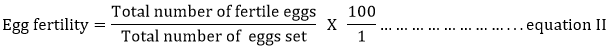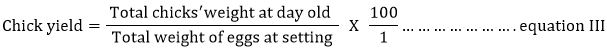Research Article
Volume 4 Issue 1 - 2022
Unfertile Eggs without Evidence of Embryo Growth or Fertile Eggs with Evidence of Embryo Growth: A Novel Categorisation of Unhatched Avian Eggs
Department of Animal Science, Nasarawa State University, Keffi, Shabu-Lafia Campus, P.M.B. 135, Lafia, 950101, Nigeria
*Corresponding Author: Kingsley Omogiade Idahor, Department of Animal Science, Nasarawa State University, Keffi, Shabu-Lafia Campus, P.M.B. 135, Lafia, 950101, Nigeria.
Received: December 25, 2021; Published: February 28, 2022
Abstract
It is believed that 100% hatchability in avian eggs incubation is not feasible due to several factors. Embryo deaths during avian eggs incubation has been reported to be a common phenomenon. The unhatched avian eggs are always discarded not minding there are some with death embryos that could be used in determining fertility of the incubated eggs. Various descriptions of embryonic deaths in incubated avian eggs have been reported yet, the dichotomy between unfertile and fertile eggs physiological status is not clear. Hence, the need to possibly classify unhatched avian eggs as either “unfertile eggs without evidence of embryo growth” or “fertile eggs with evidence of embryo growth”. A total of 1,605 Japanese quail eggs were incubated at 36°C, 37°C, 38°C, 39°C and 40°C and the unhatched eggs were opened for physiological status evaluation. Results showed that there was hatchability across the treatments and a novel categorisation of the physiological status of unhatched incubated Japanese quail eggs suspected to be “infertile”, “caked” or “not clear whether fertile or not” was proposed to be referred to as unfertile eggs without evidence of embryo growth. While those with “early dead embryos”, “fully developed but dead embryos”, “pipped and dead embryos”, “pipped and alive embryos but not able to emerge” and “hatched chicks” was proposed to be referred to as fertile eggs with evidence of embryo growth. These could possibly describe the exact physiological status of unhatched incubated eggs in avian species.
Keywords: Egg fertility; Embryo growth; Hatchability; Japanese quails; Incubation
Introduction
Incubation is the process of providing optimum temperature, air circulation and relative humidity suitable for embryo development, growth and emergence as chicks. This process could be natural, where the broody hen sits on the eggs and covers them with the feathers in order to provide suitable environmental conditions for hatching. Since Japanese quail hens do not naturally incubate and hatch their eggs, artificial incubators are used to simulate environmental conditions required to stimulate embryonic development and growth until the emergence of chicks. Woodard et al. (1973) gave 37.5°C and Musa et al. (2007) recommended 39.4°C as the optimum incubation temperature in poultry production. Meanwhile, a range of 37.5 – 38°C has been given as the optimum incubation temperature in poultry production (French, 2002; Ferguson, 1994). Yet, late hatching, low chick yield, low survivability, hatchery failure and poor post-hatch performance have been recorded hence it becomes difficult to establish the optimum temperature suitable for quail eggs incubation. Recently, French (2009) suggested that it would be more convenient to describe optimum incubation temperature in a range of values, rather than using a single value as an optimum incubation temperature. This could be largely due to fluctuations that are bound to occur in incubators, no matter the design and effectiveness.
These discrepancies may contribute to hatchability failure sometimes recorded in hatcheries thereby, forcing operators to believe that 100% hatchability is impossible in poultry eggs incubation. Fortunately, it has been reported that embryo death during eggs incubation, is a common incidence in avian species (Yilmaz et al., 2011; Romao et al. (2009a; Woodard et al. (1973). This phenomenon has resulted in unhatched eggs (even when fertile) being discarded at the end of every round of incubation period, without considering the physiological status of the incubated eggs. This would have guided the hatchery operators to be strict on egg fertility before incubation and optimum incubation temperature during incubation. The unhatched eggs often referred to as “residual eggs” are occasionally opened and those with embryo growth are included in the determination of fertility of the incubated eggs. However, it is generally assumed that only the hatched eggs are fertile, forgetting that among the unhatched eggs, there are some with death embryos at different stages of development. Thus, all unhatched eggs should be opened carefully to know what went wrong in order to improve hatchability percentage.
