Case Report
Volume 5 Issue 1 - 2024
Squamous Cell Carcinoma Developing in a Mature Cystic Teratoma of the Ovary: A Case Report
1Department of Obstetrics and Gynaecology, Kangaroo Care, Bangalore, India
2SHO at Glenfield Hospital, Department of Surgery Leceister
2SHO at Glenfield Hospital, Department of Surgery Leceister
*Corresponding Author: Al Ameen Asharaf, SHO at Glenfield Hospital, Department of Surgery Leceister.
Published: December 04, 2024
Abstract
Mature cystic teratoma is a benign condition where malignant transformation is an extremely rare event. A case of squamous cell carcinoma developing in a mature cystic teratoma of the ovary presenting at an early stage is presented here. A 48-year-old perimenopausal woman, presented with the complaints of discomfort in the lower abdomen since 1 year and a large abdomino- pelvic mass on examination. Final histopathology was reported as squamous cell carcinoma developing in a mature cystic teratoma of the right ovary and section from the tissue adherent to bladder wall showing malignant infiltration into fibro-adipose tissue. Patient was assigned to squamous cell carcinoma of the ovary arising in a mature cystic teratoma, surgical stage IIB.
Keywords: Dermoid cyst; Mature cystic teratoma; Malignant transformation; Squamous cell carcinoma of the ovary; Infiltrating into bladder wall
Introduction
Among teratoma types, mature cystic teratoma is by far the most common. This benign tumour comprises approximately 10% to 25% of all ovarian neoplasms and 60% of all benign ovarian neoplasms with a 0.17-1.4% reported incidence of malignant transformation. [1-4] Malignant transformation is typically seen in postmenopausal women. Squamous cell carcinoma is the most common type and is found in approximately 1% of mature cystic teratomas. Malignant areas are usually found as small nodules in the cyst wall or a polypoidal mass within the lumen after removal of the entire teratoma.
Platinum based chemotherapy with or without pelvic irradiation is most often used for adjuvant treatment of early stage disease. [1] It is well known that clinically advanced cases with squamous cell carcinoma arising from mature cystic teratoma have a poor prognosis partly because of its poor response to chemotherapy. [5]
Case Report
A 48-year-old perimenopausal woman, P4L4A1 presented to our out-patient department, with complains of pelvic discomfort since 1 year which increased in intensity over the past 6months. Her menstrual cycles were regular with history of spasmodic dysmenorrhea of recent onset. She reported no gastrointestinal or no genitourinary tract symptoms.
On examination: She was afebrile and her vitals were stable. Per abdominal examination revealed a tender, immobile mass of around 14 x 16 cm extending from right iliac fossa to 2cm below the umbilicus. Per speculum examination revealed an irregular, hypertrophied cervix with old tear at 3’o clock position with multiple nebothian follicles smeared with unhealthy discharge. Per vaginal examination revealed a small mobile cervix and a large abdomino-pelvic mass rising half way to umbilicus. The uterine fundus and adnexa could not be delineated from the mass. There was no cervical motion tenderness. She was admitted and further evaluated. Laboratory data like CBC, LFT, KFT, RBS and thyroid profile were within normal limits. Among Ovarian cancer markers, CA19- 9 was found to be raised (351U/ml). Rest of the markers and urine analysis were negative. Pap smear was negative for intra epithelial lesion or malignancy. Ultrasound pelvis showed uterus of normal size, shape and normal endometrial echotexture. Right ovary revealed a soft tissue lesion of 14.46 X 18.65cm having both cystic and echogenic areas seen in right adnexa suggestive of right Tubo-Ovarian mass. Left ovary was not visualised. MRI showed approximately 16X18x20cm large pelvic complex cystic lesion showing fat fluid level with Rokintansky protuberance arising from right ovary suggestive of Mature Ovarian Teratoma and left ovary seen separately and is normal. Uterus normal in shape, size and endometrial thickness. No evidence of significant regional nodes.
Patient developed fever preoperatively with Temperature of 102° F with increase in pain abdomen. Her fever profile was negative with slight.
Elevation in Total leukocyte Count (12,300cells/cumm) and marked rise in ESR (110 mm/1st Hour). She was managed conservatively with oral antibiotics and antipyretics. She was further planned for Total Abdominal Hysterectomy with bilateral Salphingo- oophorectomy. Intraoperatively, dense adhesions between the mass, omentum and anterior abdominal wall noted. Adhesiolysis done. A mass of around 20X20cm found to be arising from right ovary, which was adherent to uterine fundus, omentum and bladder. The mass was lobulated, variegated in consistency, with pre-operative rupture to omentum and bladder base with a sinus formation in the mass. The mass was careful dissected and removed. Bladder base wall was breeched upto muscle layer and was repaired by General Surgeon. Frozen section and Imprint cytology were done and no malignant cells were reported and the specimen was sent for histopathological examination. Plan for Total Abdominal Hysterectomy was cancelled due to dense adhesions and non-availability of surgical oncologist. Peritoneal lavage done and abdomen closed in layers. Patient was referred to surgical oncology for further management after the histopathology report showed malignant transformation. Patient underwent surgical staging with total abdominal hysterectomy with bilateral salphingo-oopherctomy, infracolic omentectomy and pelvic, para aortic lymph node dissection. Histopathology of the above samples did not show any malignant spread. She further received adjuvant chemotherapy.
