Research Article
Volume 3 Issue 1 - 2021
Effect of Salt and Inhibitor on the Isolation, Purification and Characterization of α -Amylase from Aspergillus niger Produced from Pigeon Pea
Department of Food Science and Technology, Federal University of Technology, Akure, Ondo State, Nigeria
*Corresponding Author: Adegbanke OR, Department of Food Science and Technology, Federal University of Technology, Akure, Ondo State, Nigeria.
Received: September 22, 2021; Published: October 09, 2021
Abstract
α-Amylase an industrially used enzyme can be obtained from Aspergillus niger and the microorganism can be produced from food sources such as pigeon pea. α-Amylase was produced from Aspergillus niger isolated from pigeon pea, purified and characterized. This process was achieved using ammonium sulphate, ion exchange DEAE column and gel filtration (Sephadex A-50 and sephadex G-100) Chromatography. The effect of salt and inhibitor was determined. Ammonium sulfate precipitation results showed that the highest specific α-amylase activity was (1.01 U/ml.mg) obtained at 11.27% saturation level, with a purity of 1.81-fold of the crude extract with 1.00% yield. Further purification using gel filtration increased the enzyme purity to 8.94-fold of the crude extract with 13.01% yield. Specific activity after purification was 4.99 U/mg. The effect of salts on α-Amylase activity increased to 258.09% when in MgSO4, while decreased to 7.71% and 21.07% when in MnSO4 and CuCl2 respectively and yielded no result when in PbNO3. Its reaction with chemical inhibitors such as Bromosuccinimide was activated to 136.465% and was inhibited at Mercaptoethanol to 0%. All these were determined using a visible spectrophotometer with an absorbance of 540nm, against the control that contains 100µL of the enzyme and 100µL of 1% starch solution.
Keywords: α-Amylase Aspergillus niger; Pigeon Pea; Purification; Characterization; Salts and Inhibitors
Introduction
Aspergillus niger is a common fungus in nature that belongs to the Aspergillus genus. It is an important industrial fermentation microorganism that is widely used for the production of industrial enzymes and organic acids (Schuster et al., 2002). According to Oludumila et al. (2015), the fungus can be grown on inexpensive substrate and is capable of producing high yield and relatively stable at the operating condition. Major advantage of using fungi for the amylase production is the economical bulk production capacity (Shah et al., 2014).
α-amylases are wide spread in occurrence which can be obtained by different resources e.g. microorganisms, animals and plants etc. However, fungi and bacteria are used for commercial production of amylases (Mathew et al., 2016; Singh et al., 2016), because of a few advantages i.e. reliability, less time and space, low cost required for enzyme production, ease of manipulation and economical bulk production capacity (Khan et al., 2019; Mahmood et al., 2016).
Pigeon pea (Cajanus cajan (L.) Huth) is one of the most common tropical and subtropical legumes cultivated for its edible seeds. Pigeon pea is fast growing, hardy, widely adaptable, and drought resistant (Bekele-Tessema, 2007). Fasoyiro et al. (2009), Amaefule and Nwagbara (2004) and Odeny (2007) reported that pigeon pea is still underutilized as food due to its tough texture, long cooking duration and lack of education on its nutritional potentials.
The objectives of this research is to produce, isolate, purify, characterize and to determine the effect of salt and inhibitor on α-Amylase produced from Aspergillus niger isolated from Pigeon pea.
Materials and Methods
Collection of the sample and preparation of seed culture
Pigeon pea was purchased from Anaye market, odo-ora, Ekiti state, Nigeria. The chemicals used were of analytical grade. The pigeon pea was fermented for 4 days (96 hours) and serial dilution was done in triplicate according to Chakraborty et al., (2000).
Pigeon pea was purchased from Anaye market, odo-ora, Ekiti state, Nigeria. The chemicals used were of analytical grade. The pigeon pea was fermented for 4 days (96 hours) and serial dilution was done in triplicate according to Chakraborty et al., (2000).
Isolation of ?-Amylase from Aspergillus niger
This was carried out according to Chakraborty et al., (2000) with slight modifications.
This was carried out according to Chakraborty et al., (2000) with slight modifications.
Assay of ?- amylase activity
Estimation of ?- amylase activity was carried out according to the dinitrosalicylic acid (DNS) method of Bernfeld (1955).
Estimation of ?- amylase activity was carried out according to the dinitrosalicylic acid (DNS) method of Bernfeld (1955).
Enzyme purification
Ammonium sulphate precipitation
The cell-free supernatant after centrifugation was subjected to ammonium sulphate precipitation and dialysis and each fraction was checked for enzyme activity as well as protein concentration. This was carried out according to Chakraborty et al., (2000).
