Research Article
Volume 1 Issue 2 - 2019
Synthesis and Characterization of Bioactive Composite Material Comprising Silver Nanoparticles and Activated Carbon to Produce Bacteria free Potable Water.
1Allama Iqbal Open University Islamabad Pakistan
2SCME National University of Science & Technology Islamabad Pakistan
2SCME National University of Science & Technology Islamabad Pakistan
*Corresponding Author: Muhammad Aslam Tahir, Allama Iqbal Open University Islamabad Pakistan.
Received: November 14, 2019; Published: November 27, 2019
Abstract
Composite material of silver nano-particles (SNPs) and activated carbon was synthesized by wet chemical method. Morphology and particle size of SNPs were investigated by Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) and X-ray powder diffraction (XRD). Particle size obtained from XRD data analysis using Debye Scherer formula comes out around 14nm while crystalline structure of SNPs was investigated as face centered cubic (fcc). Morphology of SNPs in activated carbon was studied using SEM. It shows well distributed, circular shaped SNPs and their agglomerates in pores of activated carbon. Presence of silver in the nano-composite was confirmed through Energy Dispersive X-ray (EDX) analysis. TEM shows majority of nanoparticles lying in the range between 10 to 20 nm while presence of metallic phase (fcc) of silver was further confirmed through electron diffraction studies. Microbiological activity of composite as antibacterial was examined through flow method, using open source water infected by Gram-negative (E. coli). One (1) gm. of nano-composite was found effective in sterilizing up to 55L of infected water. Antibacterial efficacy of the nano-composite was further verified against B. Subtilis and E. coli using disk diffusion method. Overall results show that composite material is a promising candidate for purification of open source water.
Key words: Silver nanoparticles; Bioactive composite; Water treatment; Nano-composite; Activated carbon.
Introduction
Effectiveness of functional material is directly related to its surface area which can be increased tremendously by using nano technology. Nano-technology is a branch of science, which is well equipped with new research methods, modern techniques and latest instruments [1]. It is being used to explore properties of material at atomic and molecular level. Such properties of material can’t be investigated and studied in its bulk form.
In recent years, development of metallic nano-particles have provided suitable solutions to cope with today’s challenges in several different areas like solar energy [2], medicine [3], chemical synthesis [4], protection of humans, animals [5] and structures from hazardous environmental effects [6]. Provision of large surface area is an important parameter of nano-scale material. Usually particle size of nano-scale material lies in the range from 1 to 100nm [7]. Nano-technology is a scientific art and modern technique which contributes effectively to reshape our future by synthesizing advanced materials.
Silver and its compounds have strong back ground related to inhibition of bactericidal effects and antimicrobial ability for fungi, and virus as well [8]. Versatile usage of silver has been reported in literature, for example in medicine, it is used to decrease infections in burn treatment, arthroplasty and to prevent bacterial growth on prostheses [9, 10]. Moreover as compared to other metals, silver is toxic to microorganisms but less toxic to mammalian cells [11] and due to this factor silver is considered as nontoxic metal [12]. Silver is also known since long time owing to its applications, however its wide spectrum usage as antimicrobial has been established in last two decades [13].
Actual mechanism as antimicrobial of SNPs on bacteria is still unknown, however possible mode of action has been proposed according to chemistry and structural changes in bacterial cell [9]. SNPs may target bacterial membrane, respiratory chain and cell division that ends to the cell’s death. Another view is that SNPs may also enter inside the bacteria and damage its phosphorus and sulfur-containing compounds such as DNA [14]. Minute quantity of SNPs may not have an acute impact on human health [15]. However, their long-term health effects still needed further study. Being small size silver ions and nano-particles may have adverse effects to human eyes, skin, respiratory track, liver, kidney and blood cells [16].
A variety of different synthetic techniques have been used to develop SNPs. Some famous methods include, electron irradiation laser ablation, γ-radiation, microwave, biological, physical, chemical, and photochemical methods [17]. Most of these methods are still in development stage and possess merits and demerits. Problems related to these methods are the lack of product stability, control over particle size, shape, and morphology [18]. In present work, wet chemical method is used to develop SNPs at normal temperature without need of any kind of energy. It is most common, simple, cost effective and stable procedure with a choice of wide variety of reducing agents [19].
