Review Article
Volume 1 Issue 1 - 2019
The Relationship between Serum Brain Derived Neurotrophic Factor Levels and Post Stroke Depression in Chinese Patients: A Meta-Analysis
The Cadre ward in Department of Neurology, the people's hospital of guangxi zhuang autonomous region, No.6?Tao Yuan Road, Nanning, Guangxi, 530021, China
*Corresponding Author: Min Han, the Cadre ward in Department of Neurology, the people's hospital of guangxi zhuang autonomous region, No.6?Tao Yuan Road, Nanning, Guangxi, 530021, China.
Received: March 19, 2019; Published: March 29, 2019
Abstract
Background: To explore the relationship between serum brain derived neurotrophic factor (BDNF) level and post stroke depression (PSD).
Methods: Prospective randomized controlled trails of the relationship between serum BDNF level and PSD were selected from database such as PubMed, CNKI, VIP Data, Wangfang Data. Review Manager 5.4 was used for the Meta analysis.
Results: 10 case-control studies were collected, there were 579 PSD patients and 639 stroke patients without depression. The results showed that PSD patients exhibited significantly lower serum BDNF levels than stroke patients without depression in the Chinese population (SMD =-5.82, 95% CI: -6.21 – -5.44). Additionally, PSD patients with mild depression showed significantly elevated serum BDNF levels compared with PSD Patients with moderate depression (SMD = 1.98, 95% CI: 1.05–2.91) or with severe depression (SMD = 4.73, 95% CI:3.78–5.67). Serum BDNF levels decreased as the degree of depression increased, and were negatively correlated with the degree of depression.
Conclusion: Our study suggested that serum BDNF levels were found significantly associated with the PSD patients in the Chinese population.
Keywords: Serum brain derived neurotrophic factor; Post stroke depression; Chinese population; and Meta-analysis
Introduction
As the third leading cause of death worldwide, and a major health issue in the elderly population, stroke not only results in physical impairments such as disability, but also leads to social nonparticipation and psychological disease. Post-stroke depression (PSD) is one of the most common neuropsychiatric sequelae of stroke that affects around 33% of stroke patients and has been associated with both poorer outcome and increased mortality. Additionally, studies suggested that the prevalence of PSD has increased from28% to 56% recently [1]. PSD has become a prominent negative factor in stroke recovery. However, benefit of present used antidepressant drug was not satisfactory [2].
Previous studies showed that PSD may be related to numerous factors, including biological, psychological and social factors [3]. Although, the pathogenesis of PSD remained unclear. Therefore, further study on the pathogenesis of PSD and development of effective prevention and treatment are in urgent needs. Recently, brain-derived neurotrophic factor (BDNF), as one of the main neuroprotective mediators in the central nervous system, attracted increasing attention from both research and clinical fields because of its important functions and its serum level notably affected in the central nervous system. Some of researches shown that BDNF was involved in delayed neurological recovery, depression, and cognitive impairment following stroke [4].
Accordingly, there is accumulating evidence for the role of BDNF in the pathophysiology of depression. However, the association between BDNF and PSD remained unelucidated, and it is still unclear whether BDNF affected PSD. Recent study showed that serum concentrations of BDNF decreased in PSD patients and BDNF may play an important role in the pathogenesis of PSD [5]. In addition?Zhang GP. et al. also found that there was an association between decreased serum BDNF and PSD [6]. Although, some studies showed conflicting results on the relation between serum BDNF and PSD. For instance, Du DB. et al. found that there were no differences in serum BDNF levels between PSD and stroke patients without depression [7]. Likewise, Guo RY. et al. also claimed that PSD patients had serum BDNF levels similar to stroke patients without depression [8]. Until now, the relationship between serum BDNF levels and PSD was controversial. To our knowledge, no previous meta-analysis article has compared the serum BDNF levels between PSD patients and the stroke patients without depression. Therefore, we performed the present meta-analysis study to determine the influence of BDNF on PSD risk. Our aim was to test the possible association between BDNF and PSD.
