Short Communication
Volume 7 Issue 1 - 2025
Clinical significance of Surface Leukemic Stem Cell Markers: CD25 Expression Restricts Prognostic Impact of CD123 in Acute Myeloid Leukemia
1Department of Personalized Cancer Immunotherapy, Mie University Graduate School of Medicine, Tsu, Japan
2Cancer Center, Mie University Hospital, Tsu, Japan
2Cancer Center, Mie University Hospital, Tsu, Japan
*Corresponding Author: Kazunori Nakase, Department of Personalized Cancer Immunotherapy, Mie University Graduate School of Medicine, Tsu, Japan.
Received: February 08, 2025; Published: February 14, 2025
Keywords: CD25; CD123; Acute myeloid leukemia
Acute myeloid leukemia (AML) is a clonal disorder of immature blast cells that originates from leukemic stem cells (LSCs). These LSCs are responsible for disease initiation and propagation, and exhibit chemotherapy resistance. Thus, eradication of LSCs is essential for preventing relapse and prolonging patient survival [1]. Cell surface markers, CD25 and CD123 are well-established LSC markers and the expression of each marker is described as an excellent predictor of unfavorable outcome of AML [2,3] However, as the distribution of the expression of CD25 and CD123 within the LSC population appreciably differ from each other, there appears to be a gap of prognostic value between these two markers. Although the combined effect of their expression on clinical outcome has been evaluated [4], little data are available regarding a difference in the prognostic effect between them in patients with AML. Analysis of this issue will provide an important information for the development of LSC targeted therapeutic strategies against AML.?
We analyzed the expression of CD25 and CD123 in 248 adult patients (age range 15-88, mean 52.0 ± 16.9) with previously untreated and de novo AML who were examined for the immunophenotyping by flow cytometry at Mie University Hospital during 1985-2020, and determined their clinical significance. Using the French-American-British (FAB) classification, patients were classified as follows: 50 as M1, 89 as M2, 27 as M3, 54 as M4, 22 as M5, 4 as M6, and 2 as M7. Induction chemotherapy was conducted in the form of an anthracycline-type drug combined with pyrimidine antagonist, which is a standard regimen for AML in Japan. M3 patients receiving all-trans retinoic acid were excluded from our prognostic assessments.
The tested markers (> 15% was positive) were as follows: HLA-DR, CD7, CD13, CD14, CD33, CD34, CD25, and CD123. Since CD25 and CD123 are cytokine receptors, the expression level (sites/cell) was analyzed based on the mean fluorescence intensity (MFI) obtained using flow cytometry. Ab binding capacities (sites/cell) were calculated based on the MFI of test samples and the control, and calibration curves were generated using DAKO QIFIKIT and TallyCAL software packages, as previously described [5]. Samples with < 200 binding sites/cell were judged as undetectable in this study. As a molecular marker, internal tandem duplications in FLT3 (FLT3-ITD) was analyzed using the TaKaRa PCR FLT3-ITD Mutation Set [6,7]. Patients were divided into three risk groups of favorable, intermediate, and adverse for cytogenetic abnormalities according to the revised Medical Research Council prognostic classification [8].
The mean expression level of CD25 was 488 sites/cell with the maximum level of 10,397 sites/cell, and those of CD123 was 861 sites/cell with the maximum level of 15,243 sites/cell. It has been reported that CD123 was expressed in the majority of AML patients and the patients exhibiting elevated levels of CD123 correlated with adverse prognosis [3]. Here, we divided the patients expressing CD123 at levels below or above 1,000 were classified as low or high expressers. We also classified the patients expressing CD25 into its low and high expressers in the same way. CD123high was detected in 60/248 (24.2 %) patients, and CD25high was in 30/248 (12.1 %) patients, respectively (Table 1). CD123high patients significantly concorded with CD25high patients (p < 0.01).
