cells, offers the potential for regenerating hair follicles and promoting hair growth [26].
There is a diverse range of stem cells that can be utilized in this manner, as detailed below:
- Embryonic Stem Cells (ESCs): These pluripotent cells can differentiate into any cell type, including hair follicle cells. However, ethical concerns and the risk of teratoma formation limit their clinical use [22].
- Induced Pluripotent Stem Cells (iPSCs): Generated by reprogramming adult somatic cells, iPSCs offer a promising alternative to ESCs. They can differentiate into hair follicle cells and have been used in preclinical models to regenerate hair follicles [27].
- Mesenchymal Stem Cells (MSCs): These multipotent cells, derived from bone marrow or adipose tissue, can differentiate into hair follicle cells and promote hair growth through paracrine signaling [28].
Stem cells can be used for hair follicle regeneration through direct differentiation or paracrine effects. With direct differentiation, stem cells can differentiate into hair follicle cells, integrating with the host tissue and promoting hair growth [29]. Through paracrine effects, stem cells secrete growth factors and cytokines that promote angiogenesis, reduce inflammation, and enhance tissue repair [26].
Biomarkers have become essential for managing hair loss, providing valuable diagnostic and prognostic information. Diagnostic Biomarkers include gene expression profiling (GEP) and circulating microRNAs (miRNAs). GEP assays measure the expression levels of specific genes associated with hair follicle health, helping to identify patients at risk of hair loss [29]. As for miRNAs, particular types in the blood can indicate the health of hair follicles and the likelihood of hair loss [29].
There are also prognostic biomarkers, such as serum biomarkers and scalp biopsy markers. For serum biomarkers, the levels of specific proteins and hormones in the blood can provide insights into the underlying causes of hair loss and the effectiveness of treatments [29]. As for scalp biopsy markers, they can predict the progression of hair loss and the response to treatment. Scalp biopsies are particularly crucial for accurately diagnosing the cause of hair loss and identifying specific markers that can guide treatment decisions [29].
Figure 2: Visual of a scalp biopsy, showing the extraction of scalp tissue and follicle specimens to be analyzed.
Histopathological examination is crucial in differentiating AGA from other forms of alopecia due to its specific features, such as follicular miniaturization, increased telogen hairs, mild perifollicular fibrosis, and minimal/absent inflammation. Histopathology provides a microscopic evaluation of scalp biopsies, essential for an accurate diagnosis and effective treatment planning for patients experiencing hair loss [30]. For example, in AGA, histopathological findings typically show miniaturization of hair follicles, fewer terminal hairs, and increased telogen hairs.
By examining the histopathological features of hair follicles, pathologists can assess the hair follicle's cycling phases (anagen, catagen, and telogen), the integrity of the follicular stem cells, and any signs of perifollicular inflammation, fibrosis, or other dermal abnormalities [30]. This can provide insight into the pathophysiology of AGA and the degree of follicular miniaturization.
Histopathology can also be used to evaluate the efficacy of treatment modalities over time. For example, examining scalp biopsies before and after treatment with drugs like minoxidil or finasteride can help determine the response of hair follicles to therapy, including any changes in follicular density or reduction in miniaturization [31].
Certain histopathological features can indicate comorbid conditions or other scalp pathologies that may coexist with AGA, such as seborrheic dermatitis or lichen planopilaris [5]. Recognizing these patterns allows for more tailored therapeutic approaches.
Histopathological examinations are typically performed using a 4-mm punch biopsy from the affected scalp area, usually from the vertex or frontal region where AGA is most prevalent [32]. The biopsies are processed with vertical and horizontal sectioning to provide a comprehensive view of the follicle's architecture, distribution, and condition.
Horizontal and vertical histopathological sections help evaluate non-scarring and cicatricial (scarring) alopecia, respectively. Horizontal sections allow for the assessment of follicular density and the hair cycle, while vertical sections help identify inflammatory infiltrates and fibrosis [33]. There are also inflammatory markers that detect the presence of lymphocytic or neutrophilic infiltrates, which can indicate specific types of alopecia (such as lichen planopilaris or folliculitis decalvans) [33].
Overall, histopathological examination remains a gold standard for diagnosing and managing hair loss disorders. It provides detailed insights into the health and behavior of hair follicles. This method complements clinical evaluation and imaging studies and is especially helpful when standard treatments fail or when the diagnosis is uncertain [2].
GEP can also be used as a molecular marker, as GEP can identify the upregulation or downregulation of genes involved in hair follicle cycling and inflammation. For example, increased expression of pro-inflammatory cytokines like IL-1 and TNF-α can indicate inflammatory alopecia [29]. Another form of molecular marking is via immunohistochemistry. This technique can detect specific proteins associated with hair follicle health. For instance, reduced expression of hair keratins and increased expression of markers like Ki-67 (a proliferation marker) can provide insights into the regenerative capacity of hair follicles [29].