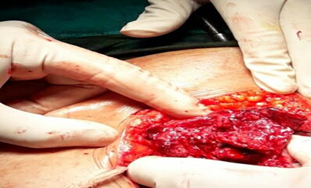
Figure 1: Intraoperative dense adhesions noted between the ovarian tumour, abdominal wall, omentum and bladder base.
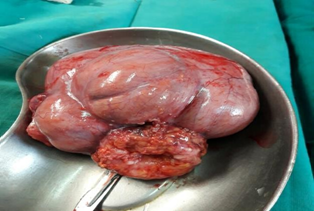
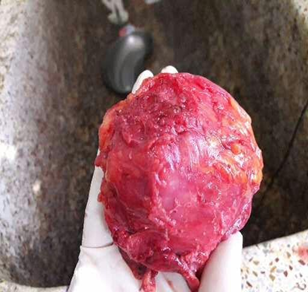
Figure 2: A lobulated and an irregulary enlarged ruptured cystic mass of right ovary with sinus formation.
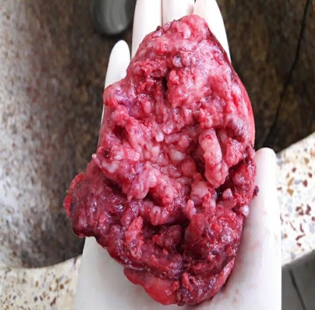
Figure 4: Gross image showing irregular, lobulated surface of the ovarian mass with papillary excrescences.
Histopathological Examination
Gross
First specimen showing an irregular bosellated grayish brown cystic tissue measuring 13x12x5cm. An irregular whitish firm area identified on the external surface measuring 6x4.6 cm. Cut section shows multiloculated cyst, largest measuring 9x7cm containing 500 ml of dirty yellow thick fluid. Hair also identified. Another cyst identified adherent to the larger cyst measuring 4.5 cm in diameter with whitish, irregular walls. Second specimen showing an irregular reddish-brown firm tissue piece measuring 7.5x6.8x3 cm. External surface showing focal areas of congestion and cut surface, whitish solid area with brownish black area suggestive of necrosis.
First specimen showing an irregular bosellated grayish brown cystic tissue measuring 13x12x5cm. An irregular whitish firm area identified on the external surface measuring 6x4.6 cm. Cut section shows multiloculated cyst, largest measuring 9x7cm containing 500 ml of dirty yellow thick fluid. Hair also identified. Another cyst identified adherent to the larger cyst measuring 4.5 cm in diameter with whitish, irregular walls. Second specimen showing an irregular reddish-brown firm tissue piece measuring 7.5x6.8x3 cm. External surface showing focal areas of congestion and cut surface, whitish solid area with brownish black area suggestive of necrosis.
Microscopy
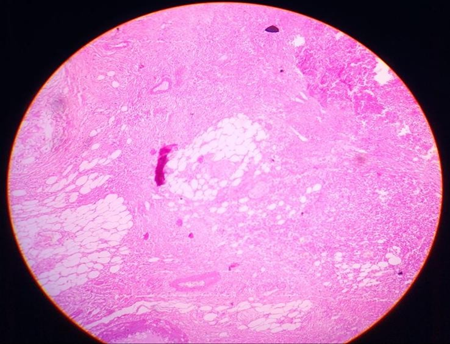
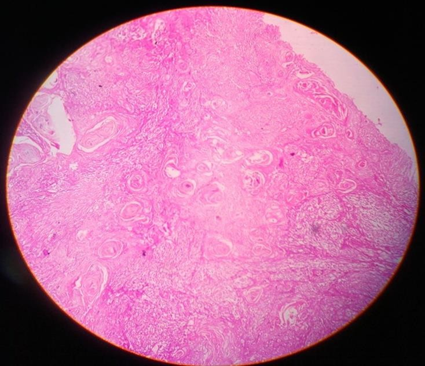
Multiple sections studied from the right cystectomy specimen show malignant squamous cells in diffuse sheets infiltrating into the ovarian stroma. Many areas show keratin pearls, focal necrosis and dystrophic calcification.
Multiple sections studied from the right cystectomy specimen show malignant squamous cells in diffuse sheets infiltrating into the ovarian stroma. Many areas show keratin pearls, focal necrosis and dystrophic calcification.
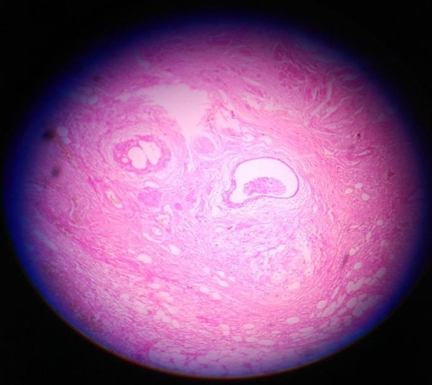
Sections taken from the thin wall area showing fibro collagenous tissue some part lined by low cuboidal epithelium and some area lined by stratified squamous epithelium with lobules of sebaceous cells, hair follicles, glands, colloid filled thyroid follicles and melanin pigment. Sections from the second tissue adherent to bladder wall also show similar malignant cells infiltrating into fibro-adipose tissue.
