Short Communication
Volume 1 Issue 2 - 2019
Young Age Colorectal Cancer, Family History of Cancer and Molecular Profiling- A Case Series of 5 Patients
1M.Sc, PhD Scholar
2MS, MCh, FRCS, FAC, FASCRS, Professor, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences Raebareli Road, Lucknow-226014, India
3MD, Professor, Departments of Surgical Gastroenterology* and Pathology** Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow-226014, India
2MS, MCh, FRCS, FAC, FASCRS, Professor, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences Raebareli Road, Lucknow-226014, India
3MD, Professor, Departments of Surgical Gastroenterology* and Pathology** Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow-226014, India
*Corresponding Author: Ashok Kumar Professor, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences Raebareli Road, Lucknow-226014, India.
Received: October 12, 2019; Published: October 23, 2019
Abstract
Hereditary colorectal carcinoma is diagnosed in patient with family history of cancer and presence of DNA mismatch repair defect. Microsatellite instability (MSI) and MMR protein expression are generally used to screen these patients. The present study was carried out on 5 young age colorectal cancer patients (4 males and one female, aged 24-50 year) who qualified for hereditary CRC based on Bethesda criteria ,We herein present the molecular profiling of these patients and its clinicopathological features,. Hematochezia, pain and altered bowel habit were the common presentations in these patients. Family history of colorectal malignancy was present in 4 (80%) patients, while 1 patient had history of lung carcinoma in the family. MSI was done by PCR-Fragment analysis and the Mismatch repair genes (MLH1, MSH2, MSH6 and PMS2) expression was done by Immunohistochemistry. MSI-high (MSI-H) was in 3 patients all these 3 patients had advance disease. MSI-Low (MSI-L) in 2 patients. In 4 patients (80) %) MMR protein intact while it was shows loss in only one patient. There was no correlation between MSI and MMR protein expression in these young CRC with family history of Cancer.
Keywords: Colorectal Cancer (CRC); young age; family history of cancer; Immunohistochemistry; Microsatellite Instability
Introduction
Colorectal cancer is the third most common cancer in men (746,000 cases, 10.0% of the total) and the second in women (614,000 cases, 9.2% of the total) worldwide [1-3]. In Asia the incidence of CRC is increasing (Countries adopting western life-style and dietary habits) and is now the third most common malignant disease. There are three broad categories of CRC- sporadic, familial and hereditary colorectal cancers. Hereditary Non polyposis colorectal cancer (HNPCC) or Lynch syndrome this disease) is the most common form of hereditary CRC and accounting for 3% of all CRCs. It is characterized by early age onset of cancer, predominance of mucin and poor degree of differentiation, proximal distribution, increased number of synchronous and metachronous cancers. [4-6]. It is an autosomal dominant syndrome which is caused by germ line mutations in Mismatch repair (MMR) genes. MMR system is a form of DNA repair mechanisms, which plays an important role in correcting the mismatch of base pairs and helps in reducing gene mutations and also keeping genome stability. Majority of HNPCC families are found to have mutations in three key Mismatch Repair genes, MLH1, MSH2, and MSH6 and other less commonly implicated genes are PMS2 and PMS1.A study reported that MSH6 and PMS2 account for approximately 7–10% and < 5%, respectively, of LS families. In human Colorectal carcinoma, 80% of the tumors are microsatellite stable, which means they have intact DNA Mismatch repair (MMR) genes which can correct single-bases and small-loop base-pair mismatches present throughout the non-coding and coding regions of the genome. Rest 20% of CRC tumors exhibit MSI due to defects in this DNA MMR pathway that corrects small base-pair mistakes in mononucleotide, di-nucleotide, and tri-nucleotide repeat region through-out the genome and are termed as high or low MSI (MSI-H or MSI-L, respectively). [7]
The present study was carried out on young age colorectal cancer patients with family history of cancer to find out its Molecular profiling (IHC and MSI testing), clinicopathological features and whether there any pointer Microsatellite instability (MSI) and Immunohistochemistry (IHC) are generally used to screen patients towards Lynch Syndrome.
