Research Article
Volume 4 Issue 1 - 2022
Evaluation of Ocular Surface (Conjunctiva and Cornea) Characteristics in Patients with Thyroid Associated Ophthalmopathy
1Department of Ophthalmology, King George’s Medical University, Lucknow, India
2Department of Pathology, King George’s Medical University, Lucknow, India
3Department of Centre for Advance Research, King George’s Medical University, Lucknow
2Department of Pathology, King George’s Medical University, Lucknow, India
3Department of Centre for Advance Research, King George’s Medical University, Lucknow
*Corresponding Author: Apjit kaur, Professor and Head, Department of Ophthalmology, King George’s Medical University, Lucknow, India.
Received: April 06, 2022; Published: May 12, 2022
Abstract
Background: Thyroid associated ophthalmopathy (TAO) is one of the most common orbital inflammatory disorders also affecting ocular surface. This study was planned to investigate the effect of severity of TAO on corneal hysteresis, and grades of squamous metaplasia.
Methods: This is a tertiary centre based observational study. 31 consecutive patients of TAO were enrolled. Grading of TAO done as per European Group of Graves’ Orbitopathy classification. Corneal hysteresis obtained on Ocular Response Analyser. Impression cytology samples obtained and evaluated for grades of squamous metaplasia.
Results: 71% patients had moderate grade TAO. Bimodal age distribution was noted. Average corneal hysteresis in mild and moderate grade TAO was 10.7 and 9.3 and average grading of squamous metaplasia in mild and moderate grade TAO was 1.8 and 2.3 respectively. The variance of corneal hysteresis and squamous cell metaplasia with severity of TAO was statistically significant. Furthermore, correlation between corneal hysteresis and intraocular pressure was also significant.
Conclusion: In this study we highlight the fact that severe grades of TAO are associated with lower values of CH, thus adversely affecting the biomechanical property of cornea. Further we reported that mild TAO patients had lesser grades of squamous metaplasia as compared with moderate TAO.
Keywords: Thyroid Associated Ophthalmopathy; Corneal hysteresis; Squamous metaplasia; EUGOGO
Introduction
Thyroid associated ophthalmopathy (TAO) is one of the most common orbital inflammatory disorders. Autoantibodies present in thyroid gland and extrathyroidal sites result in the autoimmune reaction that affects not only thyroid gland but also lacrimal gland and orbital fibroblasts leading to the progression of disease. Activation of orbital fibroblasts leads to a series of events causing inflammation and expansion of orbital tissues, which plays a vital role in the pathogenesis of the disease. This further result in structural and functional changes in cornea and conjunctiva, resulting in alteration in ocular surface. Various previous studies have established that the Dry eye disease (DED) associated with TAO is mainly responsible for the ocular discomfort seen in patients of TAO [1-4]. Symptoms of ocular surface disease include excess tearing, gritty sensation and photophobia.
Squamous metaplasia has been demonstrated on impression cytology samples in cases of DED. This has been proposed as a non-specific indicator of ocular surface disease [5-8]. Cornea is affected in all the stages of the disease activity and severity of TAO [22]. The morphological changes are manifested as alteration of corneal hydration, rigidity, and bio-elasticity [9]. These parameters are not addressed by the GAT (Goldmann Applanation Tonometer). This disadvantage of GAT adversely affects the accurate Intra ocular pressure (IOP) measurement in patients with thyroid ophthalmopath [10,11]. The risk of ocular hypertension is increased in the patients with TAO by almost 8.5% [12]. Thus, there is a need for accurate measurement of intraocular pressure to avoid irreversible consequences. The newer parameters evaluating the biomechanical characteristics of the cornea have attracted interest in the recent past. Ocular Response Analyzer (ORA) is a device which provides a non-contact method of IOP measurement, thus eliminating the bias on account of the visco-elasticity and thickness of the cornea [13,14]. Schirmer’s tests and TBUT also provide valuable information regarding the pathophysiology of DED in patients of TAO.
Changes in the biomechanical properties of cornea in patients of TAO are often overlooked which adversely affects the intraocular pressure assessment. Proper assessment of biomechanical property of cornea reflected by measurement of Corneal hysteresis values highlights the importance of Ocular Response Analyser in the understanding and management of ocular surface disorder in patients of TAO. Therefore this study was planned to evaluate the ocular surface characteristics in the form of cytological changes, and corneal biomechanics in patients of TAO and correlate the outcomes with the EUGOGO grading.
Materials and Methods
Study Design
This is a Tertiary care centre based prospective observational study from July 2020 to August 2021.
This is a Tertiary care centre based prospective observational study from July 2020 to August 2021.