Woodard et al. (1973) reported that most embryonic deaths occur during the first 3 days of incubation and at about pipping. Yilmaz et al., (2011) described embryonic deaths in incubated eggs as “early embryonic death”, "middle embryonic death” and “late embryonic death”. While Romao et al., (2009b) expressed embryonic deaths in incubated eggs as “infertile”, “early embryo death”, “intermediate embryo death”, “late embryo death” and “pipped egg with dead embryo”. In all these cases, detailed classification of the unhatched eggs physiological statuses were not given. Consequently, the present study attempted to evaluate the physiological status of unhatched Japanese quail eggs and proposed the classification of “infertile”, “caked” or “not clear whether fertile or not” as unfertile eggs without evidence of embryo growth. While unhatched eggs with “early dead embryos”, “fully developed but dead embryos”, “pipped and dead embryos”, “pipped and alive embryos but not able to emerge” and “hatched chicks” could be referred to as fertile eggs with evidence of embryo growth. This novel categorisation of the physiological status of unhatched incubated avian eggs, may guide hatchery operators to provide accurate data on incubated avian egg fertility and hatchability, pertinent in the growth of poultry industry.
Materials and Methods
Description of study area
The study was conducted at the National Veterinary Research Institute (NVRI) Vom Jos North Plateau State, Nigeria located on latitude 9° 44' 0"N, longitude 8° 47' 0"E with a population of about 900,000 residents (NPC, 2006). The altitude of Jos is about 1,264.5m above sea level with about 1,400 millimetres (55 inches) of rainfall annually. Jos enjoys a more temperate climate than the rest parts of Nigeria. Average monthly temperature range from 21 – 25°C (70 – 77°F) and from mid-November to late January, night-time temperature drops to as low as 11°C (52°F). Hails sometimes fall during the rainy season because of the cooler temperature at high altitude. This cooler temperature has from colonial times until the present day, made Jos a favourite holiday location for both tourists and expatriates based in Nigeria (Webster, 1983).
The study was conducted at the National Veterinary Research Institute (NVRI) Vom Jos North Plateau State, Nigeria located on latitude 9° 44' 0"N, longitude 8° 47' 0"E with a population of about 900,000 residents (NPC, 2006). The altitude of Jos is about 1,264.5m above sea level with about 1,400 millimetres (55 inches) of rainfall annually. Jos enjoys a more temperate climate than the rest parts of Nigeria. Average monthly temperature range from 21 – 25°C (70 – 77°F) and from mid-November to late January, night-time temperature drops to as low as 11°C (52°F). Hails sometimes fall during the rainy season because of the cooler temperature at high altitude. This cooler temperature has from colonial times until the present day, made Jos a favourite holiday location for both tourists and expatriates based in Nigeria (Webster, 1983).
Experimental design and layout
A total of 1,605 Japanese quail eggs were collected from a flock in a deep litter system in the Poultry Division, National Veterinary Research Institute (NVRI), Vom, at the peak of egg production. These eggs were gathered over a period of 5 days but were not set until the 6th day for the last clutch to cool overnight as recommended by ISA (2016). The eggs were randomly allotted based on weight to five electric incubators representing the experimental treatments designated as T1: 36°C (Very low), T2: 37°C (Low), T3: 38°C (Medium), T4: 39°C (High) and T5: 40°C (Very high). In all the incubators, 107 eggs were set in each of the upper, middle and bottom egg-trays, representing the replicates. The egg trays were turned manually every hour and the water troughs inside the incubators were refilled every morning. The study lasted for 22 days.