Ammonium sulphate precipitation
The cell-free supernatant after centrifugation was subjected to ammonium sulphate precipitation and dialysis and each fraction was checked for enzyme activity as well as protein concentration. This was carried out according to Chakraborty et al., (2000).
Ion exchange chromatography
The dialysate was further purified by applying 25 ml of dialysate on DEAE-Sephadex A50 column (1.5 x 24 cm) equilibrated with 50 mM phosphate buffer pH 6.8 at a flow rate of 1 ml/min. All the fractions were checked for enzyme activity. This was carried out according to Chakraborty et al., (2000).
The dialysate was further purified by applying 25 ml of dialysate on DEAE-Sephadex A50 column (1.5 x 24 cm) equilibrated with 50 mM phosphate buffer pH 6.8 at a flow rate of 1 ml/min. All the fractions were checked for enzyme activity. This was carried out according to Chakraborty et al., (2000).
Gel filtration
The active fractions were pooled and applied on sephadex G-100 Column (1.5 x 75 cm the resulting enzyme was utilized for the characterization of the extracellular α-Amylase. This was carried out according to Chakraborty et al., (2000).
The active fractions were pooled and applied on sephadex G-100 Column (1.5 x 75 cm the resulting enzyme was utilized for the characterization of the extracellular α-Amylase. This was carried out according to Chakraborty et al., (2000).
Determination of protein concentration
The Protein concentration was determined according to Bradford (1976) using Bovine Serum albumin and the absorbance was measured at 595nm with a spectrophotometer.
The Protein concentration was determined according to Bradford (1976) using Bovine Serum albumin and the absorbance was measured at 595nm with a spectrophotometer.
Physicochemical properties of purified enzyme
Effect of some salts on the α-Amylase activity
An assay mixture containing a final concentration of each salt were carried out according to the standard assay procedures of Ojo and Ajele (2011).
Effect of some salts on the α-Amylase activity
An assay mixture containing a final concentration of each salt were carried out according to the standard assay procedures of Ojo and Ajele (2011).
Effect of inhibitors on purified α-Amylase activity
The effect of different inhibitors on α-Amylase activity were determined according to Ojo and Ajele (2011).
The effect of different inhibitors on α-Amylase activity were determined according to Ojo and Ajele (2011).
Results and Discussion
Production of α-Amylase from Aspergillus niger
α-Amylase activities increased with the increase in time. It has its peak at 25 hrs after which it begins to decline as the starch solution has been exhausted. During the incubation incubation of A.niger in 1% starch solution, the growth increased with time and at the 30th hour, the microorganisms has its optimum growth here produced its peak number of cells due to the exhaustion of the available starch and the growth begins to retard. The protein concentration also increases with time at the inception but is stationary at the 10th to 20th hour and increased a bit till it gets to 25th hour and has a sharp increase from 25th hour to the 35th hour where it has its optimum protein concentration and then begin to decline. Figure 1 shows the production of α-amylase from A. niger in a medium containing 1% starch solution.
α-Amylase activities increased with the increase in time. It has its peak at 25 hrs after which it begins to decline as the starch solution has been exhausted. During the incubation incubation of A.niger in 1% starch solution, the growth increased with time and at the 30th hour, the microorganisms has its optimum growth here produced its peak number of cells due to the exhaustion of the available starch and the growth begins to retard. The protein concentration also increases with time at the inception but is stationary at the 10th to 20th hour and increased a bit till it gets to 25th hour and has a sharp increase from 25th hour to the 35th hour where it has its optimum protein concentration and then begin to decline. Figure 1 shows the production of α-amylase from A. niger in a medium containing 1% starch solution.