Material and Methods
A prospective study on microsatellite markers and MMR proteins was performed on 100 patients with colorectal cancer between 2016-2019. 5 patients were found to have familial history of cancer and were less or equal to 50 years of age which formed the study group. They were suspected to have HNPCC based on their clinical profile. Detailed clinicopathological information was collected from hospital informatics system on these patients. All these patients have had detailed history and clinical examination, and were evaluated by standard protocol - Colonoscopy and biopsy, CEA, CT/MRI. All of them have had surgery, MSI and IHC testing. MSI analysis was performed using 5 National Cancer Institute Microsatellite markers (BAT25, BAT26, D2S123, D5S346, and D17S250). PCR-Fragment analysis (ABI 310 Sequencer) was performed on Normal and tumour DNA samples. Loss or gain in the number of repeats in tumour DNA (case) was observed on gene mapper software which was compared with the number of repeats in the same region in non tumour DNA (control) of the resected specimen. MMR proteins expression was seen by IHC staining using 4 antibodies (MLH1, MSH2, MSH6 and PMS2; (MLH1- SC56161, Santa Cruz biotechnology, 1:50 dilution, MSH2- SC56163, Santa Cruz biotechnology, 1:150 dilution,1:50 dilution, MSH6- EP-49 Dako). MMR Protein expression, Microsatellite instability and Clinicopathological features were analysed.
Results and Discussion
There were 4 males (80%) and 1 female (20%) in the median age of 35 years (24-50 year). 3 patient had Rectal cancer and 2 colon cancer. Earlier Studied have also found a higher incidence of rectal cancer as compared to colon cancer in young age patients [9]. Histopathology revealed Stage I in 1 patient, Stage II in 1 patient, stage III disease in 2, Stage IV in 1 (table 1). 4 patient’s tumour showed normal expression of the all 4 MMR genes by IHC. However 1 had loss of PMS2 and this patient had early disease (stage I) which was indicative of germ line mutation in PMS2 protein [8]. In contrast studies have found that PMS2 expression (loss) is mostly associated with MLH1 unexpressed [9,10] MSI-H tumours were identified in 3 patients and MSI-low in 2 patients. Out of 5 markers studied for MSI both the mononucleotide markers (BAT 25 and BAT26) were found unstable in 4 patients which was similar to the published series that BAT26 marker has highest frequency amongst all. [11,12]. The 3 dinucleotide markers D2S123, D5S346, D17S250 had mixed expression (table 2). There was no any pointer towards the lynch syndrome except in one patient. We definitely cannot rule out the possibility of lunch syndrome in the remaining 4 patients due to discordant results. MMR protein assessment by IHC and MSI testing by PCR are highly concordant, but neither alone test is sufficient to identify 100 percent of tumours with defective MMR, and therefore dual PCR and IHC testing is an acceptable practice. Published literature has shown good association between MSI with MMR loss. However in our series, there was no such correlation was found. Discordant IHC and PCR results have been found in 2.2 percent of colorectal tumours; most commonly reflect mutated non-functional MMR proteins with retained antigenicity. Discordant results should be interpreted as defective MMR, and additional testing in the form of sequencing (Sanger) should be performed to rule out a germ- line mutation.
| S. No | Age/ Sex | Site of Lesion | Family History | Stage of Disease | Treatment | HPE_Differentiation (Adenocarcinoma) & Other histopathological features | MSI (NCI Panel Markers) | IHC (MMR Protein) | Remarks | ||
| Neo -Adjuvant | Surgery | Adjuvant | |||||||||
| 1 | 35/M | Rectum | Brother had died due to Abdominal malignancy | IV | Yes, CTRT | APR | - | Moderately differentiated | MSI-High | Loss of PMS2 Protein |
Liver Mets |
| 2 | 50/M | Rectum | Uncle had carcinoma rectum | IIIB | Yes CTRT |
APR | - | Poorly Differentiated, TILS | MSI-Low | Intact | HIV Positive |
| 3 | 24/F | Rectum | Great Grand Mother had Carcinoma Rectum | IVC | Yes CTRT | ULAR | - | Poorly Differentiated Signet ring cell carcinoma Presence of LVI,PNI, Necrosis, TILS, Omentum Positive. |
MSI-High | Intact | - |
| 4 | 46/M | Hepatic Flexure | 1. Mother had endometrial carcinoma at age of 60. 2. Father died of carcinoma lung |
IIIC | - | 1. Extended Right hemicolectomy 2. Excision of Parietal wall recurrence |
Yes | Poorly Differentiated Signet Ring Cell | MSI-High | Intact | Parietal wall recurrence after 7 year |
| 5 | 49/M | Caecum | Mother had Lung Carcinoma | IIA | - | Right Hemi- Colostomy | Yes | Well Differentiated | MSI-Low | Intact | - |
Table 1:.