A total of 1,605 Japanese quail eggs were collected from a flock in a deep litter system in the Poultry Division, National Veterinary Research Institute (NVRI), Vom, at the peak of egg production. These eggs were gathered over a period of 5 days but were not set until the 6th day for the last clutch to cool overnight as recommended by ISA (2016). The eggs were randomly allotted based on weight to five electric incubators representing the experimental treatments designated as T1: 36°C (Very low), T2: 37°C (Low), T3: 38°C (Medium), T4: 39°C (High) and T5: 40°C (Very high). In all the incubators, 107 eggs were set in each of the upper, middle and bottom egg-trays, representing the replicates. The egg trays were turned manually every hour and the water troughs inside the incubators were refilled every morning. The study lasted for 22 days.
Data collection
The eggs were weighed using sensitive weighing balance to obtain egg weight. The time interval between setting of eggs in the incubator and emergence of chicks was considered as incubation period. The incubation and ambient temperatures as well as relative humidity were recorded daily at 9:00 am, 12:00 noon and 3:00 pm using hygrothermometer. After 17 days of incubation when several chicks hatched, other eggs were left in the incubators for extra 5 days, in case of possible late hatching. Thereafter, all the unhatched eggs were carefully windowed to evaluate the physiological status of the developing embryos that could not emerge as chicks. Such physiological status was categorised as:
The eggs were weighed using sensitive weighing balance to obtain egg weight. The time interval between setting of eggs in the incubator and emergence of chicks was considered as incubation period. The incubation and ambient temperatures as well as relative humidity were recorded daily at 9:00 am, 12:00 noon and 3:00 pm using hygrothermometer. After 17 days of incubation when several chicks hatched, other eggs were left in the incubators for extra 5 days, in case of possible late hatching. Thereafter, all the unhatched eggs were carefully windowed to evaluate the physiological status of the developing embryos that could not emerge as chicks. Such physiological status was categorised as:
Unfertile eggs: No evidence of embryo growth
- “infertile eggs” (without any obvious signs of growth, rather intact yolk and albumen in a fluid form);
- “caked eggs” (with intact yolk and albumen but in a dried form) and
- “not clear whether fertile or not” (intact yolk and albumen but with blood spot, blacken spot and partially dried yolk and albumen)
Fertile eggs: Evidence of embryo growth
- “early dead embryos” (developed embryos with undifferentiated body parts suspected to have died on embryonic days 1 – 7);
- ii “fully developed but dead embryos” (defined body parts and downy feathers without pipping suspected to have died on embryonic days 8 – 15);
- iii “pipped and dead embryos” (fully developed embryos that pipped but suspected to have died on embryonic days 16 – 17);
- “pipped and alive but not able to emerge embryos” (alive and pipping on embryonic days 18 – 22, but could not complete the pipping process) and
- “Hatched chicks” (fully developed, pipped and emerged alive).
The hatched chicks were allowed to remain in the incubators until fluffy dried, weighed to obtain chick weight and were transferred to the brooding unit. The egg hatchability, fertility and chick yield values were calculated using equations I, II and III, respectively.
Where: Fertile eggs = the summation of “early dead embryos”, “fully developed but dead embryos”, “pipped and dead embryos”, “pipped and alive but not able to emerge embryos” and “hatched chicks”.
Data analysis
Data collected were subjected to analysis of variance procedure of SPSS (2010) and mean values were separated using Duncan Multiple Range Test of the same software package.
Data collected were subjected to analysis of variance procedure of SPSS (2010) and mean values were separated using Duncan Multiple Range Test of the same software package.
Results
Table 1 presents the incubation and ambient temperature as well as relative humidity values recorded during Japanese quail eggs incubation. There were significant differences (P<0.05) in all the parameters monitored across the treatments except the incubation relative humidity. It was shown that the incubation temperature was within the desired values calibrated to represent the treatments: Very low incubation temperature (36°C), Low incubation temperature (37°C), Medium incubation temperature (38°C), High incubation temperature (39°C) and Very high incubation temperature (40°C), respectively. However, fluctuation of a range of 0.11 to 0.27°C was recorded with a minimum temperature of 34.45°C and maximum of 41.06°C during the period.