The following are clinical case reports/trials on the above markers, highlighting the insight they provided into alopecia causes and treatment:
- A study on androgenetic alopecia reported using scalp biopsy to identify miniaturized hair follicles and perifollicular fibrosis in patients with the condition. The findings guided the use of anti-androgen therapy, which resulted in significant hair regrowth [29].
- Another report on alopecia areata highlighted the identification of lymphocytic infiltrates around hair follicles in a patient with the condition. The biopsy results supported the use of immunosuppressive therapy, which led to hair regrowth [29].
- A histopathological study emphasized the role of horizontal sections in scalp biopsy specimens for diagnosing male pattern AGA. It shows how histopathological examination can differentiate AGA from other forms of alopecia by identifying specific features like follicular miniaturization and a decrease in terminal to vellus hair ratio [31].
The following are clinical case reports/trials that have explored the use of stem cells in hair restoration:
- A study involving 22 participants (half men, half women) reported significant increases in hair growth following stem cell treatment compared to a placebo. However, some adverse effects, including post-procedure pain, were noted [34].
- Another trial demonstrated that hair follicle-derived mesenchymal stem cells (HF-MSCs) decreased hair loss and reduced inflammation in alopecia areata mouse models [35].
- A case report highlighted the successful use of stem cell therapy in a patient with androgenetic alopecia, showing significant hair regrowth and improved hair density [35].
- Another report documented hair regrowth in alopecia areata patients following Stem Cell Educator therapy, which produced lasting improvement in hair regrowth [36].
Despite the promising potential of stem cell use in hair follicle regeneration, several challenges remain. One concern is immune rejection, as ensuring the compatibility of stem cell-derived tissues with the host immune system is crucial. There is also the issue of monitoring long-term safety to identify potential adverse effects, such as tumor formation. Developing scalable and cost-effective methods for producing stem cell-derived hair follicle cells is also necessary for widespread clinical application.
Advances in hair transplantation and stem cell therapy are promising for improving outcomes in patients with hair loss. Integrating biomarkers as diagnostic and prognostic tools has further enhanced the management of hair loss. While challenges remain, ongoing research and clinical trials continue to push the boundaries of what is possible in hair regeneration. Integrating stem cell therapy into clinical practice could revolutionize the treatment landscape, offering new hope to patients experiencing hair loss.
References
- Asfour, L., Cranwell, W., Sinclair, R. (2023). Male Androgenetic Alopecia. Endotext [Internet].
- Whiting, D. A. (1993). Diagnostic and Predictive Value of Horizontal Sections of Scalp Biopsy Specimens in Male Pattern Androgenetic Alopecia. Journal of the American Academy of Dermatology, 28(5): 755-763.
- Miteva, M., & Tosti, A. (2012). Pathologic Diagnosis of Central Centrifugal Cicatricial Alopecia on Horizontal Sections. American Journal of Dermatopathology. 34(6): 637-644.
- Kossard, S. (1994). Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. 130(11): 1407.
- Headington, J. T. (1984). Transverse Microscopic Anatomy of the Human Scalp: A Basis for a Morphometric Approach to Disorders of the Hair Follicle. Archives of Dermatology, 120(4): 449-456.
- Gupta, A. K., & Charrette, A. (2015). Topical Minoxidil: Systematic Review and Meta-Analysis of Its Efficacy in Androgenetic Alopecia. Skin Therapy Letter, 20(5): 5-9.
- Devjani, S., Ezemma, O., Kelley, KJ., Stratton, E., Senna, M. (2023). Androgenetic Alopecia: Therapy Update. Drugs. 83(8): 701-715.
- Avci, P., Gupta, GK., Clark, J., Wikonkal, N., Hamblin, MR. (2014). Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med.46(2): 144-51.
- Paichitrojjana, A., Paichitrojjana, A., (2022). Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des Devel Ther. 16: 635-645.
- Gentile, P., Garcovich, S. (2020). Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int J Mol Sci. 21(8):2702.
- Yao, J., Zhu, L., Pan, M., et al. (2024). The additive value of platelet-rich plasma to topical Minoxidil in the treatment of androgenetic alopecia: A systematic review and meta-analysis. PLoS One. 19(8).
- Alves, R., Grimalt, R. (2020). Platelet-Rich Plasma and its Use for Cicatricial and Non-Cicatricial Alopecias: A Narrative Review. Dermatol Ther (Heidelb). 10(4): 623-633.