Activated carbon is a highly porous material with exceptionally large surface area, used as a part of composite material [20]. Activated carbon is a most suitable candidate for adsorption of volatile organic compounds and gases [21]. It is inert with moderate stability and is therefore, can be used effectively for purification of drinking water [22]. Along with these advantages of activated carbon, if it is impregnated with SNPs, can minimize bacterial growth or even completely eliminate it from drinking water [23]. Here antimicrobial action of composite material has been performed using open source water infected by Gram-negative (E. coli). It is a testing protocol of all the organisms that if E. coli is killed, by any means, then all other waterborne pathogens are assumed to be killed.
Experimental
For composite material, silver nitrate solutions of various concentrations 0.01-0.06 mol. /liter (table: 1)
For composite material, silver nitrate solutions of various concentrations 0.01-0.06 mol. /liter (table: 1)
| Test Pathogens | Inhibition Zone | ||
| Sample of silver 33.5% | Sample of silver 17% | Simple activated carbon | |
| B. Subtilis | 14mm | 08mm | 0 |
| E. coli | 18mm | 10mm | 0 |
Table 1: Inhibition Zone of SNPs against Gram-positive and Gram-negative bacteria.
Were prepared by dissolving silver nitrate salt in de-ionized water in glass beakers. The solutions of white color were kept in dark to avoid decomposition of AgNO3 in light. To ensure complete dissolution of salt in water, solutions were stirred continuously. For stable silver ions, ammonia solution was added drop wise during this process. Jacobi brand activated carbon of mesh size 12/40 was soaked to AgNO3 solutions prepared before. After 24h of soaking in the dark, the solutions were decanted and carbon was washed with de-ionized water to remove AgNO3 loosely attached until no AgNO3 was observed in the filtrate. This ensured that only strongly attached AgNO3 was there with carbon. Ionic silver was reduced to metallic silver by drop wise addition of sodium boro-hydrate solution along with continuous shaking.
Drop wise addition helps to avoid clusters formation and to achieve stability of SNPs [24]. Excess sodium boro-hydrate was removed by washing with de-ionized water followed by drying.
Antibacterial ability of synthesized composite material was assessed by following two methods.
- Flow method.
- Disk diffusion method.
Water disinfection study was carried out by using open source water infected by E. coli which is a testing protocol of all water borne pathogen. In flow method, water having 18+E. coli was passed@ 0.5 Lmin-1into 14gm different composite material taken in burette. Samples of effluent were collected in sterilized bottles after every 50 liter of infected water passed. Samples of treated water collected so for were tested against E. coli survivors.
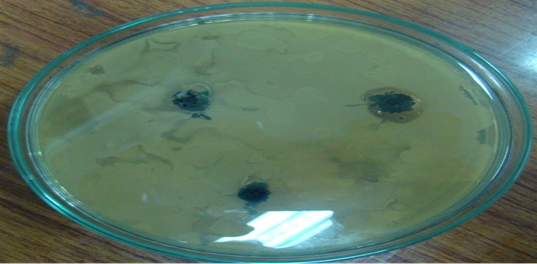
In disk diffusion method two sterilized petri dishes were used to grow B. Subtilis and E. coli bacteria culture by Mueller-Hinton Agar medium at room temperature. Then about 30mg of each sample of composite material (two samples of 0.03 and 0.06 mol. L-1AgNO3) and simple activated carbon (as a control) were loaded on petri dishes of B. Subtilis and E. coli.Petri dishes were incubated at 37°C for 24h and then diameter of inhibition zones were measured shown in table: 2. Composite material killed bacteria as seen by Inhibition zones around it shown in figure 1(A&B).
Inhibition zones were found proportional to the amount of silver in sample. No inhibition zone was seen around simple activated (control). It was also proven that SNPs were stable in carbon pores and were not washed away to mix with treated water.
Results and Discussion
SEM showed good quality surface morphology of composite material. SEM images consisted of well-defined circular shape of small and large sizes shown in figure 2A.
