Review Article
Volume 2 Issue 2 - 2020
Review on the role and Biology of Cytokines in Adaptive and Innate Immune System
APVDF-QAC, Ethiopia
*Corresponding Author: Dr. Rigbe Abraha, APVDF-QAC, Ethiopia.
Received: July 17, 2020; Published: August 11, 2020
Summery
An immune system is an intricate collection of organs, tissues, cells, and soluble factors that allow individuals to defend against harmful agents such as viruses, bacteria, fungi, parasitic organisms, and tumor cells. The ultimate goal of this system is to prevent or limit infections or damage by these agents. This system is categorized into innate and adaptive immunity, both of which are key participants in generating acute and chronic inflammatory responses. Cytokines are proteins secreted by the cells of innate and adaptive immunity that mediate many of the functions of these cells. Cytokines are produced in response to microbes and other antigens, and different cytokines stimulate diverse responses of cells involved in immunity and inflammation. In the activation phase of adaptive immune responses, cytokines stimulate the growth and differentiation of lymphocytes, and in the effector phases of innate and adaptive immunity, they activate different effector cells to eliminate microbes and other antigens. Cytokines can be classified as proteins, peptides, or glycoproteins; the term "cytokine" encompasses a large and diverse family of regulators produced throughout the body by cells of diverse embryological origin and each cytokine has a matching cell-surface receptor. Subsequent cascades of intracellular signaling then alter cell functions. This may include the up regulation and/or down regulation of several genes and their transcription factors, resulting in the production of other cytokines, an increase in the number of surface receptors for other molecules, or the suppression of their own effect by feedback inhibition. Cytokines play key roles in innate immunity to different classes of microbes are released in infections by pyogenic (pus forming) extracellular bacteria; include TNF, IL-1, IL-10, IL- 12, IL-6, IL-18, Interferons and Chemokines and cytokines that are essential for the development and effectiveness of adaptive immune system includes: - IL-2, IL-4, IL-5, TGFβ, IL-10 and IFN-γ.
Introduction
An immune system is an intricate collection of organs, tissues, cells, and soluble factors that allow individuals to defend against harmful agents such as viruses, bacteria, fungi, parasitic organisms, and tumor cells. The ultimate goal of this system is to prevent or limit infections or damage by these agents. The immune response involves recognizing any foreign material and mounting a reaction to eliminate it. Detection is complicated as pathogens can evolve rapidly, and adapt to avoid the immune system and allow the pathogens to successfully infect their hosts. To survive this challenge, multiple mechanisms evolved that recognize and neutralize pathogens. Even simple unicellular organisms such as bacteria possess enzyme systems that protect against viral infections (Don, 2002).
The immune system is composed of a large variety of cells and mediators that interact in a complex and dynamic network to protect the host against foreign pathogens and to simultaneously maintain tolerance toward self-antigens. This system is categorized into innate and adaptive immunity, both of which are key participants in generating acute and chronic inflammatory responses. Innate immune cells, such as macrophages, NK cells, and dendritic cells are the first line of defense against a foreign pathogen. These cells maintain tissue homeostasis by continuously monitoring the environment for signs of distress and are critical for the activation and modulation of the specific adaptive immune response. The adaptive immune cells such as T cells express antigen-specific receptors and generate diverse antigen recognition, stimulating B cells and ensuing acute or chronic responses dependent on the duration of the antigenic stimulus. Activated, adaptive immune cells and other immune-related components in turn activate other cells of the immune system and thereby enhance the inflammatory response to enable pathogen clearance (Nguyen et al, 2001).