- Tejapira, K., Yongpisarn, T., Sakpuwadol, N., Suchonwanit, P. (2022). Platelet-rich plasma in alopecia areata and primary cicatricial alopecias: A systematic review. Front Med (Lausanne). 9: 1058431.
- Cervelli, V., Garcovich, S., Bielli, A., et al. (2014). The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014.
- Danilenko, D. M., Ring, B. D., Yanagihara, D., et al. (1995). Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 147(1): 145-54.
- Hébert, J. M., Rosenquist, T., Götz, J., Martin, G. R. (1994). FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 78(6): 1017-25.
- Rosenquist, T. A., Martin, G. R. (1996). Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 205(4): 379-86.
- Ayatollahi, A., Hosseini, H., Shahdi, M., et al. (2017). Platelet-rich Plasma by Single Spin Process in Male Pattern Androgenetic Alopecia: Is it an Effective Treatment? Indian Dermatol Online J. 8(6): 460-464.
- Maletic, A., Dumic-Cule, I., Brlek, P., Zic, R., Primorac, D. (2022). Autologous Platelet-Rich Plasma (PRP) for Treating Androgenetic Alopecia: A Novel Treatment Protocol Standardized on 2 Cases. J Clin Med. 11(24): 7327.
- Prysak, M. H., Kyriakides, C. P., Zukofsky, T. A., et al. (2021). A retrospective analysis of a commercially available platelet-rich plasma kit during clinical use. PM R. 13(12): 1410-1417.
- Dhurat, R., Sukesh, M. (2014). Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg. 7(4): 189-97.
- Tsai, J., Garza, L.A. (2022). Cells and Structures Involved in Hair Follicle Regeneration: An Introduction. In: Jimenez, F., Higgins, C. (eds) Hair Follicle Regeneration. Stem Cell Biology and Regenerative Medicine, vol 72. Humana, Cham.
- Kang, D., Liu, Z., Qian, C., et al. (2023). 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 165: 19-30.
- Lama, S. C. (2023). Kintor Announces Update on KX-826 (Pyrilutamide) for Hair Loss. HairScience.
- Coelho, S., (2021). Stem Cell Hair Transplants: Cost, Effectiveness & More. Hims.
- Park, BS., Choi, HI. (2022). Stem Cell-Based Therapies for Hair Loss: What is the Evidence from a Clinical Perspective?. In: Jimenez, F., Higgins, C. (eds) Hair Follicle Regeneration. Stem Cell Biology and Regenerative Medicine, Humana, Cham. 72.
- Pinto, A., Terskikh, A.V. (2022). Induced Pluripotent Stem Cell Approach to Hair Follicle Regeneration. In: Jimenez, F., Higgins, C. (eds) Hair Follicle Regeneration. Stem Cell Biology and Regenerative Medicine, vol 72. Humana, Cham.
- Li, K., Liu, F., He, Y., et al. (2023). The homing of exogenous hair follicle mesenchymal stem cells into hair follicle niches. JCI Insight.
- Varothai, S., Bergfeld, W.F. (2014). Androgenetic Alopecia: An Evidence-Based Treatment Update. Am J Clin Dermatol 15: 217–230.
- Sperling, L. C., Cowper, S. E. (2006). The histopathology of primary cicatricial alopecia. Seminars in Cutaneous Medicine and Surgery. (1): 41-50.
- Rakowska, A., Slowinska, M., Kowalska-Oledzka, E., et al. (2012). Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 11(6): 753-8.
- Gurusamy, U., Venkataswamy, C., Sivaraman, A. (2018). Evaluation of Alopecia: A New Processing Technique Combining Vertical and Transverse Sections from a Single Scalp Biopsy Specimen. Int J Trichology. 10(1): 11-16.
- Wa?kiel-Burnat, A., Sadoughifar, R., Lotti, TM., Rudnicka, L. (2022). Clinical Cases in Hair Disorders. Springer Cham.
- Pozo-Pérez, L., Tornero-Esteban, P. & López-Bran, E. (2024). Clinical and preclinical approach in AGA treatment: a review of current and new therapies in the regenerative field. Stem Cell Res Ther 15: 260.
- Deng, W., Zhang, Y., Wang, W. et al. (2021). Hair follicle-derived mesenchymal stem cells decrease alopecia areata mouse hair loss and reduce inflammation around the hair follicle. Stem Cell Res Ther 12: 548.
- Li, Y., Yan, B., Wang, H. et al. (2015). Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med 13: 87.
Citation: Nepton Sheikhkhoni, Timothy Allen, Andres Felipe Gonzalez, Ariella Allen and William Moradi. (2024). Advances in Hair Loss Treatment and the Use of Stem Cells. Journal of Biotechnology and Immunology 6(1). DOI: 10.5281/zenodo.13865442
Copyright: © 2024 Timothy Allen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