Case selection
Cases were selected according to the following inclusion and exclusion criteria:
Cases were selected according to the following inclusion and exclusion criteria:
Inclusion Criteria
- Clinical diagnosis of Thyroid associated ophthalmopathy (TAO), defined by the presence of at least one of the following signs of TAO: lid lag, lid retraction, ophthalmoplegia, proptosis, periorbital oedema
- Evidence of extraocular muscle enlargement with sparing of tendons seen on orbital imaging (MRI)
Exclusion Criteria
- Patients diagnosed with pre-existing ocular surface disorders like: dry eye disease, allergic ocular surface disease, pterygium before the onset of thyroid eye disease, corneal opacity resulting from any corneal injury
- Patients who have received medications/ treatment that might affect ocular surface parameters (e.g., rheumatoid arthritis, contact lens wear, steroids, topical anti glaucoma drugs use, radiation therapy)
- Patients who had undergone previous anterior segment surgery.
Methodology
This study was approved by the institutional review board. Informed written consent was taken in each case regarding the purpose of the study and also for publication of data thereafter.
This study was approved by the institutional review board. Informed written consent was taken in each case regarding the purpose of the study and also for publication of data thereafter.
31 consecutive patients of TAO were enrolled from July 2020 to August 2021 who either consulted in ophthalmology OPD, thyroid clinic Department of ophthalmology, or were referred from the department of Medicine or Endocrinology King George’s Medical University. Selection of cases was done according to the inclusion and exclusion criteria.
Detailed history was elicited to rule out exclusion criteria. Following this, all the subjects underwent general and ophthalmological examination. This includes best corrected visual acuity (BCVA), slit lamp examination, proptosis measurement using Hertel exophthalmometer, ocular surface evaluation using tear film breakup time (TBUT), Schirmer’s test, intra-ocular pressure using Goldmann Applanation Tonometer (IOP-GAT), Central Corneal Thickness (CCT) using contact pachymeter (Pacline Optikon), Ocular response analyser (ORA) measurements, fundus examination with Indirect Ophthalmoscopy, and visual field examination in Humphrey Visual Field Analyser in suspicious optic discs. Disease activity was documented and graded according to Clinical Activity Score (CAS) and EUGOGO grading respectively. Following parameters were assessed in ORA: CH measuring corneal elasticity, CRF measuring the corneal visco-elastic property, IOPg (average of the two pressure waves generated) and IOPcc. The ORA measurements were made before instilling any topical medication and before any contact procedure. Readings with waveform <5.5 were discarded [15]. To increase the intra-observer reliability of the test, the mean of two consecutive measurements made by one operator was recorded. To increase the inter-observer reliability, the measurement done by one operator was cross checked by another operator, masked to the previous measurement, in the same session. To avoid the diurnal effects, all measurements were done in primary gaze and within minutes. Schirmer’s test was performed on every patient in a well-lit room. Two Schirmer’s strips were removed from the sterile packet, and a 90 degree bend was created at the notch at the upper end of the strip. Lower eyelid of the patient was gently pulled down and instructed to look up. The bent end of the strip was then hooked at the junction of lateral 1/3rd and medial 2/3rd. The same procedure was repeated for each eye. Patient was asked not to squeeze the eyes shut. Strip was removed carefully after 5 minutes. Using the scale given on the strips, the reading was noted in mm for each eye. TBUT testing was performed in a dark room while the patient was sitting with the help of a slit-lamp biomicroscope. After explaining the procedure, one drop of 2% fluorescein solution was instilled into the inferior fornix of the patient. Alternatively a pre-impregnated fluorescein strip moistened with preservative free lubricant drop, can also be used to deliver fluorescein into the lower fornix of the patient. Eye was examined under a slit-lamp biomicroscope with a broad beam and low magnification, to cover the whole of the cornea in a single view. Then the filter was switched to a cobalt blue filter in the slit-lamp. Patient was then instructed to blink once and to keep them open for as much time as possible. Under the cobalt blue filter with fluorescein stained ocular surface, a greenish tear film layer is seen over the cornea. A randomly distributed black spot over the cornea denotes the breakup of the tear film. The time was noted from the blink of the patient till the appearance of the first black spot in an uninterrupted eye. This gives us the TBUT. Then the fluorescein is wiped off from the patient’s eye. The procedure is repeated for each eye.
Samples for impression cytology were obtained in the department of pathology. A cellulose acetate filter paper (Millipore MAW304) was cut into 4 x 4 mm strips having a pointed tip on one of the corner which helps in easy manipulation of the filter paper by a non-toothed plain forcep. After instilling topical anaesthesia (0.5% Propacaine solution), that strip of cellulose acetate filter paper placed onto the temporal and nasal bulbar conjunctiva of both the eyes sequentially. Prism head of Goldmann applanation tonometer was used to apply pressure over the strips for 3-5 sec and then peeled off. The filter paper then transferred to glass slides and fixed with 95% ethanol. Samples were then stained with periodic acid-Schiff (PAS) stain according to the protocol described by Tseng [16], and permanently mounted. The stained slides were then examined by light microscopy for grading of squamous metaplasia. Grading of squamous metaplasia was done according to Nelson’s grading scheme used by Grene and Lankston [17]. Single slides often contain variation in the grading of squamous metaplasia. So for analysis of squamous metaplasia grade, 10 high power fields of 400X projecting 1mm x 1mm fields were examined in each slide. Average of these 10 areas were then noted.
