Research Article
Volume 2 Issue 3 - 2020
Tp 53 Oncosupressor Gene Pro47ser Polymorphism in the Development of Breast Cancer
1Institute of Biophysics and biochemistry at the National University of Uzbekistan named after Mirzo Ulugbek, Uzbekistan, Tashkent, Olmazor distr. Student campus, University street, 174.
2Scientific-Research Institute of Hematology and Blood Transfusion of the Ministry of health of Republic, Uzbekistan, Uzbekistan, Tashkent, 100097, Chilonzor distr., Bunedkor 42A.
3Tashkent city branch of the republic scientific and practical Medical Center of oncology and radiology of the Ministry of health of Republic, Uzbekistan Uzbekistan, 100054, Tashkent, Chilonzor distr., Bogustan 1 str.
4Tashkent Pediatric Medical Institute, 100140, Tashkent, Yunusabad district, Bagishamol str., 223.
5Tashkent Pharmaceutical Institute, 100015, Tashkent city, Aybek st., 45.
2Scientific-Research Institute of Hematology and Blood Transfusion of the Ministry of health of Republic, Uzbekistan, Uzbekistan, Tashkent, 100097, Chilonzor distr., Bunedkor 42A.
3Tashkent city branch of the republic scientific and practical Medical Center of oncology and radiology of the Ministry of health of Republic, Uzbekistan Uzbekistan, 100054, Tashkent, Chilonzor distr., Bogustan 1 str.
4Tashkent Pediatric Medical Institute, 100140, Tashkent, Yunusabad district, Bagishamol str., 223.
5Tashkent Pharmaceutical Institute, 100015, Tashkent city, Aybek st., 45.
*Corresponding Author: Ibragimov Zafar Zokirjonovich, Institute of Biophysics and biochemistry at the National University of Uzbekistan named after Mirzo Ulugbek, Uzbekistan, Tashkent, Olmazor distr. Student campus, University Street, 174. Uzbekistan.
Received: March 18, 2020; Published: March 27, 2020
Abstract
The relationship between the TP53 cancer suppressor genes Pro47Ser polymorphism and the risk of developing breast cancer has been studied in many countries and in different ethnic groups. However, no such studies have been conducted in among Uzbek women. This is the first article that shows results of TP53 gene Pro47Ser polymorphism genotyping in 207 Uzbek females.
The study showed that the TP53 gene Pro47Ser polymorphism’s importance and its significant role in the pathogenesis of breast cancer. The 47Ser minor allele of the Pro47Ser polymorphism was present in 5% of patients with breast cancer and 1.4% in women in the control group. It was found that polymorphism’s 47Ser allele and breast cancer correlated with odds ratio: χ2 = 3,3; p = 0,03; OR = 3,7; 95% CI: 1,0–13,6. It shows that the Ser allele increases the incidence of breast cancer by 3,7 times. The heterozygous Pro/Ser genotype of the studied polymorphism was 10% in breast cancer patients and 2,8% in conditionally healthy women. There was also a relationship between the Pro/Ser genotype and breast cancer with a odd ratio: χ2 = 5,1; p = 0.01; OR = 4,7; 95% CI: 1,28-17,1. It can be seen that the Pro/Ser genotype increases the incidence of breast cancer by 4,7 times. It should be noted that in the main and control groups, no homozygous Ser/Ser genotype was observed. In conclusion, we can see that Pro47Ser polymorphism of the TP53 suppressor gene can be used as one of the genetic markers for the prognosis of breast cancer.
Keywords: rs1800371; TP53 gene; Breast cancer; Polymorphism
Abbreviations: World Health Organization (WHO); Breast cancer (BC); International Classification of Diseases; 10th revision (ICD-10); Polymerase chain reaction (PCR); tumor protein p53 (TP53); ?ro47Ser(P47S); Ser47 (S47).
Introduction
According to the World Health Organization (WHO, 2018), 18.1 million people worldwide are diagnosed with cancer, of which 9.6 million cases ended with patient’s death and approximately 2.1 million women are diagnosed with breast cancer. That is 11.6% of the total incidence of cancer. Breast cancer (BC) is the leading cause of cancer in women, with one in four (24.2%) women diagnosed with cancer worldwide [1.4]. One year statistics in Uzbekistan show that among all women diagnosed with cancer 24.6 % had breast cancer [2.3].