The major difference between innate and adaptive immune responses is that the latter are highly specific for a particular pathogen. Moreover, although the innate immune response does not alter on repeated exposure to a given infectious agent, the adaptive response improves with each successive encounter with the same pathogen. Because innate immunity functions at times before adaptive immunity, its major role is likely to be to initiate defense early during primary infections. There is growing appreciation of the immune regulatory role of the innate immune responses both in activating cellular constituents of innate immunity and in shaping downstream acquired responses. In addition to immediately activating effector functions of the innate cellular constituents, natural killer cells and phagocytes (for example, macrophages, dendritic cells) secrete soluble mediators that can modify cell trafficking to attract effector cells to sites of infections and concentrate T and B cells of the adaptive immune system at sites of antigen presentation. Innate immune system include the emerging picture is that in response to infection, immunocytes express a finely balanced and tightly regulated pattern of cytokines, which promote the most effective immunity against the infecting agent. As a result, innate immunity functions not only to protect the host from infection while slower adaptive immune responses are developing, but also to direct the qualitative and quantitative nature of adaptive immunity (Diefenbach,et.al, 1999). The adaptive immune system evolved in early vertebrates and allows for a stronger immune response as well as immunological memory, where each pathogen is "remembered" by a signature antigen. The adaptive immune response is antigen-specific and requires the recognition of specific “non-self” antigens during a process called antigen presentation. Antigen specificity allows for the generation of responses that are tailored to specific pathogens or pathogen-infected cells. The ability to mount these tailored responses is maintained in the body by "memory cells". Should a pathogen infect the body more than once, these specific memory cells are used to quickly eliminate it includes humeral immunity involves the production of antibody molecules in response to an antigen and is mediated by B-lymphocytes and Cell-mediated immunity involves the production of cytotoxic T-lymphocytes, activated macrophages, NK cells, and cytokines in response to an antigen and is mediated by T-lymphocytes (NIAID, 2007).
The development of an effective immune response involves lymphoid cells, inflammatory cells, and hematopoietic cells. The complex interactions among these cells are mediated by a group of proteins collectively designated cytokines to denote their role in cell-to-cell communication (Roitt and Delves.2004). Cytokines are proteins secreted by the cells of innate and adaptive immunity that mediate many of the functions of these cells. Cytokines are produced in response to microbes and other antigens, and different cytokines stimulate diverse responses of cells involved in immunity and inflammation. In the activation phase of adaptive immune responses, cytokines stimulate the growth and differentiation of lymphocytes, and in the effector phases of innate and adaptive immunity, they activate different effector cells to eliminate microbes and other antigens. Cytokines also stimulate the development of hematopoietic cells (Abbas and Lichtman, 2003).
Therefore, the objective of this review work is to assess the role of cytokine in the immune development.
Properties, Structure and Receptors of Cytokines
Cytokines (Greek cyto-, cell; and -kinos, movement) are small cell-signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication. Cytokines can be classified as proteins, peptides, or glycoproteins; the term "cytokine" encompasses a large and diverse family of regulators produced throughout the body by cells of diverse embryological origin and Each cytokine has a matching cell-surface receptor. Subsequent cascades of intracellular signaling then alter cell functions. This may include the up regulation and/or down regulation of several genes and their transcription factors, resulting in the production of other cytokines, an increase in the number of surface receptors for other molecules, or the suppression of their own effect by feedback inhibition (Dowlatiet a,l 2010).
Properties, Structure and Receptors of Cytokines
Cytokines (Greek cyto-, cell; and -kinos, movement) are small cell-signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication. Cytokines can be classified as proteins, peptides, or glycoproteins; the term "cytokine" encompasses a large and diverse family of regulators produced throughout the body by cells of diverse embryological origin and Each cytokine has a matching cell-surface receptor. Subsequent cascades of intracellular signaling then alter cell functions. This may include the up regulation and/or down regulation of several genes and their transcription factors, resulting in the production of other cytokines, an increase in the number of surface receptors for other molecules, or the suppression of their own effect by feedback inhibition (Dowlatiet a,l 2010).
The nomenclature of cytokines is often based on their cellular sources. Cytokines that are produced by mononuclear phagocytes were called monokines, and those produced by lymphocytes were called lymphokines. With the development of anti-cytokine antibodies and molecular probes, it became clear that the same protein may be synthesized by lymphocytes, monocytes, and a variety of tissue cells, including endothelial cells and some epithelial cells. Therefore, the generic term cytokines is the preferred name for this class of mediators. Because many cytokines are made by leukocytes (example, macrophages or T cells) and act on other leukocytes, they are also called interleukins (Margaret, 2006).