Materials and Methods
Identification of studies
Eligible studies were identified by searching in the Cochrane Central Register of Controlled Trials (CENTRAL), Pubmed, Embase, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Database (CBM) for relevant trials without language restrictions using the following mesh search terms :serum brain derived neurotrophic factor, post stroke depression, Chinese population, stoke, depression, case-controlled trial. Terms were exploded whenever possible in each database. We fixed Sep. 2018 as the cut-off date for inclusion of studies.
Eligible studies were identified by searching in the Cochrane Central Register of Controlled Trials (CENTRAL), Pubmed, Embase, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Database (CBM) for relevant trials without language restrictions using the following mesh search terms :serum brain derived neurotrophic factor, post stroke depression, Chinese population, stoke, depression, case-controlled trial. Terms were exploded whenever possible in each database. We fixed Sep. 2018 as the cut-off date for inclusion of studies.
Inclusion criteria for relevant studies
We included studies in our review that met all of the following criteria: (1) case control design. (2) studies needed to providing information on the criteria for diagnosing stroke and depression; (3) stroke patients without depression as controls; (4) reporting results on the association between serum BDNF levels and post stroke depression (PSD), as well as stroke patients without depression; and data was expressed as mean ± standard deviation; (5) Studies would be excluded if they were: no original data, no controls, review articles and overlapping data.
We included studies in our review that met all of the following criteria: (1) case control design. (2) studies needed to providing information on the criteria for diagnosing stroke and depression; (3) stroke patients without depression as controls; (4) reporting results on the association between serum BDNF levels and post stroke depression (PSD), as well as stroke patients without depression; and data was expressed as mean ± standard deviation; (5) Studies would be excluded if they were: no original data, no controls, review articles and overlapping data.
Outcome measures for this review
Two independent reviewers obtained full manuscripts of all citations that were likely to meet the predefined selection criteria. They independently reviewed the articles and extracted the data from the included studies. Areas of disagreement or uncertainty were resolved by discussion and consultation with a third reviewer. When multiple articles were published from a single study, we selected the report that contained the most complete and relevant data on the association of serum BDNF levels between PSD patients and the stroke patients without depression. The study characteristics, number of PSD patients and the stroke patients without depression, and data of serum BDNF levels were extracted from each selected article. Standardized mean difference (SMD) and 95%confidence interval (CI) were calculated to estimate the association between serum BDNF levels and PSD. The levels of serum BDNF were all measured using enzyme-linked immunosorbent assay (ELISA) in the included studies. We calculated statistical heterogeneity by an X2 test on N-1 degrees of freedom with P < 0.1 indicating significant heterogeneity [9]. To evaluate the heterogeneity, we also used the I2 test, taking values in the range 0-100% [10]. I2 values of 25% may be represent low, 50% modest, and 75% large heterogeneity, respectively [11]. When P ≥ 0.1 or I2 ≤ 50% indicated a lack of heterogeneity, the fixed effect model was employed to pool all studies for summary SMD estimation. Otherwise, the random effect model was chosen. Potential publication bias was examined using funnel plots [12]. A sensitivity analysis was also performed by repeating the meta-analysis and omitting each study at each iteration [13]. The calculations were performed using the Revman 5.4 Statistical software.
Two independent reviewers obtained full manuscripts of all citations that were likely to meet the predefined selection criteria. They independently reviewed the articles and extracted the data from the included studies. Areas of disagreement or uncertainty were resolved by discussion and consultation with a third reviewer. When multiple articles were published from a single study, we selected the report that contained the most complete and relevant data on the association of serum BDNF levels between PSD patients and the stroke patients without depression. The study characteristics, number of PSD patients and the stroke patients without depression, and data of serum BDNF levels were extracted from each selected article. Standardized mean difference (SMD) and 95%confidence interval (CI) were calculated to estimate the association between serum BDNF levels and PSD. The levels of serum BDNF were all measured using enzyme-linked immunosorbent assay (ELISA) in the included studies. We calculated statistical heterogeneity by an X2 test on N-1 degrees of freedom with P < 0.1 indicating significant heterogeneity [9]. To evaluate the heterogeneity, we also used the I2 test, taking values in the range 0-100% [10]. I2 values of 25% may be represent low, 50% modest, and 75% large heterogeneity, respectively [11]. When P ≥ 0.1 or I2 ≤ 50% indicated a lack of heterogeneity, the fixed effect model was employed to pool all studies for summary SMD estimation. Otherwise, the random effect model was chosen. Potential publication bias was examined using funnel plots [12]. A sensitivity analysis was also performed by repeating the meta-analysis and omitting each study at each iteration [13]. The calculations were performed using the Revman 5.4 Statistical software.