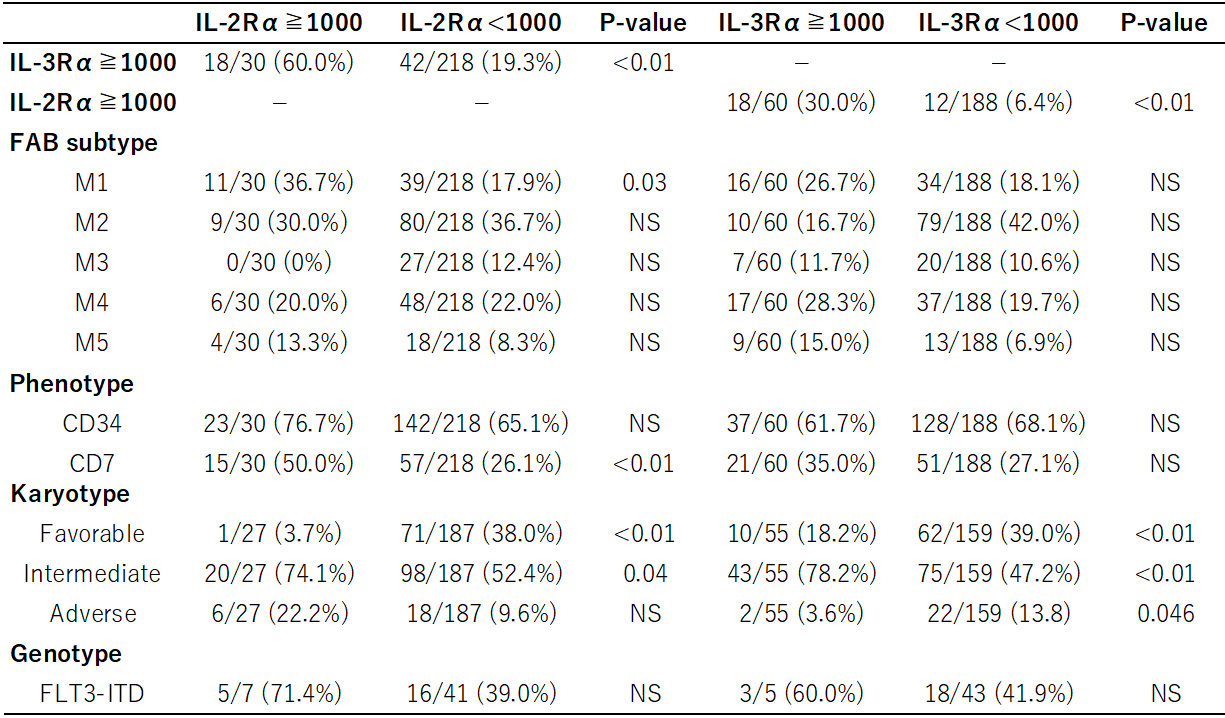
Correlations between 2 categorical variables were evaluated by the Fisher exact test.
Table 1: Correlation between the cellular features and the expression levels of CD25 or CD123.
Table 1: Correlation between the cellular features and the expression levels of CD25 or CD123.
As for a relation of these LSC markers with cellular features, CD25high patients more frequently showed M1 subtype and CD7 expression than CD25low patients. Karyotypically, both CD25low and CD123low patients were more apt to have favorable karyotypes compared to their high patients, respectively. On the other hand, both CD25high and CD123high patients tended to display intermediate karyotypes than their low patients, respectively. Unexpectedly, adverse karyotypes were more frequently observed in CD123low patients than in CD123high patients. Genetic analyses have identified several molecular markers with prognostic value in patients with AML [9]. FLT3-ITD has been demonstrated by many studies to be the most important negative indicator [10]. Previous studies doing by Pollyea DA, et al [1] and Rollins-Raval M, et al [11] suggest a correlation of FLT3-ITD with the expression of CD25 and CD123. In this study, however, the presence of FLT3-ITD was not significantly associated with both CD25high and CD123high patients. Although these data may indicate ethnic difference, additional studies are needed because the number of cases we analyzed was small.