BC – according to international classification of diseases 10th edition (ICD-10) included in the group of breast cancer C50 [5]. It is known that BC is multifactorial disease and its development occurs as a result of uncontrolled division of breast cells under the influence of external and internal factors. The basis of this uncontrolled division is the expression of mutant genes, which leads to the formation of tumor or mass in breast tissue. Most BC begins in the breast mucosa, which connects the breast to the nipple and is only detected during screening of the female, before symptoms appear or when the woman feels a nodule in the breast [4,6]. Currently, the molecular mechanisms of the occurrence and development of breast cancer are studied and makes it possible processes of early diagnosis and treatment [7].
Until the date a great deal of research is being carried out to investigate candidate genes that cause many somatic mutations in humans, including the development of breast cancer. One of these candidate genes is the TP53 gene. This TP53 gene is an oncosupressor and is located at 17p13.1 locus (OMIM, 191170) and contains 11 exons with 20 kbp [8-10].
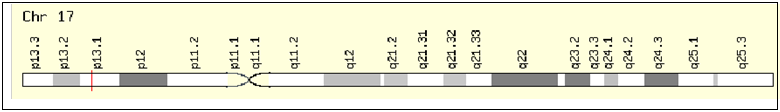
Start: 7,514,550 bp from pter. End: 7,533,728 bp from pter. Size: 19,178 bases
Figure 1: The location of the TP53 gene on the chromosome [10].
Figure 1: The location of the TP53 gene on the chromosome [10].
It contains 393 amino acid residues weighing 53 kDa, encoding the P53 nuclear phosphoprotein with 2629 nucleotide pairs [9]. The P53 protein is located in the cell nucleus and is directly linked to DNA. P53 is involved in cell cycle control, DNA repair, and apoptosis regulation. It regulates DNA repair processes caused by cell damage, including chemical and physical factors. If the DNA is mutated or damaged and cannot be restored, the P53 protein transmits a signal, then stimulates cell apoptosis and prevents cell division into tumors [7,11,12].
Somatic mutations in the TP53 gene are one of the most common genetic variations between 50-70% associated with human cancer [7,9]. About 27580 somatic mutations and 85 polymorphisms are known in TP53 [9]. The frequency of TP53 mutations registered in BC ranges from 15 to 71% [7]. These changes may affect the activity of the P53 protein and therefore may affect the risk, development, or treatment of cancer [9]. One of the most widely studied polymorphisms is codon 47 (rs1800371) of exon 4 of the TP53 gene, a unique polymorphism that leads to the substitution of C>T nucleotides in codon 47 (CCG47 TCG) and the Proline (Pro) residue of the P53 protein to serine (Ser) [13,14].
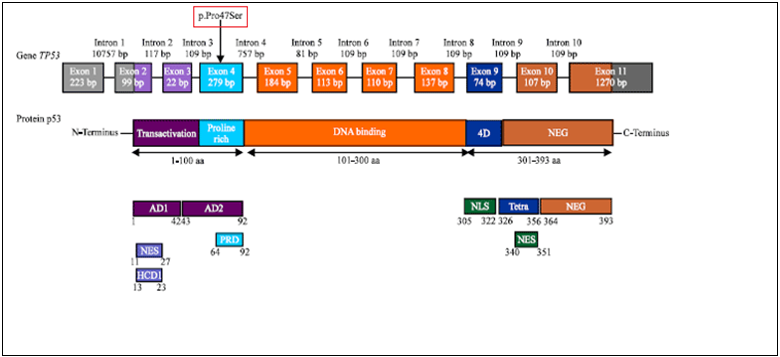
Organization of TP53 protein and p53 protein. The p53 amino terminal contains AD1 (activation domain 1), AD2 (activation domain 2), PRD (Proline-rich domain), NES (nuclear exclusion domain), and HCD I (highly conservative domain I), the Carboxyl end of p53 contains Tetra (4D) (oligomerization domain), NEG (negative regulation domain), NES (nuclear localization domain), and NLS (nuclear localization domain). The gray parts of exons 1, 2, and 11 represent UTR (untranslated regions) regions
Figure 1: Structure and role of polymorphism was studied by the TP53 gene [9].