| S.No | Patient | Site of Tumour | Microsatellite Markers | MMR Protein Expression | |||||||||
| BAT25 | BAT26 | D2S123 | D5S346 | D17S250 | Combined Microsatellite markers Phenotype |
MLH1 | MSH2 | MSH6 | PMS2 | Combined Protein Expression |
|||
| 1 | 35/M | Rectum | Instable | Instable | Stable | Instable | Stable | Microsatellite Instable High (MSI-H) | Positive | Positive | Positive | Negative | MMR Loss |
| 2 | 50/M | Rectum | Stable | Instable | Stable | Stable | Stable | Microsatellite Instable Low (MSI-L) |
Positive | Positive | Positive | Positive | MMR Intact |
| 3 | 24/F | Rectum | Instable | Instable | Instable | Stable | Stable | Microsatellite Instable (MSI-H) | Positive | Positive | Positive | Positive | MMR Intact |
| 4 | 46/M | Caecum/ Right Side | Instable | Instable | Stable | Stable | Stable | Microsatellite Instable (MSI-H) | Positive | Positive | Positive | Positive | MMR Intact |
| 5 | 49/M | Hepatic Flexure/ Right side | Stable | Stable | Stable | Instable | Stable | Microsatellite Instable Low (MSI-L) | Positive | Positive | Positive | Positive | MMR Intact |
Table 2:
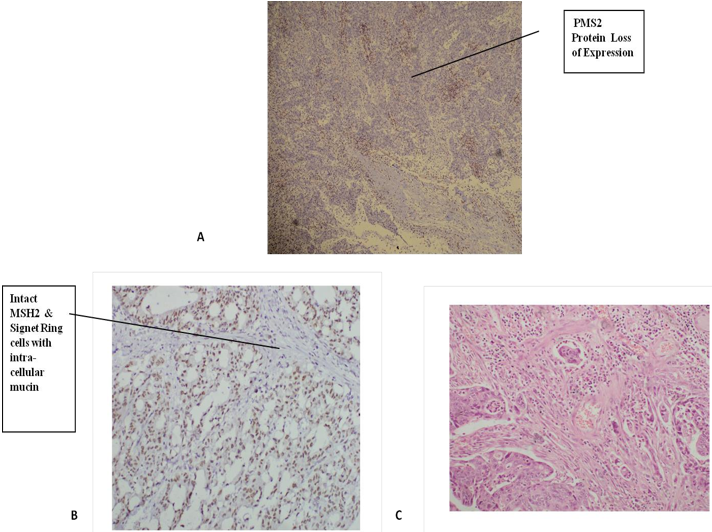
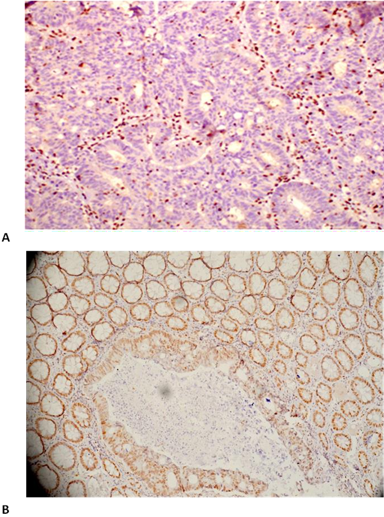
Figure 2: Immunohistochemical expression: A – 35 Y/M patient with isolated loss of PMS2 protein,
B- 24 Y/F patient with Intact MSH2 with Signet Ring cells
C- Histopathology (24 Y/F patient) showing Lymphovascular Invasion.
B- 24 Y/F patient with Intact MSH2 with Signet Ring cells
C- Histopathology (24 Y/F patient) showing Lymphovascular Invasion.