Discussion
The commonly encountered malignancies in mature cystic teratoma of ovary include squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, melanoma, sarcoma, carcinoid and germ cell neoplasms.6 Malignant transformation arising from mature cystic teratoma occurs in 1-2% of the cases and pre- operative diagnosis is often difficult. Kikkawa et al [7] reported that the survival was 94.7%, 80% and 12% for stages I to IV diseases respectively. Tseng CJ et al [8] showed that the disease free survival at 2 years was 100%, 100%, 30% and 0% for stage I to IV disease respectively. Therefore, it is especially important to seek for a method to diagnose malignant transformation from benign mature cystic teratoma. Among them, the SCC antigen appears to be the most useful as it is sensitive and specific compared to other markers such as CA 125 or CA 19-9 both of which were normal in our case. However, if restricted to stage Ia cases, it is not sufficiently sensitive or specific. The rise would depend on the stage of tumor and may not be suitable for early detection of small tumours since the level depends on the tumor volume. [9] In this respect, a combination of serum SCC measurements with other markers or with clinical factors such as patient age or tumor size may give more sensitivity and/or specificity to the diagnosis.
In summary, our data and that of others indicate that in malignant transformation of mature cystic teratoma the stage of disease is the most important prognostic factor and considering the relatively poor chemo- response, early diagnosis is especially important. Since extra-ovarian involvement subsequent to malignant transformation may occur rapidly in some cases, relatively frequent follow-ups are to be recommended. If malignant transformation was suspected due to serum marker elevation, the employment of other diagnostic methods including imaging study such as USG and MRI should be considered. It is particularly important to inform the patient of any risk if present and to give options for the surgical confirmation. In this case, laparoscopic surgery should be avoided as there is potential risk of intra- operative tumor spillage and if the patient desires a conservative follow-up, repeat blood tests and monitoring tumor size are useful in decreasing false positive cases.
I am writing in response to "Against Homeopathy: a Utilitarian Perspective," [1] by Kevin Smith, Ph. D., whom I commend for the clarity of his writing and the thoroughness of his logic. I suppose I should also derive some comfort from the fact that, contrary to the advice he gives to his readers, he takes homeopathy at least seriously enough to go to such trouble to denounce it.
References
- Kong CS, Longcare TA, Hendrickson MR. (2012). Vol. 38. Philadelphia: Lippincott, Williams & Wilkins; Gynaecologic Oncology. 176-8
- Williams Gynecology, (2016). Third edition: edited by Hoffman BL, Schorge JO, Bradshaw KD, Halvorson LM, Schaffer JI, Corton MM; April. Gynecological Oncology. 765-6.
- Mori Y, Nishii H, Takabe K, Shinozaki H, Matsumoto N, Suzuki K, et al. (2003). Preoperative diagnosis of malignant transformation arising from mature cystic teratoma of the ovary. Gynecol Oncol 90(2): 338-41.
- Ranu P. (2014). Squamous cell carcinoma arising in mature cystic teratoma of the ovary. J Midlife Health. 0ct-Dec. 5(4): 195-97.
- Kido A, Togashi K, Konishi I, Kataoka ML, Koyama T, Ueda H, et al. (1999). Dermoid cysts of the ovary with malignant transformation: MR appearance. AJR Am J Roentgenol. 172:445-9
- Chiang AJ, Chen MY, Weng CS, Lin H, LU CH, Wang PH et al. (2017). Malignant transformation of ovarian mature cystic teratoma into squamous cell carcinoma: a Taiwanese Gynecologic Oncology Group study. J Gynecol Oncol. 28(5): e69.
- Kikkawa F, Ishikawa H, Tamakoshi K, Nawa A, Suganuma N, Tamoda Y. (1997). Squamous cell carcinoma arising from mature cystic teratoma of the ovary: a clinicopathological analysis. Obstet. Gynecol. 89: 1017-1022.
- Tseng CJ, Chou HH, Huang KG, Chang HH, Liang CC, Lai CH. (2015). Squamous Cell Carcinoma Arising in Mature Cystic Teratoma of the Ovary. Gynecol Oncol. 25(1): 1-6.
- Black JD, Roque MD, Pasternak, MC, Buza N, Rutherford TJ, Schwartz PE et al. (2015). A Series of Malignant Ovarian Cancers Arising from Within a Mature Cystic Teratoma: A Single Institution Experience. Int J Gynecol Cancer25 (5): 792-97.
Citation: Sushma HP, Yashika Shivanna and Al Ameen Asharaf. (2024). Sqamous cell Carcinoma in a Mature Cystic Teratoma. Journal of Gynaecology and Paediatric Care 5(1).
Copyright: © 2024 Al Ameen Asharaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.