All patients underwent serum T3, T4 and TSH analysis in department of Biochemistry to evaluate the thyroid status of the patients. Also, all the patients underwent treatment for hyperthyroidism with antithyroid medications and hypothyroid patients were given dose adjusted oral thyroid hormone as per the Medicine or Endocrinology opinion.
Covid-19 protocol is followed strictly during the conduct of this study.
Statistical Analysis
All the statistical analysis were performed using IBM SPSS 2.0 and Microsoft excel 2016 for windows. Descriptive analysis was performed to characterize the study population. Descriptive statistics were also used to characterize IOP, CH, grades of Squamous metaplasia, TBUT and Schirmer’s test in patients of mild and moderate EUGOGO grade of TAO.
All the statistical analysis were performed using IBM SPSS 2.0 and Microsoft excel 2016 for windows. Descriptive analysis was performed to characterize the study population. Descriptive statistics were also used to characterize IOP, CH, grades of Squamous metaplasia, TBUT and Schirmer’s test in patients of mild and moderate EUGOGO grade of TAO.
Charan Biswas formula [18] was used for calculation of sample size:
Sample Size = Z2(p) (1 – p)/C2
Where, Z= 1.96 for 95 percent confidence level
p (prevalence) = 2%
C (margin of error) = 0.05
Sample Size = Z2(p) (1 – p)/C2
Where, Z= 1.96 for 95 percent confidence level
p (prevalence) = 2%
C (margin of error) = 0.05
To analyse the variation of CH, IOP, grades of Squamous metaplasia, TBUT and Schirmer’s test among the mild and moderate EUGOG grade of TAO unpaired student-t test was applied. Pearson correlation test was used to analyse the relationship between CH and IOP on applanation tonometer. It was also used to study the corelation of TBUT and Schirmer’s test with grades of Squamous metaplasia. P value <0.05 is taken as significant.
Results
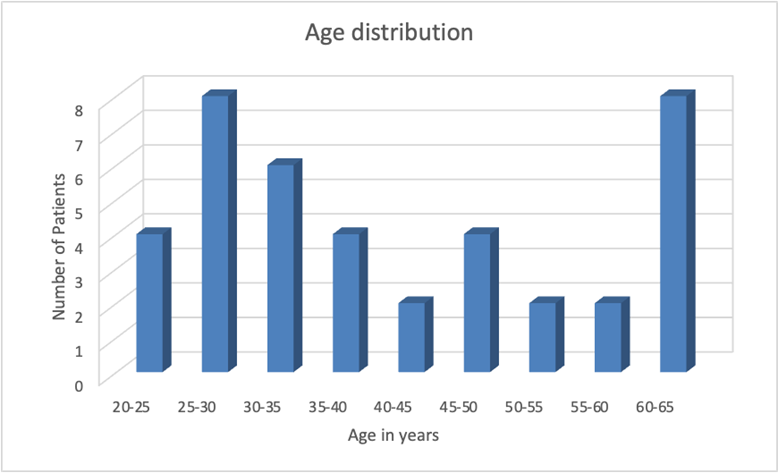
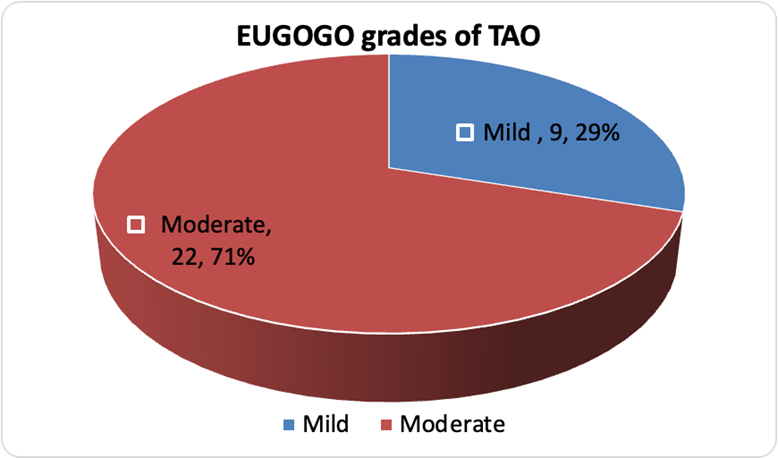
31 patients of TAO were enrolled in the study. 62 eyes of 31 patients were evaluated. Mean age group was 41.45 years (Table 1). A bimodal distribution of age groups was seen, with first peak during 2nd-3rd decade and second peak during 6th decade (Figure 1). Out of the 31 patients, 17 (55%) were males, and 14 were (45%) were females. The total number of patients with mild and moderate grade of TAO were depicted in the figure (Figure 2).