| Parameters | Treatments | Statistics | |||||||
| T1 | T2 | T3 | T4 | T5 | Min | Max | Overall mean | SEM | |
| 36°C | 37°C | 38°C | 39°C | 40°C | |||||
| IT (°C) | 36.11e | 37.20d | 38.22c | 39.27b | 40.12a | 34.45 | 41.06 | 38.37 | 0.08 |
| AT (°C) | 27.67b | 27.61b | 28.82a | 29.00a | 28.30ab | 20.52 | 38.28 | 28.43 | 0.13 |
| IRH (%) | 27.47 | 27.30 | 27.44 | 27.35 | 27.48 | 27.04 | 38.02 | 26.44 | 0.26 |
| ARH (%) | 10.04d | 10.89c | 20.14a | 12.90b | 20.14a | 10.04 | 22.06 | 14.74 | 0.22 |
a,b,c,d,e: Mean values on the same row with different superscript differ statistically at 5% probability test; SEM: Standard error of means; IT: Incubation temperature; AT: Ambient temperature; IRH: Incubation relative humidity; ARH Ambient relative humidity; Min: Minimum value; Max: Maximum value.
Table 1: Temperature and relative humidity during Japanese quail eggs incubation.
Table 1: Temperature and relative humidity during Japanese quail eggs incubation.
While the ambient temperature values did not differ significantly (P>0.05) between T3 (28.82°C) and T4 (29.00°C), they were statistically different (P<0.05) from T1 (27.67°C) and T2 (27.61°C) that were similar and T5 (28.3°C) was somewhat similar to all the observed values in other treatments. Incubation relative humidity values ranged from 27.47% (T1) to 27.48% (T5) and ambient relative humidity was least (10.04%) in T1 and highest (20.14%) in T3 and T5.
Physiological status of Japanese quail eggs incubated at 36 to 40°C is presented in table 2. There were no statistical differences (P>0.05) in total egg weight, average egg weight, infertile eggs, hatched chicks, fertile eggs, average chick weight, hatchability, fertility and chick yield.
| Parameters | Treatments | SEM | ||||
| T1 | T2 | T3 | T4 | T5 | ||
| 36°C | 37°C | 38°C | 39°C | 40°C | ||
| Total eggs set | 321 | 321 | 321 | 321 | 321 | - |
| TEW (g) | 3361.18 | 3306.68 | 3238.87 | 3222.82 | 3352.13 | 3.11 |
| AEW (g) | 10.34 | 10.28 | 10.14 | 10.00 | 10.42 | 0.04 |
| IP (days) | 20 | 18 | 17 | 16 | 18 | - |
| Unfertile eggs: No evidence of embryo growth | ||||||
| Infertile eggs | 10.68 | 8.30 | 7.67 | 7.64 | 12.31 | 1.22 |
| Caked eggs | 19.78ab | 25.70a | 16.53b | 22.64ab | 15.71b | 1.64 |
| NCWFN | 15.24a | 10.67b | 16.00a | 15.72a | 12.51b | 1.35 |
| Fertile eggs: Evidence of embryo growth | ||||||
| EDE | 12.03b | 27.70a | 15.13b | 18.32ab | 21.67a | 7.68 |
| FDDE | 28.60a | 8.06c | 14.67b | 12.67b | 14.33b | 2.37 |
| PDE | 4.67c | 3.57c | 13.00a | 7.67b | 10.13a | 0.48 |
| PAE | 1.33a | 0.00b | 0.00b | 0.00b | 0.00b | 0.17 |
| Hatched chicks | 14.67 | 23.00 | 24.00 | 22.34 | 20.34 | 3.05 |
| Egg fertility, chick hatchability and yield | ||||||
| Fertile eggs | 61.30 | 62.33 | 66.80 | 61.00 | 66.47 | 4.83 |
| Fertility (%) | 57.29 | 58.25 | 62.43 | 57.09 | 62.12 | 2.64 |
| Hatchability (%) | 23.93 | 36.90 | 35.93 | 36.62 | 30.60 | 4.15 |
| TCW (g) | 96.89b | 125.78ab | 183.02a | 147.22ab | 122.89ab | 20.31 |
| ACW(g) | 6.67 | 5.45 | 6.64 | 6.66 | 6.10 | 0.17 |
| Chick yield (%) | 64.50 | 53.02 | 65.48 | 66.60 | 58.54 | 3.76 |
a,b,c: Mean values on the same row with different superscript differ statistically at 5% probability test; SEM: Standard error of means; TEW: Total egg weight; AEW: Average egg weight; IP: Incubation period; NCWFN: Not clear whether fertile eggs or not; EDE: Early dead embryos; FDDE: Fully developed but dead embryos; PDE: Pipped and dead embryos; PAE: Pipped and alive but not able to emerge embryos; TCW: Total chick weight; ACW: Average chick weight.