Result
139 studies were identified from the initial search, we identified 10 studies for the meta-analysis [7,8, 14-21]. All these studies were the PSD and the levels of serum BDNF correlation study. Three studies of them showed the association between the degree of PSD and serum BDNF levels [18, 20, 21] . We excluded 129 studies for the following reasons: reviews or case reports, the publications dealt with other topics, duplication of data, using other diseases as control or not available as full text articles. All eligible studies were considered case–control in design. Selected characteristics of the included studies are summarized in Table 1. These studies represented data from 579 PSD patients and 639 stroke patients without depression in the Chinese population.
| year | Author | N | serum BDNF levels(ug/L) | ||
| PSD | N-PSD | PSD | N-PSD | ||
| 2010 | Guo | 46 | 50 | 25.8 ± 8.35 | 24.2 ± 7.48 |
| 2010 | Du | 38 | 30 | 12.44 ± 4.33 | 8.56 ± 3.55 |
| 2011 | Wang | 46 | 50 | 25.42 ± 8.32 | 23.87 ± 7.26 |
| 2013 | Lu | 86 | 68 | 17.2 ± 3.48 | 18.4 ± 4.15 |
| 2013 | Zhu | 40 | 38 | 24.87 ± 4.02 | 33.61 ± 2.62 |
| 2015 | Zhao | 33 | 55 | 22.9 ± 4.86 | 28.24 ± 4.68 |
| 2016 | Li | 40 | 50 | 16.75 ± 4.45 | 29.55 ± 3.21 |
| 2016 | Zhang | 43 | 57 | 18.76 ± 2.47 | 38.52 ± 4 |
| 2016 | Liu | 168 | 211 | 7.1 ± 2.28 | 11.43 ± 2.89 |
| 2016 | Li | 38 | 30 | 21.38±4.26 | 28.75±3.58 |
Table 1: The characteristics of all studies included in the meta-analysis.
PSD: post stroke depression, N-PSD: stroke patients without depression, BDNF: brain derived neurotrophic factor.
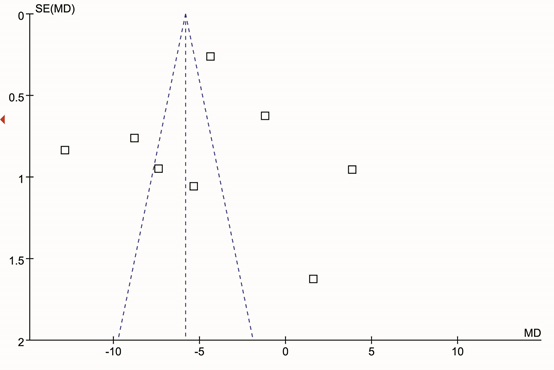
There was significant statistical heterogeneity among the studies, thus, a random effect model was employed. Pooling all studies for summary SMD estimation, PSD patients have significant lower BDNF levels than stroke patients without depression (SMD =-5.82, 95% CI: -6.21 – -5.44 (Figure 1). There was an evidence of an association between the reduced serum BDNF levels and PSD patients. In the subgroup analysis, we studied the association between the degree of PSD and serum BDNF levels. For the subgroup analysis, data for 251 patients of three studies were available. The PSD patients with mild depression showed significantly elevated serum BDNF levels compared with PSD patients with moderate depression (SMD = 1.98, 95% CI: 1.05–2.91 (Figure 2) or with severe depression (SMD = 4.73, 95% CI: 3.78–5.67 (Figure 3). In addition, the PSD patients with moderate depression also showed significantly elevated serum BDNF levels compared with severe depression (SMD = 3.95, 95% CI: 1.37–6.53 (Figure 4). Serum BDNF levels decreased as the degree of depression increased, and were negatively correlated with the degree of depression. The results remained the same when we performed a secondary analysis by repeating the meta-analysis and omitting each study at each iteration. Publication bias may be acceptably low because all the funnel plots for the included studies did not reveal obvious signs of publication bias (Figure 5).