Particle sizes were found in between 40 to 70nm. This large size might be due to study of images at low resolution in SEM. Morphology of SNPs varied from single particle to agglomerates (bright spots) shown in figure 2B
Some areas of pores were also investigated where SNPs were not uniformly impregnated. This deficiency may be improved by increasing stirring time, reduction process and temperature for which R&D is underway. Energy Dispersive X-ray (EDX) analysis of SNPs impregnated on activated carbon showed high intensity silver and carbon peaks, with very low intensity peak of Oxygen. First, it verified that white spots were of silver particles. Secondly, it showed a very small amount of Oxygen which might be due to instrumental error. In fact relative atomic weight percent of elements, carbon and silver (obtained from EDX), were consistent with relative weight percent in composite material. Two samples with silver, 17 and 33.5% by weight in final product was verified by EDX also.
TEM study was performed to determine whether the silver nano-particles were occurring freely dispersed, as aggregated silver particles or in the form of complex colloids.
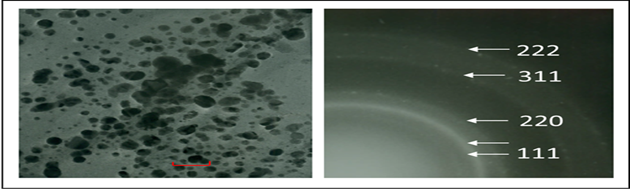
TEM shows typical images of silver nano-particles dispersed freely along with some aggregates too.TEM showed that sizes of majority of SNPs were lying in between 10-20nm.This size range of silver nano-particles was comparable to the size calculated by Debye Scherer formula based on XRD data analysis (14nm).Presence of metallic phase (fcc) of silver was further confirmed through electron diffraction study as shown above in figure 3.
Composite material was also studied by taking its scan in XRD in the range of 10 to 90° shown in fig: 4.
XRD confirmed face-centered cubic crystal structure of pure SNPs which was determined earlier in diffraction pattern [25].
Five prominent Bragg reflections at 38.115°(111), 44.299°(200), 64.443°(220), 77.397°(311) and 81.541°(222) were closely related to literature values (JCPDS No. 04-0783) [26] given in table 3.
| 2θ | I | h | k | l |
| 38.115 | 999 | 1 | 1 | 1 |
| 44.299 | 457 | 2 | 0 | 0 |
| 64.443 | 225 | 2 | 2 | 0 |
| 77.397 | 222 | 3 | 1 | 1 |
| 81.541 | 61 | 2 | 2 | 2 |
Table 2: Five XRD Bragg reflections of composite material.
These values can be indexed according to the facts of face centered cubic crystal structure of silver [27]. Size of SNPs was also calculated using XRD peak of maximum intensity (38.115o) by Debye Scherer formula given as
Dp = 0.94 ? / β1/2 cos θ
Where Dp = is average crystallite size.
β1/2 = 0.6 {full widths at half maximum (FWHM)}
θ = Bragges angle (19.06)
λ = X-ray wavelength (Cuk1=1.5406 Å)
Dp = 14nm.
Dp = 0.94 ? / β1/2 cos θ
Where Dp = is average crystallite size.
β1/2 = 0.6 {full widths at half maximum (FWHM)}
θ = Bragges angle (19.06)
λ = X-ray wavelength (Cuk1=1.5406 Å)
Dp = 14nm.
Conclusion
Composite material of silver and activated carbon has been developed by wet chemical method. Morphology, phase and particle sizes of SNPs were characterized by XRD, SEM, EDX and TEM. Crystal structure of silver nano-particle was face centered cubic (fcc).Majority of particles were of circular shape and were well distributed in carbon pores along with some agglomerates. Particle size most of the particle lying in between 10-20nm. Activated bacterial activity of composite has been tested against open source water infected by B. Subtilis and E. coli. Overall results show the potential of composite material for use in water purification to produce portable quality water.
Currently, in world, safe drinking water is made using chemicals, UV lamps or by heating/ boiling. Chemical treatment is easy but not necessarily safe for long run. Other methods required electricity/fuel which makes it costly and impossible in the areas where there is no electricity. In all these circumferences silver composite is safe and most suitable alternate for purification of drinking water.
References
- Whitesides, G.M., (2005). Nanoscience, nanotechnology, and chemistry. Small. 1(2): p. 172-179.