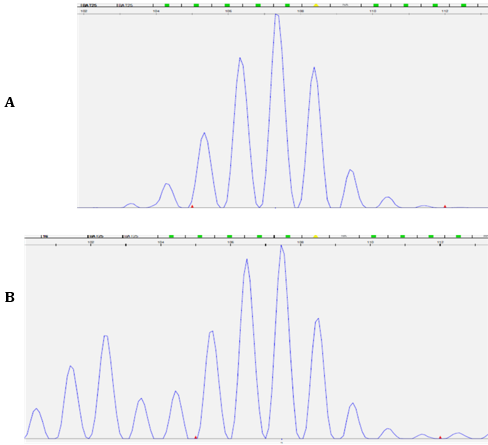
Figure 3: A. The Electropherogram of the mononucleotide repeat marker BAT26 in normal sample (top panel).
B. The appearance of numerous extra alleles at lower molecular weights in the tumor sample (bottom panel) indicates significant genomic instability.
B. The appearance of numerous extra alleles at lower molecular weights in the tumor sample (bottom panel) indicates significant genomic instability.
Conclusion
Most of the young colorectal cancer patients in this study had advanced disease and had poor degree of differentiation. There were male preponderance and that of rectal cancers. There was no correlation found between MSI and IHC results except one patient. Further genetic testing is suggested to detect germ line mutation. All the newly diagnosed colorectal carcinoma should undergo testing for MMR status to rule out the implications such as disease prognosis, surveillance, and predicted responses to therapy.
References
- Ferlay, J., et al. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer, 2010. 127(12): p. 2893-917.
- Center, M.M.,et al. (2009). Worldwide variations in colorectal cancer. CA Cancer J Clin, 2009.59(6): p.366-78.
- Haggar, F.A. and R.P. Boushey (2009). Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 22(4): p. 191-7.
- Wang, Y., et al. (2009), Clinicopathological features in endometrial carcinoma associated with Lynch syndrome in China. Int J Gynecol Cancer, 2009. 19(4): p. 651-6.
- Shpitz, B., et al. (2006), Predominance of younger age, advanced stage, poorly-differentiated and mucinous histology in Israeli Arab patients with colorectal cancer. Anticancer Res, 2006. 26(1B): p. 533-7.
- Lynch., et al. (2008), Hereditary colorectal cancer syndromes: molecular genetics, genetic counseling, diagnosis and management. Fam Cancer. 2008; 7 (1): 27-39.
- Boland et al. (2010), Microsatellite instability in colorectal cancer. Gastroenterology. 2010 Jun; 138(6):2073-2087.
- Alpert et al (2018), Colorectal Carcinomas with Isolated Loss of PMS2 Staining by Immunohistochemistry. Arch Pathol Lab Med. 2018 Apr; 142 (4):523-528.
- Rosty C et al. (2016), Germline mutations in PMS2 and MLH1 in individuals with solitary loss of PMS2 expression in colorectal carcinomas from the Colon Cancer Family Registry Cohort. BMJ Open. 2016 Feb 19; 6 (2).
- Hall et al. (2019), Gene-specific features (MLH1, MSH2, MSH6, PMS2) of mismatch repair (MMR) protein expression and somatic mutations (muts), microsatellite instability (MSI) and tumor mutational burden (TMB) in MSI-H and MMR-mutated tumor genomic profiles (TGPs). Journal of Clinical Oncology 37, no. 4_suppl (February 1 2019) 505-505.
- Pyatt, et al; Polymorphic Variation at the BAT-25 and BAT-26 Loci in Individuals of African Origin, Am J Pathol. 1999 Aug; 155 (2): 349–353.
- Samowitz et al, BAT-26 and BAT-40 instability in colorectal adenomas and carcinomas and germline polymorphisms. Am J Pathol. 1999 Jun; 154 (6):1637-41.
Citation: Alka Yadav, Ashok Kumar and Niraj Kumari. (2019). Young Age Colorectal Cancer, Family History of Cancer and Molecular Profiling- A Case Series of 5 Patients. Journal of Medical Research and Case Reports 1(2).
Copyright: © 2019 Ashok Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.