| Age distribution in years | |
| Mean | 41.45 |
| Median | 37 |
| Mode | 20 |
| Standard Deviation | 15.1419777 |
| Sample Variance | 229.279487 |
| Kurtosis | -1.2447865 |
| Skewness | 0.34007168 |
| Range | 45 |
| Minimum | 20 |
| Maximum | 65 |
| Count | 62 |
Table 1: Table showing descriptive statistical analysis of age (in years) distribution in patients of TAO.
The average IOP in mild and moderate grade TAO was 12.7 mmHg and 15.2 mmHg respectively (Table 2). The variance of IOP among the patients of mild and moderate TAO was found to be statistically significant (p= 0.003).
| Applanation IOP (in mmHg) in mild TAO | Applanation IOP (in mmHg) moderate TAO | ||
| Mean | 12.7083333 | Mean | 15.18214286 |
| Median | 12 | Median | 14.25 |
| Mode | 12 | Mode | 12 |
| Standard Deviation | 1.52402478 | Standard Deviation | 3.325997881 |
| Sample Variance | 2.32265152 | Sample Variance | 11.0622619 |
| Kurtosis | 0.25296091 | Kurtosis | -1.496684589 |
| Skewness | 0.88012485 | Skewness | 0.105494552 |
| Range | 5 | Range | 10 |
| Minimum | 11 | Minimum | 10 |
| Maximum | 16 | Maximum | 20 |
| Count | 18 | Count | 44 |
Table 2: Table showing descriptive statistical analysis of Intraocular pressure values in patients with mild and moderate EUGOGO grade of TAO.
Average CH in mild grade TAO was 10.7 and in moderate grade TAO was 9.3. (Table 3 & Figure 3). The variance of CH among the patients of mild and moderate TAO was found to be statistically significant (p= 0.01). A statistically significant correlation was found between CH and IOP on applanation tonometer (p = 0.001, r = -0.59) (Figure 4).
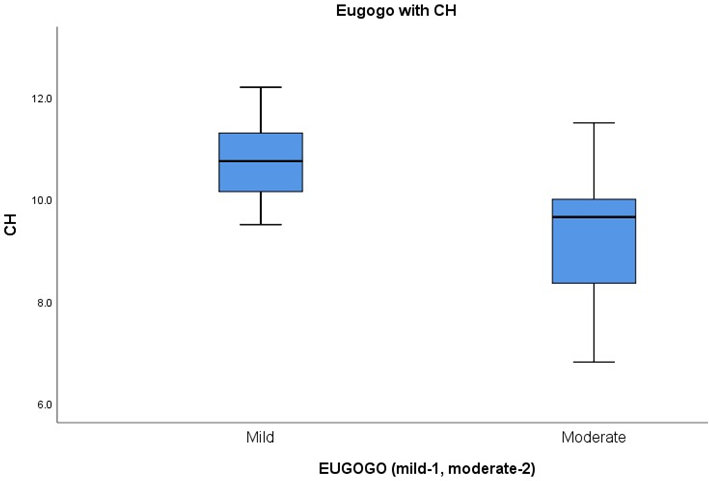
Figure 3: Box and Whisker plot of Corneal hysteresis, in patients of mild and moderate EUGOGO grade of TAO.
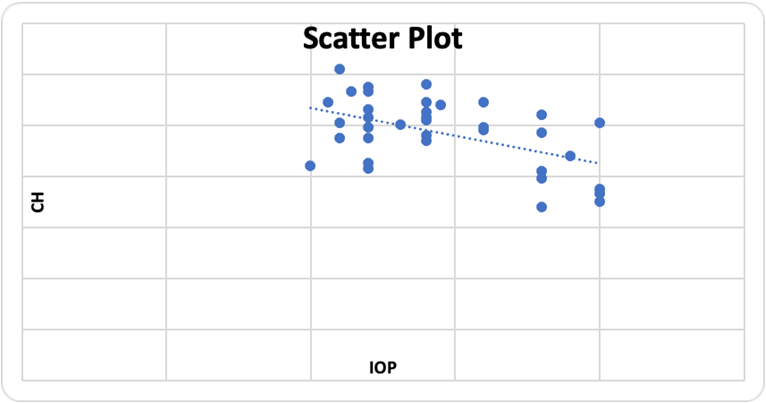
Figure 4: Scatter plot with trend line depicting the correlation between Intraocular pressure and Corneal Hysteresis, in patient of TAO.