Purification of α-Amylase from Aspergillus. Niger
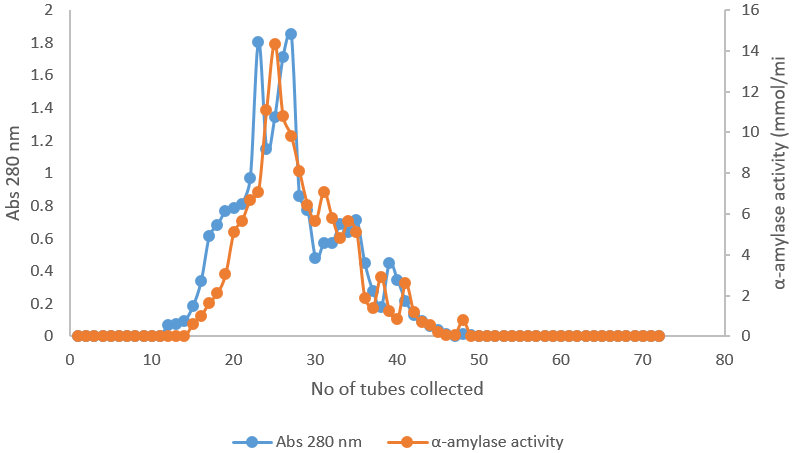
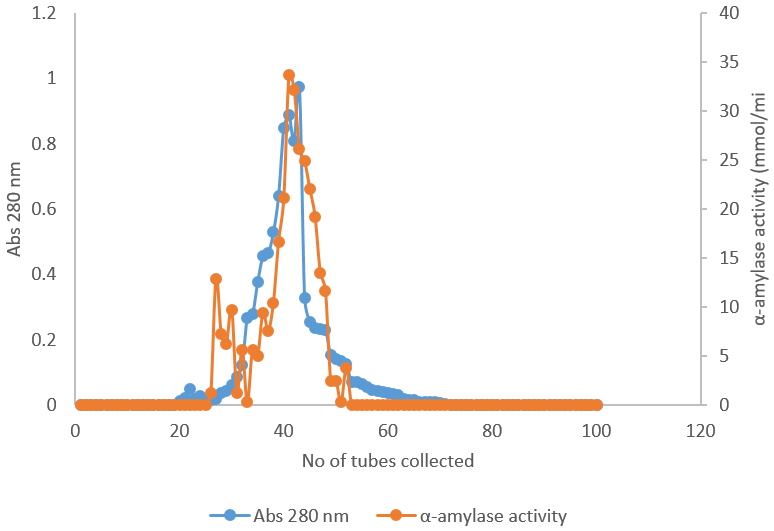
The elution profile of Alpha amylase from ion exchange chromatography on DEAE-sephadex A50 was shown in Figure 2. The elution profile shows one protein and activity peaks and the fraction containing α-amylase activity was pooled and used for further purification on gel filtration chromatography while in Figure 3, the elution profile of α-amylase from gel filtration chromatography produced one activity peak which was also pooled for further analysis, the results were similar with Samson et al., (2001), who purified A. niger from food substrate. The summary of purification of alpha amylase from A. niger is as shown in Table 1. After the purification, the activity of the enzyme increased from 8.85 to 32.7 mmol/min, the specific activity was 4.99 mmol/min/mg, the yield of the enzyme was 13.01% and the purification fold was 8.94. This was in accordance with Shafiei et al., 2010, which carried out enzyme activity, specific activity and purification fold of enzyme purified from A niger.
The elution profile of Alpha amylase from ion exchange chromatography on DEAE-sephadex A50 was shown in Figure 2. The elution profile shows one protein and activity peaks and the fraction containing α-amylase activity was pooled and used for further purification on gel filtration chromatography while in Figure 3, the elution profile of α-amylase from gel filtration chromatography produced one activity peak which was also pooled for further analysis, the results were similar with Samson et al., (2001), who purified A. niger from food substrate. The summary of purification of alpha amylase from A. niger is as shown in Table 1. After the purification, the activity of the enzyme increased from 8.85 to 32.7 mmol/min, the specific activity was 4.99 mmol/min/mg, the yield of the enzyme was 13.01% and the purification fold was 8.94. This was in accordance with Shafiei et al., 2010, which carried out enzyme activity, specific activity and purification fold of enzyme purified from A niger.
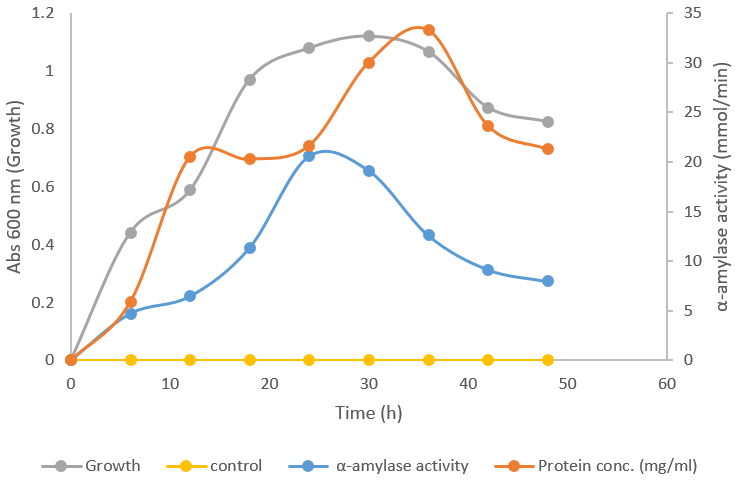
Figure 1: Alpha amylase production from Aspergillus niger in a medium containing 1% starch solution.