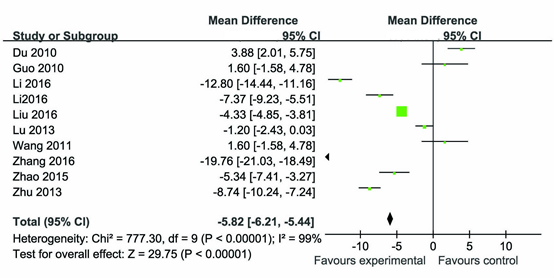
Figure 1: The serum BDNF levels in PSD patients compared with stroke patients without depression in the Chinese population.

Figure 2: The serum BDNF levels in PSD patients with mild depression compared with PSD Patients with moderate depression in the Chinese population.

Figure 3: The serum BDNF levels in PSD patients with mild depression compared with PSD Patients with severe depression in the Chinese population.

Figure 4: The serum BDNF levels in PSD patients with mild depression compared with PSD Patients with severe depression in the Chinese population.
Discussions
PSD is one of the most frequent neuropsychiatric consequences of stroke. Depression also negatively impacts stroke outcome with increased morbidity, mortality and poorer functional recovery [22]. Therefore, early diagnosis of PSD would greatly benefit patients with relevant disorders in terms of decreasing their disability and mortality. However, the pathogenesis of PSD is not unclear and the identification of PSD is still a significant clinical problem. So the clinicians and researchers have tried to identify predictors that indicated patients at risk of developing PSD. One of the most widely potential biomarkers is BDNF which is a neurotrophin that is involved in neuronal cell growth, survival, and synaptic plasticity [23]. Recent years, there have been many documents which shown a strong relationship between BDNF levels and PSD [24, 25]. Furthermore, in stroke survivors diagnosed with PSD, serum BDNF concentrations were found to be decreased at 3–6 months post-event [25]. Several studies also reported that reduced BDNF levels were associated with an increased risk of subsequent depression [18, 20]. However, it is still unclear whether BDNF affects PSD. We believe this is the first meta-analysis study to examine relationship between serum BDNF levels and PSD. Overall, we found that PSD patients had lower serum BDNF levels than the stroke patients without depression. Our findings are consistent with most prior studies of BDNF levels and PSD [5, 6].
In Addition, the subgroup analysis confirmed that the degree of depression was negatively related to the levels of BDNF?It is currently accepted that stroke may cause the reduction or even deficiency of BDNF, which is an important factor in the cause of PSD [26]. So, we though that serum BDNF levels should be used as the degree of depression in patients with stroke. And the BDNF level differences between PSD and the stroke patients without depression suggested that a possible association between expression of BDNF in the pathogenesis of PSD. The complex interaction between BDNF and PSD might be explained by the follow reasons. First, BDNF protects against ischaemic brain injury and attenuates apoptosis in cultured neurons after glucose deprivation [27, 28]. In addition, BDNF is promising as a candidate molecule underlying the structural changes associated with ischemia damage, and as a potential target for cerebral ischemia injury [29, 30]. Furthermore, BDNF signalling is crucially involved in hippocampal neurogenesis [31]. Besides, BDNF in periinfarct cortex improves functional recovery after stroke [32].