| CH in Mild TAO | CH in Moderate TAO | ||
| Mean | 10.7416667 | Mean | 9.28571429 |
| Median | 10.75 | Median | 9.65 |
| Mode | 11.3 | Mode | 9.9 |
| Standard Deviation | 0.77160792 | Standard Deviation | 1.22888516 |
| Sample Variance | 0.59537879 | Sample Variance | 1.51015873 |
| Kurtosis | -0.3726171 | Kurtosis | -0.5187144 |
| Skewness | 0.29813327 | Skewness | -0.4828603 |
| Range | 2.7 | Range | 4.7 |
| Minimum | 9.5 | Minimum | 6.8 |
| Maximum | 12.2 | Maximum | 11.5 |
| Count | 18 | Count | 44 |
Table 3: Table showing descriptive statistical analysis of CH values in patients with mild and moderate EUGOGO grade of TAO.
Squamous metaplasia evaluation on impression cytology revealed average grading of squamous cells in mild grade TAO as 1.8 and in moderate grade TAO as 2.3 (Table 4 & Figure 5). The variance of the squamous metaplasia grades among the patients of mild and moderate TAO was found to be statistically significant (p= <0.001).
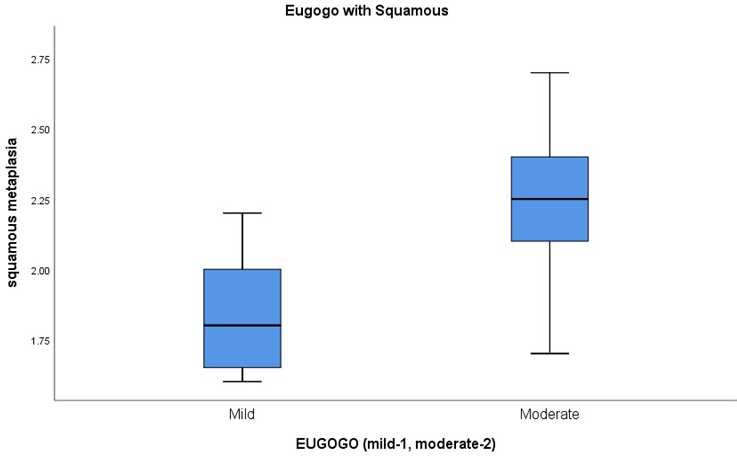
Figure 5: Box and Whisker plot of Squamous metaplasia, in patients of mild and moderate EUGOGO grade of TAO.
| Squamous metaplasia in Mild TAO | Squamous metaplasia in Moderate TAO | ||
| Mean | 1.8375 | Mean | 2.261111111 |
| Median | 1.8 | Median | 2.25 |
| Mode | 2 | Mode | 2.4 |
| Standard Deviation | 0.213390989 | Standard Deviation | 0.252374346 |
| Sample Variance | 0.045535714 | Sample Variance | 0.06369281 |
| Kurtosis | -0.702228374 | Kurtosis | -0.042831611 |
| Skewness | 0.523755508 | Skewness | -0.319540878 |
| Range | 0.6 | Range | 1 |
| Minimum | 1.6 | Minimum | 1.7 |
| Maximum | 2.2 | Maximum | 2.7 |
| Count | 18 | Count | 44 |
Table 4: Table showing descriptive statistical analysis of Squamous metaplasia in patients with mild and moderate EUGOGO grade of TAO.
Mean TBUT in mild grade of TAO was 9.37 sec while in moderate grade TAO the mean was 6 sec. A statistically significant difference of TBUT among the patients of mild and moderate grade TAO was found (p= 0.007). An insignificant negative correlation was found between TBUT and squamous metaplasia, (p= 0.725r= -0.072).
The average Schirmer’s value in mild TAO was 24.1 mm and in moderate TAO the average value of Schirmer’s was 14.6 mm. This difference of Schirmer’s test in patients of mild and moderate grade TAO was not statistically significant (p= 0.078). Pearson correlation analysis showed an insignificant correlation between Schirmer’s values and squamous metaplasia (p= 0.587, r= -0.112).
Discussion
Thyroid associated ophthalmopathy (TAO) also known as Thyroid eye disease (TED) is one of the most common orbital inflammatory disorders. With this study the authors aim to analyze the ocular surface characteristics in the patients of TAO.