As regards clinical significance of these LSC markers, we found some interesting findings that give the perspectives regarding this observation. As previously described [2,5], CD25high patients obviously showed shorter overall survival (OS) than CD25low patients (Figure 1A, p = 0.006). CD123high patients had also worse OS compared to CD123low patients (Figure 1B, p = 0.049) [3]. Given the high concordance of CD25high and CD123high patients, and these clinical results, we tried to investigate the prognostic relevance of CD25 status in high and low expressers of CD123 in AML. In CD123high AML, CD25high patients had significantly poorer OS than CD25low patients (Figure 1C, p = 0.038). Namely, CD123high patients showing unfavorable prognosis exclusively displayed the high expression levels of CD25. Furthermore, OS in CD25low AML indicated that there was no statistically significant difference between CD123high and CD123low patients (Figure 1D, p = 0.40), suggesting that low CD25 expression levels outweigh the prognostic power of high CD123 expression levels in AML. Therefore, the adverse prognostic impact of elevated CD123 levels appeared to be restricted to CD25high patients.
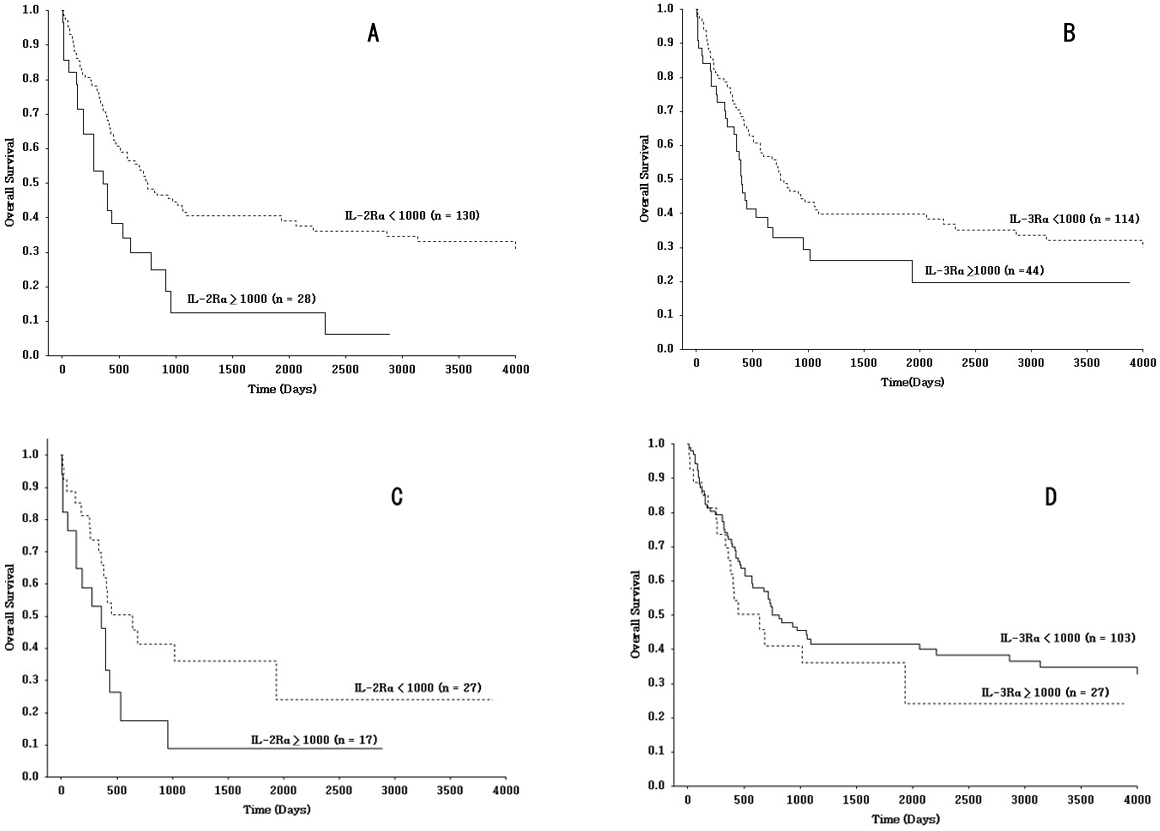
Figure 1: Kaplan-Meier curves of overall survival (OS) according to leukemic stem cell (LSC) markers CD25 and CD123. Survival differences were evaluated by the log-rank test. Statistical significance was defined as a p-value of less than 0.05. (A) CD25high patients had significantly poorer OS than CD25low patients (p = 0.006). (B) CD123high patients had significantly poorer OS than CD123low patients (p = 0.049). (C) In CD123high patients, CD25high patients had significantly shorter OS than CD25low patients (p = 0.038). (D) In CD25low patients, no significant difference in OS was observed between CD123high patients and CD123low patients (p = 0.40).