As previously noted, some publication reported that antidepressants have been shown to increase BDNF levels in the brain, and higher serum BDNF may predict better antidepressant response[33]. Furthermore, BDNF produces antidepressant effects in behavioural models of depression and confers resilience to chronic stress[34]. Therefore, it is estimated that serum BDNF levels can be used as the antidepressant effect of reference index?Most importantly, a number of studies have also reported beneficial effects of antidepressants and especially of selective seroto-nin reuptake inhibitors (SSRIs) on post- stroke outcome including activities of daily living as well as cognitive and executive functioning [23, 35]. Antidepressant treatment initiated soon after stroke may prevent the emergence of PSD [5, 36].
Despite an extensive research effort, the exact etiology of PSD remains elusive. Thus, polymorphisms of BDNF have been investigated as candidate genes for PSD. Recently, the BDNF Val66Met polymorphism have been found to modify the association between stroke and depression [37], which provides a direction for the investigation of mechanisms underlying the pathogenesis of PSD and brings promise for the effective prevention, diagnosis, and therapy of PSD [38]. Although?Lu L., et al. found that no significant differences were demonslrated in Val66Met BDNF genotype (P=0.844) or allele frequencies(P=0.899)between PSD patients and the controls in the Chinese patients [14]. So, it remains unclear whether BDNF gene was associated with the risk of PSD. With the development of biomedical techniques, increasing studies are undertaken to investigate the targets of BDNF gene, which not only is beneficial to elucidate the mechanisms underlying the development of PSD, but also provides theoretical evidence for the diagnosis and treatment of PSD.
Conclusion
Our results showed that there was an evidence of an association between the reduced serum BDNF levels and PSD patients. These findings suggested a possible association between expression of BDNF in the the pathogenesis of PSD. Future attempts should be done to unravel this neurotrophin in the pathogenesis of PSD.
Acknowledgments
We would like to acknowledge the investigators for their helpful comments on this paper.
We would like to acknowledge the investigators for their helpful comments on this paper.
Conflict of interest
We have no conflicts of interest with regard to the content of this article.
We have no conflicts of interest with regard to the content of this article.
References
- Adachi, N., et al. (2014). New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J Biol Chem, 5 (4): p. 409-28.
- Kohn, N., et al., (2014). Neural correlates of effective and ineffective mood induction. Soc Cogn Affect Neurosci, 9 (6): p. 864-72.
- Feng C, Fang M, and L. XY., (2014). The neurobiological pathogenesis of poststroke depression. ScientificWorldJournal, 04 p. 20.
- Zhou, Z., et al., (2015). Association between Single-Nucleotide Polymorphisms of the Tyrosine Kinase Receptor B (TrkB) and Post-Stroke Depression in China. PLoS One, 10 (12).
- Rasmussen A, et al.,(2003). A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychoso-matics., 44: p. 216-21.
- Zhang GP, Wang LL, and Wang HY. (2015). Correlation of post—stroke depression with stroke deficit scale and serum brain-derived neurotrophie factor levels in the elderly Chin J Geriatr, 34 (6): p. 612-615.
- Du DB. (2010). Correlation of post—stroke depression and serum brain-derived neurotrophie factor levels. medical information, 05 (12): p. 3544-3545.
- Guo RY, et al., (2010). Study on the serum leptin and IGF-l in the elderly patients with post-stroke depression .Chin J Behav Med?Brain Sci, 19 (9): p. 798-800.
- Bowden, J., et al., (2011). Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol, 11: p. 41.
- Higgins, J.P., et al., (2003). Measuring inconsistency in meta-analyses. BMJ, 327 (7414): p. 557-60.
- Ioannidis, J.P., N.A. Patsopoulos, and E. Evangelou, (2007). Uncertainty in heterogeneity estimates in meta-analyses. BMJ, 335 (7626): p. 914-6.
- Egger, M., et al., (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315 (7109): p. 629-34.
- Saltelli, A., (2002). Sensitivity analysis for importance assessment. Risk Anal, 22 (3): p. 579-90.
- Lu Lp, Zhu YB and Cheng JY, (2013). The Correlation between Vai66Met Polymorphism in Brain-derived Neurotrophic Factor and Post-stroke Depression. Chin J Clin Neuro sci, 21(4): p. 413-417.
- Wang CX, (2011). Serum marker for predicting mechanism and the diagnosis effect of poststroke depression, Qingdao University.