This study was conducted during the surge of novel COVID-19 infection, from July 2020 to August 2021. The interpretations were derived keeping in mind the effect that COVID-19 might have in the outcome of the study. A wide range of age distribution was noted which was suggested by the large sample variance on descriptive analysis. Most of the patients in our study were middle aged individuals, which correlates with the previous published literature on TAO [19]. Our study documented a bimodal age distribution with first peak during 2nd to 3rd decade and second peak during 6th decade. Previous study had also reported similar observation [1]. However, in contrast to the published literature [20,21], male predominance was seen in our study. This might be due to the restrictions imposed during the COVID-19 surge. Hence it is proposed that males are more likely to present to the hospital while females are likely to restrain themselves at home during this COVID-19 pandemic. We document 70% patients with moderate and 30% with mild EUGOGO grade and no case of severe grade TAO. This finding is in contrast to what previously reported in literature [22]. Reddy et.al. in 2014 reported 83% prevalence of mild disease and 15% prevalence of severe disease in their study from northern India [22]. This disparity again, might be due to the ongoing COVID-19 pandemic situation where mild cases being less severe and having less impact on quality of life, do not venture out to seek medical advice considering the risk of getting COVID infected. Whereas patients with moderate grade TAO, which had a major impact on quality of life, had to seek medical help in spite of COVID-19 situation. Severe grade TAO, must be having other systemic comorbidities because of the thyroid disease, thus ophthalmic complaints might not be such a major concern for them.
It is now well established that inflammation and expansion of orbital tissues plays a key role in the pathogenesis of TAO, that results in structural and functional changes in cornea and conjunctiva, resulting in alteration in ocular surface. Following flow chart simplifies the pathophysiologic processes happening in a patients of TAO that leads to the constellation of signs and symptoms (Figure 6).
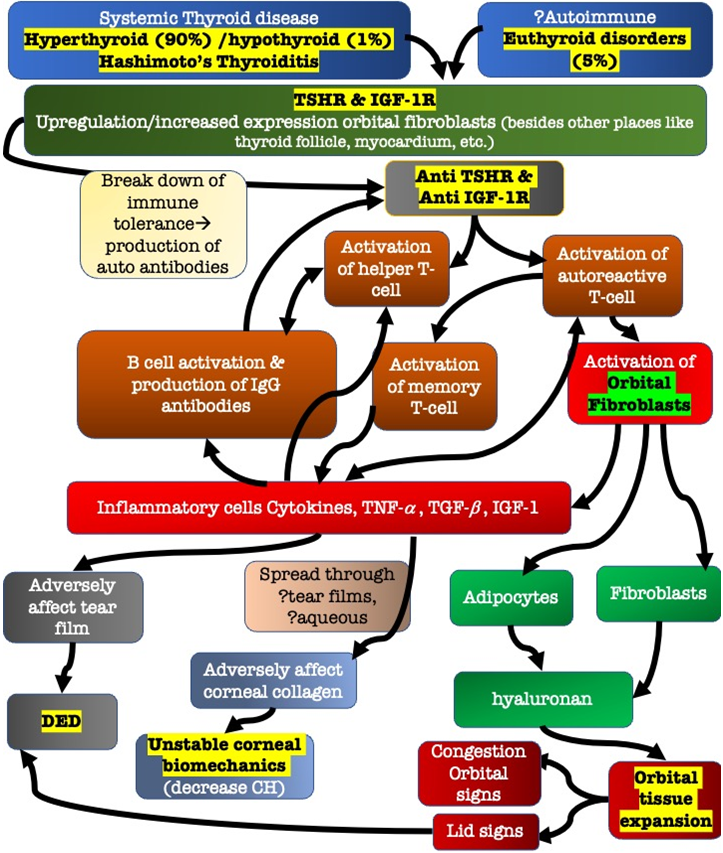
Figure 6: This figure depicts a simplified version of the pathophysiologic process happening in patients of TAO. The autoimmune basis of TAO is established where autoantibodies against TSHA & IGF-1R activates the immune system of our body. This further ignites a cascade of processes which activate orbital fibroblasts and inflammatory mediators, which further leads to the clinical manifestation of TAO. This chart also shed light upon the pathophysiologic process of DED and adversely affected corneal biomechanics in patients of TAO.
CH-Corneal Hysteresis, DED-Dry Eye Disease, IGF-1-Insulin like Growth Factor-1, IGF-1R-Insulin like Growth Factor-1 Receptor, TSHR-Thyroid Stimulating Hormone Receptor/Thyrotropin Receptor, TGF ?-Transforming Growth Factor-Beta, TNF?-Tumour Necrosis Factor-alpha.
CH-Corneal Hysteresis, DED-Dry Eye Disease, IGF-1-Insulin like Growth Factor-1, IGF-1R-Insulin like Growth Factor-1 Receptor, TSHR-Thyroid Stimulating Hormone Receptor/Thyrotropin Receptor, TGF ?-Transforming Growth Factor-Beta, TNF?-Tumour Necrosis Factor-alpha.
