Short Communication
Volume 1 Issue 1 - 2019
Adrenocortical Response in Influenza
Center of Theoretical Problems of Physico-Chemical Pharmacology (until 2007), Moscow, Russia
*Corresponding Author: Irma Th. Rass, Center of Theoretical Problems of Physico-Chemical Pharmacology (until 2007), Moscow, Russia.
Received: November 19, 2019; Published: December 03, 2019
Abstract
Blood levels of 11-oxycorticosteroids and tyrosine were determined in thirty-three patients hospitalized because of severe influenza. The data were treated based on the assumption that the extremely high blood level of tyrosine could be caused by the combined action of two factors: 1. Intoxication of the liver resulting in its inability to synthesize tyrosine aminotransferase (TAT), the major enzyme of tyrosine metabolism and 2. Insufficient entrance of glucocorticoids required for induction of TAT synthesis. The adrenocortical response was dramatically different in 11 patients with severe and long-term course of the disease and in 9 patients with relatively light course of influenza who initially had nearly the same level of the liver intoxication. Thus, the course of the acute infection was associated with the presence or absence of the pronounced and well-timed adrenocortical response.
Key words: Influenza; Adrenocortical response; Blood tyrosine
During epidemics, influenza affects sometimes to 50% of population and up to now is not controlled sufficiently because of diversity and variability of influenza viruses. However, besides the influenza virus, there is a host organism for which activation of the hypothalamic-pituitary-adrenal axis seems to be the major feature of the organism response to stress, in particular, caused by the infection with the influenza virus. Nevertheless, it is unclear whether the contribution of this activation to the course of the disease is favorable or unfavorable, because of the pleiotropic effects of glucocorticoid hormones [1, 2]. On the other hand, glucocorticoid preparations are commonly prescribed at severe influenza (usually at influenza pneumonia), but results of clinical studies on their effect are controversial and there is uncertainty over their potential benefits or harm [reviews 3, 4]
The work I would like to remind is only a remark to the discussion about the role of glucocorticoid hormones in influenza and about the reason for using glucocorticoid preparations.
This work, initiated by Prof. RM Zaslavskaya, was performed in February 1977 in Moscow during epidemics of influenza caused by the group a virus. The results were published in Russian [5], but, despite the small number of patients under study, they seem to contribute to understanding the role of adrenocortical response in the course of influenza.
Thirty-three patients hospitalized because of their severe conditions were under study. Blood samples were taken on empty stomach on the next morning (usually it was the 3rd–4th day from the beginning of the disease) and then on the 7th–8th, 13th–14th, and 20th–21st days of the disease. In these samples contents of 11-oxycorticosteroids and tyrosine were determined. 11-oxycorticosteroids were measured as described in [6], blood tyrosine was measured as described in [7].
The level of 11-oxycorticosteroids characterized the general activity of the adrenal cortex. Blood tyrosine is not a routine laboratory parameter, but we measured it basing on some features of its metabolism [8] and on data of the works [9, 10] (reproduced in English in [11, 12]). Blood tyrosine level virtually depends on activity of the liver enzyme tyrosine aminotransferase (TAT) synthesized by the liver cells as tyrosine enters the liver, but for this synthesis glucocorticoids are also required. Thus, it was reasonable to assume that TAT synthesis, and as a result the level of blood tyrosine should depend on two factors: 1. the functional competence of the liver and 2. Entrance of glucocorticoids into the liver.
The data were processed after the patients were discharged from the hospital. According to the clinical course of influenza, the patients were subdivided by the clinicians into two groups: 11 patients (15–69 years old, on average 40 years) with a long-term severe complicated course of the disease (pneumonia in 9 (bilateral in 6, acute tracheobronchitis and lacunar angine in 2 patients) (the 1st group) and 22 patients with a relatively light course of the disease (the 2nd group). The 1st group patients were in the hospital from 15 to 55 days (26.5 days, on average), the 2nd group patients were in the hospital from 5 to 16 days (10.1 days, on average).
In the 1st group blood tyrosine level was significantly increased in all patients in 22 of 40 samples during the hospitalization, being on average 102.5 ± 19.4 µg/ml on the 3d day of the disease. In 16 healthy donors blood tyrosine level was 16.2 ± 0.9 µg/ml. When processing the data for the 2nd group, we distinguished the patients with blood tyrosine level above 53 µg/ml at least in one sample. This threshold value corresponding to the doubled upper limit of the normal value was taken arbitrarily, but on taking into account data of the previous work at exacerbation in SLE (on average 49.1 ± 0.8 µg/ml) [8]. In the 2nd group values above this conventional threshold were recorded in 9 patients (16–70 years old, on average 31 years) only at the first determination and was, on average (90.2 ± 11.4 µg/ml). In five of these 9 patients of the 2nd group there were no complications, in one was local pneumonia and in three – acute tracheobronchitis.
We assumed that such very high values of blood tyrosine could be a result not only of a probable insufficient entrance of glucocorticoids, but also of the liver intoxication resulting in the inability of the liver cells to synthesize the required amount of TAT. Thus, it was admitted that 11 patients with the severe course of influenza and these 9 patients of the 2nd group with the relatively light course had virtually the same “intoxication start”.