CD25 is the interleukin-2 receptor α-chain (IL-2Rα) and CD123 is the IL-3Rα. The IL-2R consists of three subunits, CD25, β-chain and common γ-chain (γc), and both β chain and γc are responsible for cytoplasmic IL-2 signal transduction. In AML, most cases express γc but lack β chain [5], whereas only 10–20 % cases express CD25 [2,5]. Regardless of the presence of any IL-2R subunit, AML cells are generally unresponsive to IL-2 because of lacking functional IL-2R [2,5]. On the other hand, IL-3R consists of two subunits, CD123 and β-chain. Testa et al. [3] demonstrated that AML cells expressing elevated levels of CD123 responded to IL-3, and STAT5 was subsequently activated at higher levels in these AML cells. STAT5 is the key transcription factor in IL-3 signaling, and CD25 is transcriptionally regulated by STAT5. Some investigators have described that IL-3 regulates the expression of CD25 on normal immature myeloid cells [12] or on IL-3 dependent bone marrow derived cell lines [13]. Accordingly, CD25 may be induced by STAT5 that is activated by IL-3 in some AML cells expressing high levels of CD123. Since OS in AML has been shown to worsen as the CD25 levels increased [5], highly induced CD25 by chance in CD123high patients seems to strongly affect the clinical prognosis of AML. Unlike CD123, CD25 expression was associated with unfavorable cellular features, M1 subtype and CD7 expression [14]. However, we think that the presence of CD25 molecule may predominantly play a key role in the dismal clinical outcome because aggressive biological functions of CD25 have been demonstrated in AML cells [15,16].
CD123 is adopted as a suitable and attractive target for AML therapy, and a variety of CD123 targeting treatments have been developed and evaluated at clinical levels [17]. However, their therapeutic efficacy is still unsatisfactory. We think that CD25 status can be considered as an important marker in the selection of patients for CD123 targeted therapies for the depletion of LSCs. In addition, treatment strategies including CD25-directed therapies may be also expected to improve the prognosis of this type of AML.
Article Information
Conflicts of interest
None
Conflicts of interest
None
Sources of Funding
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the promotion of Science [Grant 63570570 and 06454346].
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the promotion of Science [Grant 63570570 and 06454346].
Acknowledgment
We gratefully acknowledge the late Dr. K. Kita (Japan Baptist Hospital, Kyoto, Japan) for suggesting the conception of this paper. We are also grateful to the late Dr. T. Uchiyama (Kyoto University, Kyoto, Japan), Dr. T. Kitamura (Tokyo University, Japan), and Dr. R. Ueda (Aichi Cancer Center, Japan) for supplying monoclonal antibodies. We thank Drs. T. Kyo (Hiroshima Red Cross Atomic-Bomb Survivors Hospital, Hiroshima, 5 Japan), T. Ueda (Fukui University, Fukui, Japan), K. Nasu (Osaka Red Cross Hospital, Osaka, Japan), and staffs in the Department of Hematology and Oncology, Mie University Hospital for the provision of data for this study.