Table 2: Incubated Japanese quail eggs physiological status.
Table 2: Incubated Japanese quail eggs physiological status.
While the average egg weight value was approximately 10g across the treatments, the incubation period was observed to be shortest (16 days) in T4 (39°C) and as long as 20 days in T1. Infertile eggs ranged from 7.64 (T4) to 12.31 (T5), more early dead embryos were recorded in T2 (27.70) and T5 (21.67) but these values were statistically similar (P>0.05) to 18.32 recorded in T4 whose value was similar to 15.13 (T3) and 12.03 (T1).
Fertile eggs ranged from 61.00 (T4) to 66.80 (T3), hatched chicks were more in T3 (24.00) slightly followed by T2 (23.00), T4 (22.34) and fewer (14.67) in T1. Average chick weight value varied from 5.45g (T2) to 6.67g (T1), hatchability varied from 23.93% in T1 to 36.90% in T2 and fertility rate value ranged from 57.09% (T4) to 62.43% (T3). Chick yield value was lowest (53.02%) in T2 and was as high as 66.60% in T4.
Discussion
The incubation temperatures were somewhat out of a range of 37 – 38°C reported as the optimum incubation temperatures for avian species (Archer and Cartwright, 2018; Sartell, 2018). However, it was apparently within 33 – 40°C, reported to be the lower and upper limits of incubation temperatures, required for optimal embryo development in avian species (Pam, 2015; Mani et al., 2008; G?th and Booth, 2005). According to Wilson (1991), deviations from this optimum incubation temperature values could have a major impact on hatching success. Therefore, the incubation temperatures used in the study were probably suitable for avian embryo development. The incubation relative humidity recorded in each of the treatments was close to 36% observed in Japanese quail eggs incubation (Romao et al., 2009b). However, it was lower than a range of 45 – 55% recorded in waterfowl, 35 – 45% (parrots) and more than 65% in all avian species (Cutler and Abbott, 1991). Thus, low incubation relative humidity, may not play a vital role in Japanese quail eggs incubation. The ambient temperature values recorded were within 20 – 34°C, but the ambient relative humidity was less than 60 – 80% reported to be the ideal values in rearing poultry species (Poultry CRC, 2016).
Average weight of the incubated eggs across the treatments was within 7.69 – 10.80g reported in Japanese quails (Indreswari et al., 2019; Ajide 2011), but somewhat less than 10 – 12g speculated to be the normal size of Japanese quail eggs (FAO, 2003). Therefore, the eggs used in this study were perhaps normal. Meanwhile, the observed incubation period was within the range of 16 – 18 days recorded when Japanese quail eggs were subjected to microclimatic conditions (Sellier et al., 2006). The shortest incubation period (16 days) recorded in T4 (39°C), probably implied economic benefit as the total hatchery cost could be cut down. This observation agreed with the report of Romao et al. (2009a) that higher incubation temperature at 40°C resulted in 16 days incubation period (early hatching) in Japanese quails, compared to those incubated at 35°C that hatched on day 23.
At hatch, the chicks weighed close to a range of 7.0 – 7.96g reported by Farghly et al. (2015) when Japanese quails of similar age were subjected to photo stimulated conditions during egg incubation. This probably reflected that the hatchlings were seemingly healthy with little or no adverse effects on their health as a result of the incubation temperatures.