Blood tyrosine behavior during the disease was essentially different in these groups (Figure 1) tyrosine level remained increased in all the “severe” patients, whereas in 9 patients of the 2nd group it decreased nearly to normal level already by the 14th day (Figure 1). In the remaining 13 patients of the 2nd group blood tyrosine was slightly above normal during the hospitalization (in 9 patients there were no complications, in two – local pneumonia, in two – acute bronchitis).
Changes in the level of 11-oxycorticosteroids characterized the adrenocortical response during the disease. This response occurred to be dramatically different in the patients of these two groups. In the “severe” patients, the level of hormones by the moment of hospitalization (i.e. by the 3rd–4th day of the disease was, on average, not higher than in normal limits (19.3 ± 1.4 µg/100 ml for 25 healthy donors), and began to increase later. In 9 patients of 2nd group with the “same intoxication start” the level of hormones at the first measurement was significantly increased and remained elevated during the acute period of the disease (Figure 2). At the remaining 13 patients of the 2nd group the level of 11-oxycorticosteroids was above the normal level during the hospitalization.
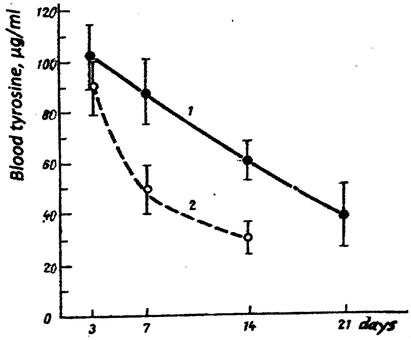
Figure 1: Blood level of tyrosine in patients with influenza during the hospitalization.
Curve1 – patients of the 1st group
Curve 2 – patients of the 2nd group with the high initial level of tyrosine.
Curve1 – patients of the 1st group
Curve 2 – patients of the 2nd group with the high initial level of tyrosine.
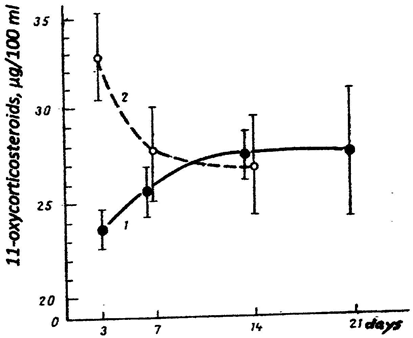
Figure 2: Blood level of 11-oxycorticosteroids in patients with influenza during the hospitalization.
Curve 1 – patients of the 1st group
Curve 2 – patients the 2nd group with the high initial level of tyrosine.
Curve 1 – patients of the 1st group
Curve 2 – patients the 2nd group with the high initial level of tyrosine.
Thus, at the severe course of influenza there was no normal adrenocortical response at the beginning of the disease, but, probably, the delayed increased activity of the adrenal cortex could create a background for secondary infections in this group. The relatively light course of influenza in the 2nd group patients was associated with a pronounced and well-timed response of the adrenal cortex at the beginning of the disease.
Therefore, our remark to the discussion is the following: it seems physiologically justified to give glucocorticoid preparations in influenza at severe conditions, but only during the first 1-3 days.
References
- Jamieson AM, Shuang Y, Annicelli CH and Medzhidov R. (2010). Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe 2010 7(2): 103-114.
- Quatrini L, Wieduwild E, Escaliere B, Filtjens J, Chasson L et al. (2018). Endogenous glucocorticoids control host resistance through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol 19(9): 954-962.
- Ni Yn, Chen G, Sun j, Liang BM, Liang ZA. (2019). The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 23(1): 99.
- Lansbury L, Rodrigo C, Leonardi-Bee J, Nguen-Van-Tam J, Lim WS. (2019). Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Library, published 24 February 2019.
- Rass IT, Zaslavskaya RM, Zolotaya RD and Shekhaeva OM (1978). Changes in the adrenal cortex activity and blood tyrosine content in influenza (in Russian), Terap Arkhiv 60 (12): 107-109.
- Pankov YA, Usvatova IY. (1965). Fluorimetric determination of 11-oxycorticosterids in peripheral blood, in Methods of Investigation of Some Hormones and Mediators (in Russian), Moscow 137-145.
- Udenfriend S and Cooper JR. (1952). The chemical estimation of tyrosine and tyramine. J Biol Chem 196: 227-233.
- Knox WE. (1955). Metabolism of phenylalanine and tyrosine, in A Symposium on Amino Acid Metabolism. Baltimore 836-866.
- Rass IT, Borisov IA, Nikishova TA and Sura VV. (1977). Blood tyrosine dynamics and treatment with corticosteroids in systemic lupus erythematosus (in Russian). Terap Arkhiv 59(8): 110-115.
- Rass IT. (1978). The usage of corticosteroid hormones and tyrosine metabolism (in Russian). Patol Fiziologiya No. 2: 87-91.
- Rass IT. (2010). Blood content of tyrosine is an index of glucocorticoid action on metabolism. Biochemistry (Moscow) 75(3): 431-446.
Citation: Irma Th. Rass. (2019). Adrenocortical Response in Influenza. Journal of Biotechnology and Immunology 1(1).
Copyright: © 2019 Irma Th. Rass. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