As the structure of many different cytokine has been elucidated, it has become possible to show that cytokine molecules can be arranged four structural groups. The largest family, the group one cytokines (or hematopoiteins) includes IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-1O, 1L-11, IL-13, G-CSF, M-CSF, GM-CSF, and the interferones. All of these molecules are characterized by a structure of fourα-helices bundled together. They can further sub divided on the basis of chain length. For example IL-2, IL-3, IL-4, IL-7, and IL-9 have short chains and IL-6, IL-10 and IL-11 have long chain. The tumor necrosis factors (TNF), the IL-1 family, and TGF-β are grouped in group two cytokines characterized by having long chain β-sheet structures. Group three cytokines are small proteins with α-helices and β-sheets. These includes the chemokines (IL-8, MIP, MCP) and related molecules. Group four cytokines have mosaic structures- mixture of different structural motifs.IL-12 belongs to group four. When classified according to structure, patterns can be seen in the biological activities of cytokine. Thus, the group one molecules are all involved in immune regulation or hematopoiesis. The group two cytokines are mainly involved in the growth and regulation of tissues, cell death and the acute-phase response to injury. The group three cytokines are involved in inflammation. The activity of group four cytokines depends on their subcomponents. For example, IL-12 is a mosaic of a group one structure and a hematopoietin receptor. It acts like group one cytokine (Tizard, 1998).
Cytokine receptors are integral membrane proteins with both intracellular and extracellular domains. They usually consists at least two functional units, one for ligand binding and one for signal transduction, which may or not be on the same peptide. According to the extracellular cytokine-binding domain, cytokine receptors are divided into 5 families: Immunoglobulin (Ig) super family receptors (IL-1), Class I cytokine receptors (hematopoietin family), Class II cytokine receptors (interferon family), TNF receptor family, and Chemokine family (Wenny, 2007).
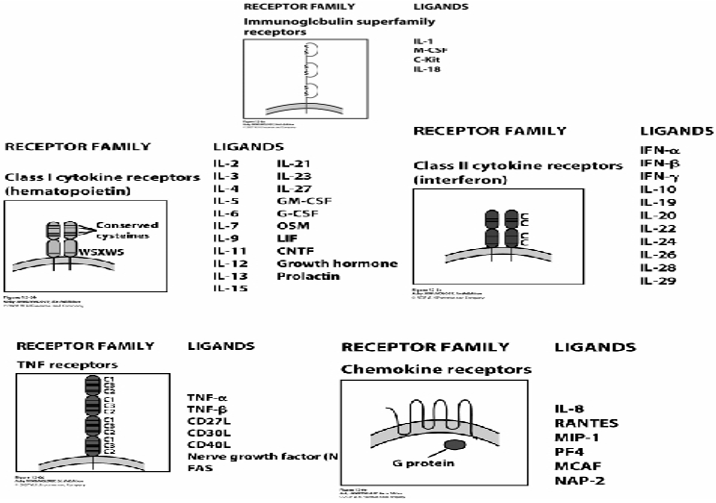
Figure 1: Clinically detectable opaque nonuniform or incomplete ring at the corneal margins – Grade 3.
Summary of cytokine receptors and their ligands, Source: -Wenny's Immunology Web notes (Wenny, 2007).