Figure 1: Structure and role of polymorphism was studied by the TP53 gene [9].
Aim. TP53 Gene Pro47Ser polymorphism incidence and assessment of its role in the pathogenesis of breast cancer in Uzbek women.
Materials and Methods or Experimental Procedures
207 Uzbek women were included in the study, of which 100 patients with breast cancer were the main group, while 107 were included in the control group. Based on the results of mammography and histological examination, women with a diagnosis of breast cancer were taken from the mammology department of the Tashkent city branch of the Republican Specialized Oncology and the Scientific and Practical Medical Center for Oncology of the Ministry of Health of the Republic of Uzbekistan.
A molecular genetic part of this study was carried out at the Department of Molecular Medicine and Cellular Technologies of the Research Institute of Hematology and Blood Transfusion. The peripheral blood of the studied groups was separated using the AmpliPray Ribo-prep DNA amplification kit (Next Bio LLC, Russia) and Diatom tm DNA Prep 100 (Isogen Laboratory, Russia). Quantification and purity of DNA was investigated on a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). The TP53 gene was used to detect Pro47Ser polymorphism using the Polymerase chain reaction (PCR) Litex (01338-100 NPF Litex Russia) 2720 Applied Biosystems (USA). The presence of PCR products was measured on a transillinator (Biocom UVT1) after electrophoresis on a 3% agarose gel [Figure 3].
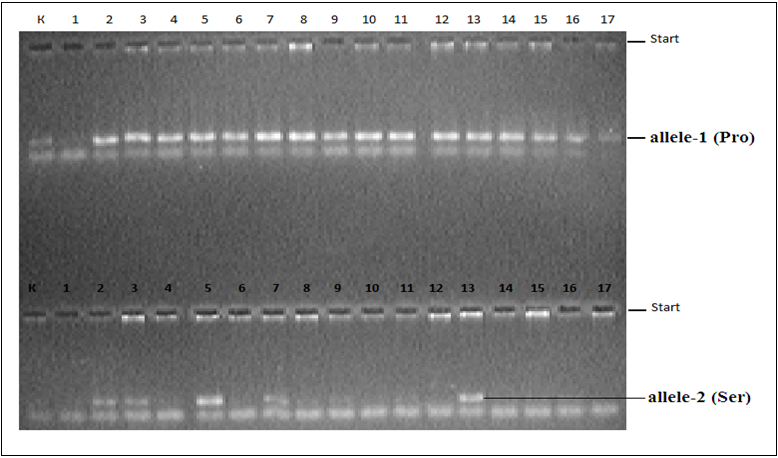
Note: K (-) control, K (+) control, 2,3,5,7,13 heterozygous lines Pro/ Ser:
4,6,8,9,10,11,12,14,15,16,17 normals of the Pro / Pro series
Figure 3: Electro photogram of the TP53 gene polymorphism detector Pro47Ser.
4,6,8,9,10,11,12,14,15,16,17 normals of the Pro / Pro series
Figure 3: Electro photogram of the TP53 gene polymorphism detector Pro47Ser.
Statistical analysis of the results was carried out using statistical computer programs "WINPEPI 2016, Version 11.65" and "EpiCalc 2000, version 1.02".
Results
A total of 207 women were examined, 100 of whom were patients with B? with an average age of 34-72 years, and 107 were collected biomaterials from healthy women with an average age of 32-72 years. In our research groups, we identified TP53 (P47S) gene polymorphism [4 picture].