When the unhatched eggs were windowed after embryonic day 22, it was observed that some of the incubated eggs were “infertile”, “caked” and “not clear whether fertile or not”. The caked eggs were probably due to cracks that led to loss of water leaving the egg yolk and albumen in dried form. While the “infertile” eggs were probably due to the improper sex ratio of the flock, mating or fertilization failure, the “not clear whether fertile or not eggs” were perhaps due to failure of the zygote to cleave and differentiate. This probably resulted in the regression or death of the zygote that left a stain (brown, black or blood) on the yolk, making it difficult to ascertain whether the egg was fertile or not. This observation was similar to a condition of the tiny spot, occasionally found on egg yolk described as blood spots or egg spots, which do not however signify fertility in poultry eggs (AEB, 2018). It was stated that this condition could be due to rupture of blood vessels on the yolk surface or accidental oviductal wall rupture during egg formation. Therefore, unhatched incubated Japanese quail eggs with the suspicion of “infertile”, “caked” or “not clear whether fertile or not” may be categorised as unfertile eggs with no evidence of embryo growth.
In some other unhatched Japanese quail eggs windowed, it was observed that the embryos were at different physiological stages of growth and development that were either dead or could not emerge as hatchlings after embryonic day 22. Such developmental stages were described in this study as “early dead embryos” [suspected to have died on embryonic days 1 – 7], “fully developed but dead embryos” [suspected to have died on embryonic days 8 – 15], “pipped and dead embryos” [suspected to have died on embryonic days 16 – 17] as well as “pipped and alive embryos but not able to emerge” [still alive on embryonic days 18 – 22]. However, these stages were similar to the conditions described earlier as “infertile”, “early embryo death”, “intermediate embryo death”, “late embryo death” and “pipped egg with dead embryo” (Romao et al., 2009a). Meanwhile, Yilmaz et al. (2011) simply described similar stages as “early”, “middle” and “late embryonic death”. Although the causes of hatching failure were not clear according to Ramteke et al. (2013), the causes of the observed different physiological statuses of the embryos that could not emerge as hatchlings, could be purely unfavourable microclimatic conditions that perhaps resulted in metabolic disorder. Also, it could be partly due to microbial contamination resulting in infection of the developing embryos and possibly due to the poor nutritional plane of the hens, resulting in low quality yolk and albumen that were seemingly inadequate or probably unsuitable for the developing embryos.
However, Woodard et al. (1973) reported that most embryonic deaths occur during the first 3 days of incubation and/or just prior to hatching. Also, it was stated that most eggs removed at the first candling (8 days of incubation) were infertile and early embryo deaths. It was further stressed that fatal mortality was largely due to the inability of the developing embryos to form vital organs or malfunctioning of the organs during development. Critical functions in such conditions include a change in position of the embryo prior to pipping, utilization of the remaining albumen, absorption of the yolk sack and change from allantoic to pulmonary respiration. It was added that there was an indication of a slight mid-incubation peak of mortality, suggestive of chickens formally associated with dietary deficits.
It was observed that the hatchability was poor and less than a range of 78.67 – 85.56% reported in Japanese quails (El-Kholy et al., 2019), probably due to fairly low egg fertility of 49 – 61% recorded in the present study. In any case, the observation was similar to 2.9 – 75.0% reported when Japanese quail eggs were incubated at different temperatures (Romao et al., 2009a) and when Japanese quail eggs were stored for 20 days before incubating at 39°C (Mani et al., 2008). The observed 41 – 67% chick yield was less than 72% recorded when Akpinar et al. (2019) evaluated growth traits in Japanese quails and 67 – 68% described as optimum chick yield in poultry species (Aviagen, 2015). However, chick yield in T3 (38°C) and T4 (39°C) were similar to 67% that Poultry Site (2014) reported as an ideal target for best chick quality. Although chicks hatched in all the incubators set at 36°C (Very low), 37°C (Low), 38°C (Medium), 39°C (High) and 40°C (Very high) incubation temperatures, there was reduced incubation period of 16 days in chicks hatched at 39°C, followed by 38°C, but chick yield was best at 38°C, followed by 39°C whereas, hatchability was best at 37°C, followed by 39°C, 38°C and 40°C in that order.