In this study we found that patients with moderate grade of TAO have lower values of CH, while those with mild grade of TAO have higher values of CH. The higher sample variance found on the descriptive statistics, suggests more variable corneal biomechanics in patients of moderate EUGOGO grade TAO. The difference of CH in mild and moderate grade of TAO was statistically significant, giving the impression that higher grades of TAO adversely affect the corneal biomechanical properties. The pathogenesis regarding how the corneal biomechanical property is affected is poorly understood. The inflammatory mediators playing a crucial role in the pathogenesis of TAO is expected to cause the unstable biomechanical property of the cornea. These mediators may reach the corneal tissue via tear film dynamics and aqueous current. Further studies are required for the in-depth understanding of the pathogenesis of biomechanical derangements of the cornea in patients of TAO. This study also demonstrated an increase in the mean IOP with an increase in the grade of TAO. However, the mean IOP in both mild and moderate grade TAO were not significantly raised. Thus, CH is negatively correlating with grade of TAO as well as IOP values of the patient. In other words, it can be said that the stress produced due to increase in IOP with higher grades of TAO along with the pathological changes happening in TAO, adversely affect the corneal biomechanics. This in turn affects the IOP measurement of patients with higher grades of TAO. In such patients IOPcc might be a more reliable measure of IOP assessment. Similar findings were also noted in other previous studies [23-28]. This emphasizes the importance of assessment of CH as a marker of corneal biomechanics, before initiating the anti-glaucoma treatments in higher grades of TAO. Or in other words a single value of raised IOP in higher grades of TAO doesn’t warrant initiation of anti-glaucoma treatment until and unless it is also associated with other clinical signs of glaucoma.
In our study we found that mild TAO patients had lesser grades of squamous metaplasia as compared with moderate TAO which was statistically significant. A statistically significant lower values of TBUT were found in moderate EUGOGO grade of TAO, while no statistically significant difference in Schirmer’s values were noted among the patients of mild and moderate EUGOGO grade TAO. This emphasizes the importance of both aqueous deficiency as well as meibomian gland dysfunction in the pathogenesis of DED in TAO. However, the statistically insignificant difference in Schirmer’s values, gives the impression that there is minimal effect on tear production in TAO, and emphasizes the role of evaporative dry eye in the causation of DED in TAO. The higher grades of squamous metaplasia in moderate grade TAO are due to the higher prevalence of DED in patients of TAO. However, in our study we couldn’t establish a statistically significant correlation between either TBUT or Schirmer’s values with grades of squamous metaplasia. A longitudinal follow-up of the patients is required to establish a stronger correlation.
With this study we emphasize the importance of the role of evaporative dry eye in the causation of DED in TAO. Conjunctival characteristics in the form of squamous metaplasia grades showed that severe grades of TAO are associated with higher grades of squamous metaplasia. This highlights the importance of initiation of dry eye specific treatment which can not only alleviate the patients of their symptoms but also will prevent further damage of conjunctiva. Furthermore, we study the corneal characteristics in the form of CH, which represents the biomechanical property of cornea. We highlight the fact that severe grades of TAO are associated with lower values of CH, thus adversely affecting the biomechanical property of cornea. Changes in the biomechanical properties of cornea in patients of TAO are often overlooked which adversely affects the intraocular pressure assessment. Proper assessment of biomechanical property of cornea reflected by measurement of CH values highlights the importance of ORA in the understanding and management of ocular surface disorder in patients of TAO.
Acknowledgement
The authors are grateful to the Vice Chancellor LT. GEN. (DR.) Bipin Puri, Mr. Akash Verma, staff in the record section, Ophthalmology and the residents in the department of ophthalmology,King George’s Medical University (KGMU) Lucknow, for the encouragement and support for this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors are grateful to the Vice Chancellor LT. GEN. (DR.) Bipin Puri, Mr. Akash Verma, staff in the record section, Ophthalmology and the residents in the department of ophthalmology,King George’s Medical University (KGMU) Lucknow, for the encouragement and support for this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Financial support: NIL
Proprietary interests/ conflict of interest: None of the authors have any proprietary interests or conflicts of interest related to this submission
Declaration: This submission has not been published anywhere previously and it is not simultaneously being considered for any other publication.
Ethic statement: This study was approved by the institutional review board of King George’s Medical University, Lucknow.
References
- Gürdal C, Saraç Ö, Genç ?, K?r?ml?o?lu H, Takmaz T, Can ?. (2011). Ocular surface and dry eye in Graves’ disease. Curr Eye Res. 36(1): 8-13.
- Versura P, Campos EC. (2010). The ocular surface in thyroid diseases. Curr Opin Allergy Clin Immunol. 10(5): 486-92.
- Ismailova DS, Fedorov AA, Grusha YO. (2013). Ocular surface changes in thyroid eye disease. Orbit. 32(2): 87-90.
- Selter JH, Gire AI, Sikder S. (2014). The relationship between Graves' ophthalmopathy and dry eye syndrome. Clin Ophthalmol. 9: 57-62.
- Adams GG, Dilly PN, Kirkness CM. (1988). Monitoring ocular disease by impression cytology. Eye. 2(5): 506-16.