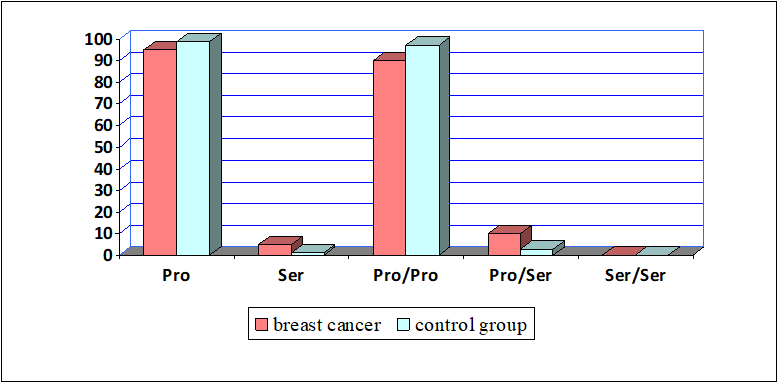
The figure above shows the distribution levels of alleles and genotypes. In this case, The Ser minor allele of the Pro47Ser polymorphism was present in 5% of patients with breast cancer and 1,4% in women in the control group, while the main Pro allele of this polymorphism was 95% in breast cancer and 98,6% in healthy women. The correlation of alleles between breast cancer and healthy women suggests that the Pro allele is more common in healthy women, indicating that it is protected from breast cancer. When comparing breast cancer and healthy women, it was found that the minor allele has a statistically significant correlation with breast cancer (χ2=3,3; p=0,03; OR=3,7; 95% CI: 1,0–13,6). It can be seen, the Ser allele increases the incidence of breast cancer by 3,7 times.
Analysis of the Pro47Ser polymorphism genotype for the TP53 gene showed that Pro/Pro genotype was present in 90% of cases in main group and 97.2% in the control group. Accordingly, correlations between high incidence of Pro/Pro genotype in control group and reduced risk of BC have been found. The Pro/Ser heterozygous genotype of this polymorphism was present in 10% of women in cancer group and 2,8% in healthy women. Consequently, we determined a statistically significant correlation of the Pro/Ser heterozygous genotype with BC (χ2=5,1; p=0,01; OR=4,7; 95% CI: 1,28–17,1). It can be seen, the Pro/Ser genotype increases the incidence of breast cancer by 4,7 times. It should be noted that Ser/Ser genotype was not observed in the main and control groups.
Thus, based on the results of the study, it was revealed that the Pro47Ser polymorphism of the TP53 gene is important in the mechanism of breast cancer development and can be considered as one of the genetic markers for prediction of breast cancer.
Discussion
There are several risk factors for breast cancer, and one of these risk factors is the polymorphism of the TP53 oncosuppressor gene [15]. One such polymorphism is Pro47Ser, with little information about the status of its distribution in populations [16]. Several studies have examined the participation of this polymorphism in the risk of breast cancer, including differences in ethnicity or race [17, 18]. However, there is no information about the role of Pro47Ser polymorphism in the TP53 gene in women with breast cancer in Uzbek women. Thus, this study evaluates the role of Pro47Ser polymorphism in the P53 gene of breast cancer P53 in Uzbek women.
The distribution of alleles and genotypes of the Pro47Ser polymorphism of the TP53 gene was determined in the main and control groups and compared with that in other populations. Pro47Ser polymorphism in African populations has an S47 allele frequency of 2-4% and ~ 1.2% in African Americans, while this allele has not been identified in Caucasian and Arab countries [17,19,20]. According to the results of our studies, the frequency of the S47 allele in the healthy control group of the ?ro47Ser polymorphism of the TP53 gene in Uzbek women was 1.4%.
Conclusion
- The frequency of alleles of the minor Ser-allele of the Pro47Ser polymorphism of the TP53 gene in the control group was 1,4%.
- Pro allele and Pro/Pro genotype of TP53 gene is correlated with reduced risk of BC.
- Ser allele and Pro/Ser genotype of TP53 gene is correlated with BC predisposition.
Acknowledgments
We thank the Director of the Institute of Biophysics and biochemistry at the National University of Uzbekistan's Sobirov, R. Z. for practical collaboration. Also, we would like to thank the head of the Department Karimov H.Y in Scientific research Institute of Hematology and blood transfusion and Ministry of health of the Republic of Uzbekistan for the support, staff of the Tashkent branch of the Republican specialized scientific-practical medical centre of Oncology and radiology.
We thank the Director of the Institute of Biophysics and biochemistry at the National University of Uzbekistan's Sobirov, R. Z. for practical collaboration. Also, we would like to thank the head of the Department Karimov H.Y in Scientific research Institute of Hematology and blood transfusion and Ministry of health of the Republic of Uzbekistan for the support, staff of the Tashkent branch of the Republican specialized scientific-practical medical centre of Oncology and radiology.
References
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D. M., Piñeros M., Bray F. 2019, Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International journal of cancer. 8(144): 1941-1953.