- Kamat, P.V., (2007). Meeting the clean energy demand: nanostructure architectures for solar energy conversion. The Journal of Physical Chemistry C, 111(7): p. 2834-2860.
- Liao, H., C.L. Nehl, and J.H. Hafner, Biomedical applications of plasmon resonant metal nanoparticles. 2006.
- Sau, T.K. and A.L. Rogach, (2010). Nonspherical noble metal nanoparticles: colloid?chemical synthesis and morphology control. Advanced Materials. 22(16): p. 1781-1804.
- Ahamed, M., M.S. AlSalhi, and M. Siddiqui. (2010). Silver nanoparticle applications and human health. Clinica chimica acta. 411(23): p. 1841-1848.
- Fabrega, J., et al., (2011). Silver nanoparticles: behaviour and effects in the aquatic environment. Environment international, 37(2): p. 517-531.
- Zhang, H., (2013). Application of silver nanoparticles in drinking water purification. Citeseer.
- Cho, K.-H., et al., (2005). The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochimica Acta, 51(5): p. 956-960.
- Rai, M., A. Yadav, and A. Gade, (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology advances. 27(1): p. 76-83.
- Alt, V., et al., (2004). An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 25(18): p. 4383-4391.
- Zhao, G. and S.E. Stevens Jr, (1998). Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals. 11(1): p. 27-32.
- Percival, S., P. Bowler, and D. Russell, (2005). Bacterial resistance to silver in wound care. Journal of hospital infection. 60(1): p. 1-7.
- Lok, C.-N., et al., (2006). Proteomic analysis of the mode of antibacterial action of silver nanoparticles. Journal of Proteome research. 5(4): p. 916-924.
- Hatchett, D.W. and H.S. White, (1996). Electrochemistry of sulfur adlayers on the low-index faces of silver. The Journal of Physical Chemistry. 100(23): p. 9854-9859.
- Yang, J., et al., (2010). Common SNPs explain a large proportion of the heritability for human height. Nature genetics. 42(7): p. 565-569.
- Panyala, N.R., E.M. Peña-Méndez, and J. (2008). Havel, Silver or silver nanoparticles: a hazardous threat to the environment and human health. J Appl Biomed. 6(3): p. 117-129.
- Iravani, S., et al., (2014). Synthesis of silver nanoparticles: chemical, physical and biological methods. Research in pharmaceutical sciences. 9(6): p. 385.
- Baleanu, D., Z.B. Güvenç, and J.T. Machado, (2010). New trends in nanotechnology and fractional calculus applications: Springer.
- Iravani, S., (2011). Green synthesis of metal nanoparticles using plants. Green Chemistry. 13(10): p. 2638-2650.
- Mochida, I., et al., (2000). NO oxidation over activated carbon fiber (ACF). Part 1. Extended kinetics over a pitch based ACF of very large surface area. Fuel. 79(14): p. 1713-1723.
- Bansal, R.C. and M. Goyal, (2005). Activated carbon adsorption: CRC press.
- Neely, J.W. and E.G. Isacoff, (1982). Pollution engineering and technology series. Volume 21. Carbonaceous adsorbents for the treatment of ground and surface waters.
- Lee, H., S. Yeo, and S. Jeong, (2003). Antibacterial effect of nanosized silver colloidal solution on textile fabrics. Journal of Materials Science. 38(10): p. 2199-2204.
- Sondi, I. and B. Salopek-Sondi, (2004). Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Journal of colloid and interface science. 275(1): p. 177-182.
- Martinez-Castanon, G., et al., (2008). Synthesis and antibacterial activity of silver nanoparticles with different sizes. Journal of Nanoparticle Research, 10(8): p. 1343-1348.
- Baker, C., et al., (2005). Synthesis and antibacterial properties of silver nanoparticles. Journal of nanoscience and nanotechnology. 5(2): p. 244-249.
- Prakash, P., et al., (2013). Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids and Surfaces B: Biointerfaces, 108: p. 255-259.
Citation: Muhammad Aslam Tahir., et al. (2019). Synthesis and Characterization of Bioactive Composite Material Comprising Silver Nanoparticles and Activated Carbon to Produce Bacteria free Potable Water. Archives of Chemistry and Chemical Engineering 1(2).
Copyright: © 2019 Muhammad Aslam Tahir. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.