| Step | Vol. of solution | α-amylase activity | Protein Concentration | Total α-amylase activity | Total Protein Concentration | Specific activity | Yield | Fold |
| Crude extract | 850 | 8.85 | 15.87 | 7529.52 | 13493.75 | 0.56 | 100.00 | 1.00 |
| Amm ppt/ Concentra | 63.6 | 13.34 | 13.25 | 848.83 | 842.72 | 1.01 | 11.27 | 1.81 |
| Ion exchange | 41.8 | 17.44 | 9.62 | 729.03 | 402.32 | 1.81 | 9.68 | 3.24 |
| Gel | 32.7 | 29.96 | 6.00 | 979.71 | 196.21 | 4.99 | 13.01 | 8.94 |
Table 1: Purification of α-Amylase.
Effect of salts on α- Amylase activity
Effects of metal ions of nitrate salts on the partially purified α-amylase activity is shown in Table 2. Metal such as CuCl2 and MnSO4 decreased the α-amylase activity; however, PbNO3 reduced the activity of purified α-amylase to zero. MgSO4 increased the activity of α-amylase to over 200% followed by the control (100%) and CoCl2 (77.12%). The results were similar to the report of Bajpai et al., (1992).
Effects of metal ions of nitrate salts on the partially purified α-amylase activity is shown in Table 2. Metal such as CuCl2 and MnSO4 decreased the α-amylase activity; however, PbNO3 reduced the activity of purified α-amylase to zero. MgSO4 increased the activity of α-amylase to over 200% followed by the control (100%) and CoCl2 (77.12%). The results were similar to the report of Bajpai et al., (1992).
Effects of Inhibitors on α-Amylase Activity
When inhibitor binds reversibly to the active side of the enzyme it is known as a competitive Inhibitor. Often a competitive inhibitor is a similar shape to the substrate. Its association with the active site of the enzyme reduces the rate of binding between the substrate and the enzyme, thus lowering the rate of reaction. However in comparison to literature, this type of inhibition can be overcome by increasing the substrate concentration as this will decrease the chances of enzyme and inhibitor binding (Reece et al., 2011).
When inhibitor binds reversibly to the active side of the enzyme it is known as a competitive Inhibitor. Often a competitive inhibitor is a similar shape to the substrate. Its association with the active site of the enzyme reduces the rate of binding between the substrate and the enzyme, thus lowering the rate of reaction. However in comparison to literature, this type of inhibition can be overcome by increasing the substrate concentration as this will decrease the chances of enzyme and inhibitor binding (Reece et al., 2011).
| Metallic salts | Activity | R.A (%) |
| KCl | 8.30 | 54.24 |
| PbNO3 | 0.00 | 0 |
| MgSO4 | 39.52 | 258.09 |
| MnSO4 | 1.18 | 7.712 |
| NaCl | 13.70 | 89.46 |
| CuCl2 | 3.22 | 21.07 |
| CoCl | 11.81 | 77.12 |
| Control | 15.31 | 100.00 |
Table 2: Effect of metal ion on the partially purified activity of α-amylase.
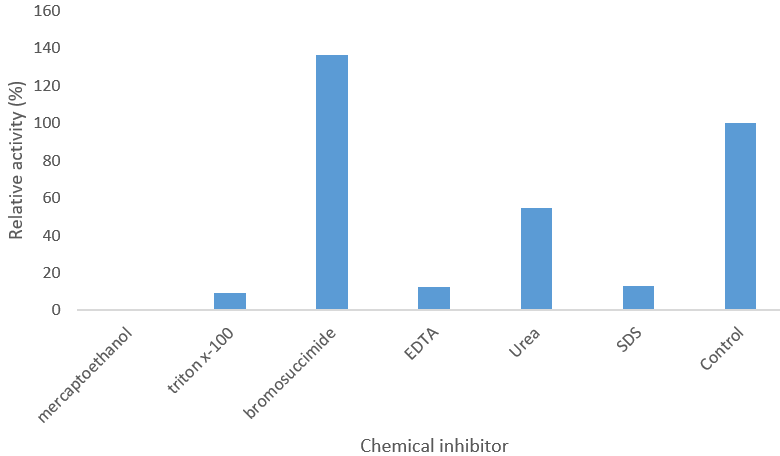
The relative activity of some chemical inhibitors and their effects on the purified α-Amylase activity is shown in Figure 4. This was determined using a visible spectrophotometer with an absorbance of 540nm, against the control that contains 100µL of the enzyme and 100µL of 1% starch solution, after testing the chemical inhibitors against the control, the results gotten are as follows; Mercaptoethanol 0%, Triton X-100 9.172%, Bromosuccinimide 136.465%, EDTA 12.527%, Urea 54.809%, Sodium Dodecyl Sulfate (SDS) 12.751%. The values of the chemical inhibitors higher than 100% of the control activated the activity the enzyme, while the values lower than the control inhibited the activity of the enzyme. Putting this into consideration, this makes Mercaptoethanol with 0% the chemical compound with the highest inhibiting power, followed by Triton X-100 with 9.172%, EDTA with 12.527%, Sodium Dodecyl Sulfate (SDS) with 12.751%, and Urea with 54.809%. This makes Bromosuccinimide, with 136.465%, an activator.