- Navruzov S. N., Alieva D. A. 2016, Oncology of Uzbekistan: achievements and prospects. Russian journal of Oncology. 21: 1-2.
- Khudaykulov T. K., Khudaykulov A. T. 2016, Incidence of breast cancer in Uzbekistan. Volga cancer Bulletin. no. 2 (24).
- Atlan M., Neman J. 2019, Targeted Transdermal Delivery of Curcumin for Breast Cancer Prevention. International Journal of Environmental Research and Public Health. 24(16): 4949.
- International classification of diseases of the tenth revision (ICC-10). Tashkent 2004: 406.
- Semiglazov V. F., Semiglazov V. V. 2010, Breast cancer Screening. Prakt. onkol. 11(2): 60-65.
- Huszno J., Grzybowska E. TP53 mutations and SNPs as prognostic and predictive factors in patients with breast cancer. Oncology letters. – 2018. – ?. 16. – ?. 1. – ?. 34-40.
- Hainaut P., Wiman K. G. (ed.). 25 years of p53 research. – Netherlands : Springer, 2005.-?-55.
- Chandel N., Chauhan A., Guleria K. 2013, The p. Pro47Ser Polymorphism of TP53: A systematic review. Int. J. Cancer Res. 9: 1-8.
- Campo E. Cymbalista, .., Ghia, P., Jäger, U., Pospisilova, S., Rosenquist, R., Stilgenbauer, S. 2018, TP53 aberrations in chronic lymphocytic leukemia: an overview of the clinical implications of improved diagnostics. Haematologica. 12(103): 1956-1968.
- Laptiev S. A., Korzhenevskaya M. A., Imyanitov E. N. 2017, Molecular genetic "portrait" of breast cancer. Scientific notes of Spbsmu. IP Pavlova. 2(24).
- Varna, M., Bousquet, G., Plassa, L. F., Bertheau, P., Janin, A. 2011, TP53 status and response to treatment in breast cancers. BioMed Research International.
- Sameer, A. S., Shah, Z. A., Syeed, N., Banday, M. Z., Bashir, S. M., Bhat, B. A., Siddiqi, M. A. 2010, TP53 Pro47Ser and Arg72Pro polymorphisms and colorectal cancer predisposition in an ethnic Kashmiri population. Genet Mol Res, 9(2): 651-660.
- Škere?ová, M., Halašová, E., Matakova, T., Jesenská, ?., Jure?eková, J., Šarlinová, M., Dobrota, D. 2017, Low variability and stable frequency of common haplotypes of the TP53 gene region in colorectal cancer patients in a Slovak population. Anticancer research, 37(4): 1901-1907.
- Jennis,M., Kung, C.P., Basu, S., et al. 2016, An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 30: 918–930.
- Basu S. Barnoud, T., Kung, C. P., Reiss, M., Murphy, M. E. 2016, The African-specific S47 polymorphism of p53 alters chemosensitivity. Cell cycle. 19(15): 2557-2560.
- Leu,J.I., Murphy, M.E., and George, D.L. 2019, Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc. Natl Acad. Sci. USA 116: 8390–8396.
- Budina-Kolomets, A., Barnoud, T., and Murphy, M.E. (2018). The transcription-independent mitochondrial cell death pathway is defective in non-transformed cells containing the Pro47Ser variant of p53. Cancer Biol. Ther. 19: 1033–1038.
- Alawadi Sh., Ghabreau L., Alsaleh M., Abdulaziz Z., Rafeek M., Akil N., Alkhalaf M. (2011). P53 gene polymorphisms and breast cancer risk in Arab women. Med. Oncol. 28: 709–715.
- Barnoud, T., Parris, J. L., Murphy, M. E. (2019). Common genetic variants in the TP53 pathway and their impact on cancer. Journal of molecular cell biology, 11(7): 578-585.
Citation: Kodirova Dilbar Abdullaevna, Avezov Nodirjon Shaxriqulovich, Ibragimov Zafar Zokirjonovich., et al. (2020). Tp 53 Oncosupressor Gene Pro47ser Polymorphism in the Development of Breast Cancer. Journal of Medical Research and Case Reports 2(3).
Copyright: © 2020 Ibragimov Zafar Zokirjonovich. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.