- Zhu JY, et al., (2013). The significance of serum brain-derived neurotrophic factor in post-stroke depression., Chinese Journal of Practical Nervous Diseases Dec, 16 (23): p. 89-90.
- Zhao K, Gu DD, and YangY, (2015). Study on the Content of Serum BDNF in Early Stage and Related Factors in Patients with Depression after Acute Stroke., Prevention and Treatment of CardioCerebral Vascular Disease (3): p. 194-196.
- Li W, (2016). The correlation and significance between depression after stroke and BDNF. Guide of China Medicine, 14 (9): p. 153-154.
- Li J, et al., (2016). Correlation between selllm brain-derived neurotrophic factor level and depression after ischemic stroke. Int J Cerebrovasc Dis, 24 (9): p. 815-818.
- Zhang HM, Zhang HY,and Liang W, (2016). Correlation between depression after stroke and serum brain-derived neurotrophic factor in senile patients. Chinese Journal of Practical Nervous Diseases, 19 (16): p. 111.
- Liu X and Yang WD, (2016). Post-stroke depression level and the level of brain derived neurotrophic factor correlation studies. Chinese geriatric health care medicine, 14 (1): p. 21-23.
- Loubinoux, I., et al., (2012). Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med, 16 (9): p. 1961-9.
- Jorge RE, Acion L, and e.a. Moser D, (2010). Escita-lopram and enhancement of cognitive recovery following stroke. Arch Gen Psy-chiatry. , 67.2: p. 187–96.
- Yang L, et al., (2011). Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int J Geriatr Psychiatry, 26: p. 485-502.
- Zhou Z, et al., (2011). Decreased serum brain?derived neurotrophic factor (BDNF) is associated with post?stroke depression but not with BDNF gene Val66Met polymorphism. . Clin Chem Lab Med. 49: p. 185-9.
- Yan, H., M. Fang, and X.Y. Liu, (2013). Role of microRNAs in Stroke and Poststroke Depression. ScientificWorldJournal, 2013.
- Jiang Y, Wei N, and e.a. Lu T, (2011). Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience., 172: p. 398–405.
- Tong L and P.-P. R., (1998). Brain-derived neurotrophic factor (BDNF) protects cultured rat cerebellar granule neurons against glucose deprivation-induced apoptosis. J Neural Transm., 105: p. 905–14.
- Pizarro JM, et al., (2004). Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res, 1025: p. 10-20.
- Zhao Y, et al., (2015). Minocycline upregulates cyclic AMP response element binding protein and brain-derived neurotrophic factor in the hippocampus of cerebral ischemia rats and improves behavioral deficits. Neuropsychiatric Dis Treat, 11: p. 507–16.
- Chan JP, et al., Calderon GA, (2008). Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 39: p. 372–83.
- Clarkson AN, et al., Zhong S, (2011). AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci., 31: p. 3766–75.
- Kurita M, et al., (2012). Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PloS One, 7: p. e39212.
- Shirayama Y, et al., (2002). Nakagawa S, Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci., 22: p. 3251-61.
- Acler M, R. E?et al., (2009). A dou-ble blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol., 256: p. 1152–8.
- Moser DJ, et al., (2008). Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA., 299: p. 2391–400.
- Kim JM, Stewart R, and Kim SW, (2008). BDNF genotype potentially modifying the association between incident stroke and depression. Neurobiol Aging, 29: p. 789–92.
- Lasek-Bal, A., et al., (2015). Low Concentration of BDNF in the Acute Phase of Ischemic Stroke as a Factor in Poor Prognosis in Terms of Functional Status of Patients. Med Sci Monit, 21: p. 3900-5.
Citation: Bo Liu Luo Wenjing Yingmin Mo Chunying Wei Ran Tao and Min Han. (2019). The Relationship between Serum Brain Derived Neurotrophic Factor Levels and Post Stroke Depression in Chinese Patients: A Meta-Analysis. Journal of Brain and Neurological Disorders 1(1).
Copyright: © 2019 Min Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.