Therefore, when the structural resemblance of the competitor matches that of the substrate, it binds to the active site competitively, but when competitive inhibitor’s concentration exceeds that of the substrate, competitive inhibitor binds to the active binding site to form enzyme-inhibitor complex (EI) and finally no product is formed (Thoma and Koshland, 1960; Hegyi et al., 2013) which makes all the compounds but Bromosuccunimide inhibitors in respect to their mechanism of actions.
Conclusion
Conclusively, with the use of pigeon pea as substrate for this experiment, the following has been observed: the activity of α-amylase depends on increased activity in the presence of MgSO4, and a decrease in MnSO4 and CuCl2. The activity was inhibited using EDTA, SDS, Urea, Mercaptoethanol, and Triton X-100 while Bromosuccinimide activated the enzyme, increasing its activity. The experiment shows that the activities of enzymes can be activated/increased or inhibited/decreased, which are useful in fermentation, and in prolonging the shelf life of foods respectively. These could be achieved with the use of suitable mineral salts, or inhibitors.
References
- Thoma J. A., and Koshland Jr D. E. (1960). Competitive inhibition by substrate during Enzyme action. Evidence for the induced-fit theory1, 2. J Am Chem Soc. 82(13): 3329-33.
- Tiwari, K.L., Jadhav, S.K. and Fatima, A., (2007). Culture conditions for the production of thermostable amylase by Penicillium rugulosum. Globle J. Biotechnol. Biochem., 2: 21-24.
- Hegyi G., Kardos J., and Kovács M. (2013) Introduction to Practical Biochemistry. ELTE Faculty of Natural Sciences. Institute of Biology. Eötvös Loránd University.
- Reece, J.B., Urry, L.A., Cain, M.L., Wasserman, S.A., Minosky, P.V. and Jackson, R.B. (2011). Campbell Biology, Pearson, ISBN 10: 0321739752.
- Oludumila Omolara Racheal, Abu Temitope Folagbade Ahmed, Enujiugha Victor Ndigwe, Sanni David Morakinyo. (2015). Extraction, Purification and Characterization of Protease from Aspergillus niger Isolated from Yam Peels. International Journal of Nutrition and Food Sciences. Vol. 4, No. 2, pp. 125-131.
- Bajpai, P. K. Gera, P. K. Bajpai, (1992). Optimization studies for the production of alphaamylase using cheese whey medium. Enzyme Microbial. Technol., 14, 679.
- Samson RA, Houbraken J, Summerbell RC, Flannigan B, Miller JD. (2001). Common and important species of fungi and actinomycetes in indoor environments.In: Microorganisms in Home and Indoor Work Environments. New York: Taylor & Francis, pp. 287-292. ISBN
- Shafiei, M., Ziaee, A. A. & Amoozegar, M. A. (2010). Purification and characterization of an organic-solvent-tolerant halophilic α-amylase from the moderately halophilic Nesterenkonia sp. Strain F. J Ind Microbial Biotechnol, 38, 275-281.
- Bekele-Tessema, A., (2007). Profitable agroforestry innovations for eastern Africa: experience from agroclimatic zones of Ethiopia, India, Kenya, Tanzania and Uganda. World Agroforestry Centre (ICRAF), Eastern Africa Region
- Fasoyiro SB, Akande BR, Arowora KA, Sodeko OO, Sulaiman PO, Olopade CO, Odini CE (2010). Physico-Chemical and Sensory Properties of pigeon Pea (Cajanus cajan) Flours. Afr. J. of Food Chem Sciences 4: 120-126.
Citation: Adegbanke OR, Isimoya PC, Omodunoye TR and Adewole OA. (2021). Effect of Salt and Inhibitor on the Isolation, Purification and Characterization of α -Amylase from Aspergillus niger Produced from Pigeon Pea. Archives of Nutrition and Public Health 3(1).
Copyright: © 2021 Adegbanke OR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.