- Kinoshita S, Kiorpes TC, Friend J, Thoft RA. (1983). Goblet cell density in ocular surface disease: a better indicator than tear mucin. Arch Ophthal. 101(8): 1284-7.
- Rivas L, Oroza MA, Perez?Esteban A, Murube?del?Castillo J. (1991). Topographical distribution of ocular surface cells by the use of impression cytology. Acta ophthalmol. 69(3): 371-6.
- Rolando M, Terragna F, Giordano G, Calabria G. (1990). Conjunctival Surface Damage Distribution in Keratoconjunctivitis sicca An Impression Cytology Study. Ophthalmologica. 200(4): 170-6.
- Yamada M, Li AW, Wall JR. (2000). Thyroid-associated ophthalmopathy: clinical features, pathogenesis, and management. Crit Rev Clin Lab Sci. 37(6): 523-49.
- Manni G, Oddone F, Parisi V, Tosto A, Centofanti M. (2008). Intraocular pressure and central corneal thickness. Prog Brain Res. 173: 25-30.
- Cross JM, Girkin CA, Owsley C, McGwin G. (2008). The association between thyroid problems and glaucoma. Br J Ophthalmol. 92(11): 1503-5.
- Behrouzi Z, Rabei HM, Azizi F, Daftarian N, Mehrabi Y, Ardeshiri M, et al. (2007). Prevalence of open-angle glaucoma, glaucoma suspect, and ocular hypertension in thyroid-related immune orbitopathy. J Glaucoma.16(4): 358-62.
- Goebels SC, Seitz B, Langenbucher A. (2012). Precision of ocular response analyzer. Curr Eye Res. 37(8): 689-93.
- Abitbol O, Bouden J, Doan S, Hoang?Xuan T, Gatinel D. (2010). Corneal hysteresis measured with the ocular response analyzer® in normal and glaucomatous eyes. Acta ophthalmol. 88(1): 116-9.
- Vantomme M, Pourjavan S, Detry-Morel M. (2013). The range of the waveform score of the ocular response analyzer (ORA) in healthy. Bull Soc Belg Ophthalmol. 322: 91-7.
- Tseng SC. (1985). Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 92(6): 728-33.
- Grene RB, Lankston P. (1990). Cartography of impression cytology. Cornea. 9(4): 275-8.
- Charan J, Biswas T. (2013). How to calculate sample size for different study designs in medical research?. Indian J Psychol Med. 35(2), 121-6.
- Sahli E, Gündüz K. (2017). Thyroid-associated ophthalmopathy. Turk J Ophthalmol. 47(2): 94.
- Bartley GB. (1994). The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 92: 477.
- Dolman PJ. Natural History of Thyroid Eye Disease. In Thyroid Eye Disease 2015 Springer, New York, NY. (pp. 13-22).
- Reddy SV, Jain A, Yadav SB, Sharma K, Bhatia E. (2014). Prevalence of Graves’ ophthalmopathy in patients with Graves’ disease presenting to a referral centre in north India. Indian J Med Res. 139(1): 99.
- Karabulut GO, Kaynak P, Altan C, Ozturker C, Aksoy EF, Demirok A, et al. (2014). Corneal biomechanical properties in thyroid eye disease. Kaohsiung J Med Sci. 30(6): 299-304.
- Zhang Y, She XX, Yu XJ, Chen LF, Shen LJ. (2016). The corneal biomechanical properties of patients with Graves' orbitopathy. [Zhonghua yan ke za zhi] Chin J Ophthalmol. 52(4): 263-7.
- Sullivan-Mee M, Billingsley SC, Patel AD, Halverson KD, Alldredge BR, Qualls C. (2008). Ocular Response Analyzer in subjects with and without glaucoma. Optom Vis Sci. 85(6): 463-70.
- Pniakowska Z, Klysik A, Gos R, Jurowski P. (2016). Corneal biomechanical changes and intraocular pressure in patients with thyroid orbitopathy. Int J Ophthalmol 9(3): 439.
- Moghimi S, Safizadeh M, Mazloumi M, Hosseini H, Vahedian Z, Rajabi MT. (2016). Evaluation of corneal biomechanical properties in patients with thyroid eye disease using ocular response analyzer. J Glaucoma. 25(3): 269-73.
- Pandey N, Kaur Chhabra A. (2021). Evaluation of corneal biomechanical properties on ocular response analyzer and their correlation with the clinical profile of the patients with thyroid-associated ophthalmopathy. Orbit. 40(3): 193-8.
Citation: Ashutosh Maharana, Nibha Mishra, Shalini Bhalla, Shailandra K. Saxena and Apjit Kaur. (2022). Evaluation of Ocular Surface (Conjunctiva and Cornea) Characteristics in Patients with Thyroid Associated Ophthalmopathy. Journal of Ophthalmology and Vision Research 4(1). DOI: 10.5281/zenodo.6544970
Copyright: © 2022 Apjit kaur. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.