Conclusion
Hatchability was recorded in all the treatment incubators thus, a range of 36 – 40°C may be recommended as the optimum incubation temperature in Japanese quails. Unhatched eggs may be left in the incubator for extra 5 days in case of late hatchability. Thereafter, all the unhatched eggs should be windowed carefully and those with embryo growth included in the determination of the incubated eggs fertility. The unhatched incubated eggs suspected to be “infertile”, “caked” or “not clear whether fertile or not” could be referred to as “unfertile eggs without evidence of embryo growth”. While unhatched eggs with “early dead embryos” (embryonic days 1 – 7), “fully developed but dead embryos” (embryonic days 8 – 15), “pipped and dead embryos” (embryonic days 16 – 17), “pipped and alive embryos but not able to emerge” (embryonic days 18 – 22) and hatched chicks could be referred to as “fertile eggs with evidence of embryo growth”. This novel categorisation of the physiological status of unhatched incubated eggs, may guide hatchery operators to provide accurate data on incubated avian egg fertility and hatchability, needed for growth of the poultry industry.
References
- AEB, (2018). The incredible edible egg™ EGGCYCLOPEDIA. American Egg Board (Fifth Edition), Rev. 3/12, E-0024.
- Ajide, S. O. (2011). Effect of age and egg size of Japanese quail hens on hatchability and post-hatch performance of quail chicks fed different dietary protein levels. MSc Dissertation, Ahmadu Bello University, Zaria. Pp. 108.
- Akp?nar G. Ç. & Günenç A. (2019). Effects of transportation and storage duration of Japanese quail eggs on hatchability. South African Journal of Animal Science, 49 (2): 253–261.
- Archer G.S. and Cartwright A.L. 2018. Incubating and hatching eggs. Texas A&M AgriLife Extension Service, AgriLifeBookstore.org. EPS-001 7/13.
- Aviagen, (2015). How to measure chick yield. Accessed in November, 2018 at: http://en.aviagen.com/assets/Tech_Center/BB_Resources_Tools/Hatchery_How_Tos/02HowTo2MeasureChickYield.pdf Pp. 4.
- Cutler, B. A. & Abbott U. K. (1991). Effects of temperature on the hatchability of artificially incubated cockatiel eggs (Nymphicus hollandicus). Proceedings of 35th Western Poultry Disease Conference, Pp. 32-35.
- El-Kholy, M. S., Ibrahim, Z. A., El-Mekkawy, M. M. & Alagawany, M. (2019). Influence of in ovo administration of some water-soluble vitamins on hatchability traits, growth, carcass traits and blood chemistry of Japanese quails. Annals of Animal Science, 19 (1): 97–111.
- FAO, (2003). Good practices in planning and management of integrated commercial poultry production in South Asia. Food and Agriculture Organization of the United Nations, Rome, FAO Animal Production and Health 159, Pp106.
- Farghly, M. F. A., Mahrose, K. H. & Abou-Kassem, D. E. (2015). Pre- and post-hatch performance of different Japanese quail egg colours incubated under photo stimulation. Asian Journal of Poultry Science, 9(1):19-30. DOI: 10.3923/ajpsa.2015.19.30.
- Ferguson, M. W. J. (1994). Temperature dependent sex determination in reptiles and manipulation of poultry sex by incubation temperature. Proceedings 9th European Poultry Conference, Glasgow, UK, Pp. 380-382.
- French, N. A. (2009). Incubation and hatching. In: Biology of Breeding Poultry, (Hocking, P. M., Ed), Pp. 206-223. CAB International, Wallingford, Oxfordshire, UK.
- French, N. A. (2002). The critical importance of incubation temperature. In Practical Aspects of Commercial Incubation, (Deeming, D. C., Ed), Ratite Conference Books, Lincolnshire, UK. Pp. 17-20.
- G?th, A. & Booth, D.T. (2005). Temperature-dependent sex ratio in a bird. Biology Letters, 1: 31-33.