We gratefully acknowledge the late Dr. K. Kita (Japan Baptist Hospital, Kyoto, Japan) for suggesting the conception of this paper. We are also grateful to the late Dr. T. Uchiyama (Kyoto University, Kyoto, Japan), Dr. T. Kitamura (Tokyo University, Japan), and Dr. R. Ueda (Aichi Cancer Center, Japan) for supplying monoclonal antibodies. We thank Drs. T. Kyo (Hiroshima Red Cross Atomic-Bomb Survivors Hospital, Hiroshima, 5 Japan), T. Ueda (Fukui University, Fukui, Japan), K. Nasu (Osaka Red Cross Hospital, Osaka, Japan), and staffs in the Department of Hematology and Oncology, Mie University Hospital for the provision of data for this study.
References
- Pollyea DA, Jordan CT. (2017). Therapeutic targeting of acute myeloid leukemia stem cells. Blood 129(12): 1627-35.
- Gönen M, Sun Z, Figueroa ME, et al. (2012). CD25 expression status improves prognostic risk classification in AML independent of established biomarkers: ECOG phase 3 trial, E1900. Blood 120(11): 2297-306.
- Testa U, Riccioni R, Militi S, et al. (2002). Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 100(8): 2980-88.
- Aref S, Azmy E, El Ghannam D, et al. (2020). Clinical value of CD25/CD123 co-expression in acute myeloid leukemia patients. Cancer Biomark. 29(1): 9-16.
- Nakase K, Kita K, Kyo T, Ueda T, Tanaka I, Katayama N. (2015). Prognostic relevance of cytokine receptor expression in acute myeloid leukemia: Interleukin-2 receptor α- chain (CD25) expression predicts a poor prognosis. PLoS One 10: e0128998.
- Kiyoi H, Naoe T. (2002). FLT3 in human hematologic malignancies. Leu Lymphoma 43(8):1541-7.
- http://www.takara-bio.co.jp
- Grimwade D, Hillis RK, Moorman AV, et al. (2010). Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116(3): 354–65.
- Patel JP, Gonen M, Figueroa ME, et al. (2012). Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Eng J Med 366(12): 1079-89.
- Yanada M, Matsuo K, Suzuki T, et al. (2005). Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: meta- analysis. Leukemia 19(8): 1345–49.
- Rollins-Raval M, Pillai R, Warita K, et al. (2013). CD123 immunohistochemical expression in acute myeloid leukemia is associated with underlying FLT3-ITD and NPM1 mutations. Appl Immunohistochem Mol Morphol. 21(3): 212-7.
- Gazzola MV, Collins NH, Tafuri A, et al. (1992). Recombinant interleukin 3 induces interleukin 2 receptor expression on early myeloid cells in normal human bone marrow. Exp Hematol. 20(2): 201-8.
- Le Gros GS, Shackell PS, Le Gros JE, et al. (1987). Interleukin 2 regulates the expression of IL 2 receptors on interleukin 3-dependent bone marrow-derived cell lines. J Immunol 138(2): 478-83.
- Pinheiro LHS, Trindade LD, Costa FO, et al. (2020). Aberrant Phenotypes in acute myeloid leukemia and its relationship with prognosis and survival: a systematic review and meta-analysis. Int J Hematol Oncol Stem Cell Res. 14(4): 274-88.
- Nguyen CH, Schlerka A, Grandits AM, et al. (2020). IL2RA promotes aggressiveness and 7 stem cell-related properties of acute myeloid leukemia. Cancer Res. 80(20): 4527-39.
- Nakase K, Kita K. (2024). IL-2/CD25 axis mediates cellular networks promoting the growth of CD25+ acute myeloid leukemia cells. Leuk Res Rep. 21: 100454.
- Pelosi E, Castelli G, Testa U. (2023). CD123 a therapeutic target for acute myeloid leukemia and blastic plasmacytoid dendritic neoplasm. Int J Mol Sci. 24(3): 2718.
Citation: Kazunori Nakase, Hiroshi Miwa and Yoshihiro Miyahara. (2025). Clinical significance of Surface Leukemic Stem Cell Markers: CD25 Expression Restricts Prognostic Impact of CD123 in Acute Myeloid Leukemia. Journal of Biotechnology and Immunology 7(1). DOI: 10.5281/zenodo.14874237
Copyright: © 2025 Kazunori Nakase. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