- Indreswari, R., Ratriyanto, A. & Nugroho, T. (2019). Performance of Japanese quails (Coturnix coturnix japonica Temminck & Schlegel, 1849) fed hatchery waste meal. In The UGM Annual Scientific Conference Life Sciences 2016, KnE Life Sciences, Pp. 281–287.
- ISA, (2016). ISA Brown Commercial Stock. A Hendrix Genetics Co. Accessed in October, 2016 at: http://www.isapoultry.com/en/Products/ISA/ISA%20Brown.aspx
- Mani, A. U., Garndawa, I. I. & Usman, B. A. (2008). Effects of pre-incubation storage on the hatchability of quail (Coturnix coturnix japonica) eggs in the Sahel region of Nigeria. International Journal of Poultry Science, 7 (4):350-354. ISSN 1682-8356.
- Musa, U., Haruna, E.S. & Lombin, L.H. (2007). Quail production in the tropics. NVRI Press, Vom. Pp. 158.
- NPC, (2006). National population and housing census. National Population Commission, Abuja, Federal republic of Nigeria.
- Pam, T. (2015). Hatching eggs: A step by step guide. Pp. 3. Accessed in December, 2015 at: www.canteach.ca
- Poultry CRC, (2016). Climate in poultry houses. Poultry Cooperative Research Centre, Australia.
- Poultry Site, (2014). Investigating hatchery practice - monitoring egg and chick weights. Poultry news, health, welfare, diseases, markets and economics. Accessed in November, 2018
- Ramteke, J., Charde, P., Zade, S. & Gabhane, R. (2013). Comprehensive Study of organogenesis during embryonic development of Japanese quail. International Journal of Life Science, 1(3):193-197.
- Romao, J. M., Moraes, T. G. V., Teixeira, R. S. C., Buxade, C. C. & Cardoso, W. M. (2009a). Incubation of Japanese quail eggs at different temperatures: hatchability, hatch weight, hatch time and embryonic mortality. Archives of Veterinary Science, 14(3):155-162.
- Romao, J. M., Moraes, T. G. V., Teixeira, R. S. C., Buxade, C. & Cardoso, W. M. (2009b). Effect of relative humidity on incubation of Japanese quail eggs. LRRD, 21(3).
- Sartell, J. (2018). Incubation station: a reference guide to incubation terms, temperatures, times and humidity levels. Community Chickens Newsletter. Accessed in July 2018 at: https://www.communitychickens.com/category/coops/https://www.beautyofbirds.com/eggincubationtemperature.html
- Sellier, N., Brillard, J.P., Dupuy, V. & Bakst, M. R. (2006). Comparative staging of embryo development in chicken, turkey, duck, goose, guinea fowl, and Japanese quail assessed from five hours after fertilization through seventy-two hours of incubation. Journal of Applied Poultry Research, 15:219-228.
- SPSS, (2010). Statistical package for social sciences, SPSS Inc., 444. Michigan Avenue, Chicago, IL 60611.
- Webster, J. B. (1983). Studies in the history of Plateau State Nigeria. In: Isichei E. (Editor) Partisan Picture, African Affairs, 82(326): 138–139. https://doi.org/10.1093/oxfordjournals.afraf.a097491
- Wilson, R. H. (1991). Effect of egg size on hatchability, chick size and post-hatching growth. In Avian Incubation, (Hocking, P. M., Ed), CAB International, Wallingford, Oxfordshire, UK, Pp. 279-283.
- Woodard, A. E., Abplanalp, H., Wilson, W.O. & Wohra, P. (1973). Japanese quail husbandry in the laboratory, Department of Avian Sciences, University of California. Pp. 22.
- Y?lmaz, A., Tepeli, 1. C., Garip, M. & Ça?layan, T. (2011). The effects of incubation temperature on the sex of Japanese quail chicks. Poultry Science, 90:2402-2406.
Citation: Kingsley Omogiade Idahor. (2022). Unfertile Eggs without Evidence of Embryo Growth or Fertile Eggs with Evidence of Embryo Growth: A Novel Categorisation of Unhatched Avian Eggs. Journal of Agriculture and Aquaculture 4(1).
Copyright: © 2022 Kingsley Omogiade Idahor. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.