Cytokines bind to specific receptors on the membrane of target cells, triggering signal-transduction pathways that ultimately alter gene expression in the target cells. The susceptibility of the target cell to a particular cytokine is determined by the presence of specific membrane receptors (Jason et al, 2001). External signals regulate the expression of cytokine receptors and thus the responsiveness of cells to cytokines. For instance, stimulation of T or B lymphocytes by antigens leads to increased expression of cytokine receptors. For this reason, during an immune response, the antigen-specific lymphocytes are the preferential responders to secreted cytokines. This is one mechanism for maintaining the specificity of immune responses, even though cytokines themselves are not antigen specific. Receptor expression is also regulated by cytokines themselves, including the same cytokine that binds to the receptor, permitting positive amplification or negative Feedback (Abbas and Lichtman, 2003). In general, the cytokines and their receptors exhibit very high affinity for each other, with dissociation constants ranging from 10–10 to 10–12 M. Because their affinities are so high, cytokines can mediate biological effects at Pico molar concentrations. A particular cytokine may bind to receptors on the membrane of the same cell that secreted it, exerting autocrine action; it may bind to receptors on a target cell in close proximity to the producer cell, exerting paracrine action; in a few cases, it may bind to target cells in distant parts of the body, exerting endocrine action (Jason et al, 2001). Cytokines regulate the intensity and duration of the immune response by stimulating or inhibiting the activation, proliferation, and/ or differentiation of various cells and by regulating the secretion of antibodies or other cytokines. Binding of a given cytokine to responsive target cells generally stimulates increased expression of cytokine receptors and secretion of other cytokines, which affect other target cells in turn. Thus, the cytokines secreted by even a small number of lymphocytes activated by antigen can influence the activity of numerous cells involved in the immune response. For example, cytokines produced by activated TH cells can influence the activity of B cells, T cells, natural killer cells, macrophages, granulocytes, and hematopoietic stem cells, thereby activating an entire network of interacting cells (Roitt and Delves, 2004).
Cytokines exhibit the attributes of pleiotropy, redundancy, synergy, antagonism, and cascade induction, which permit them to regulate cellular activity in a coordinated, interactive way. A given cytokine that has different biological effects on different target cells has a pleiotropic action. Two or more cytokines that mediate similar functions are said to be redundant; redundancy makes it difficult to ascribe a particular activity to a single cytokine. Cytokine synergism occurs when the combined effect of two cytokines on cellular activity is greater than the additive (Charles et al, 2001).
The cellular responses to most cytokines consist of changes in gene expression in target cells, resulting in the expression of new functions and sometimes in the proliferation of the target cells. Many of the changes in gene expression induced by cytokines result in differentiation of T and B lymphocytes and activation of effector cells such as macrophages. For instance, cytokines stimulate switching of antibody isotopes in B cells, differentiation of helper T cells in to TH1 and TH2 subsets, and activation of microbicidal mechanisms in phagocytes. Exceptions to the rule that cytokines work by changing gene expression patterns are chemokine’s, which elicit rapid cell migration, and a cytokine called tumor necrosis factor (TNF), which induces apoptosis by activating cellular enzymes, without new gene transcription or protein synthesis. And Cytokines often influence the synthesis and actions of other cytokines. The ability of one cytokine to stimulate production of others leads to cascades in which a second or third cytokine may mediate the biologic effects of the first. Two cytokines may antagmizeeach other's action, produce additive effects, or, in some cases, produce greater than anticipated or synergistic effects (Abbas and Lichtman, 2003).
Cytokines Involved in Innate Immune Response
Microorganisms such as bacteria that penetrate the epithelial surfaces of the body for the first time are met immediately by cells and molecules that can mount an innate immune response. Phagocytic macrophages conduct the defense against bacteria by means of surface receptors that are able to recognize and bind common constituents of many bacterial surfaces. Bacterial molecules binding to these receptors trigger the macrophage to engulf the bacterium and also induce the secretion of biologically active molecules. Activated macrophages secrete different cytokines, which are defined as proteins released by cells that affect the behavior of other cells that bear receptors for them. Macrophages encountering bacteria in the tissues are triggered to release cytokines that increase the permeability of blood vessels, allowing fluid and proteins to pass into the tissues. They also produce chemokine’s that direct the migration of neutrophils to the site of infection. The stickiness of the endothelial cells of the blood vessels is also changed, so that cells adhere to the blood vessel wall and is able to crawl through it; first neutrophils and then monocytes are shown entering the tissue from a blood vessel. The accumulation of fluid and cells at the site of infection causes the redness, swelling, heat, and pain, known collectively as inflammation (Charleset al, 2001).
Cytokines play key roles in innate immunity to different classes of microbes are released in infections by pyogenic (pusforming) extracellular bacteria; include TNF, IL-1, IL-10, IL- 12, IL-6,IL-18, Interferons and Chemokine’s.
Tumor necrosis factor alpha (TNF-α)
Tumor necrosis factor alpha is produced by activated macrophages in response to microbes, especially the lipopolysaccharide of Gram negative bacteria. It is an important mediator of acute inflammation. It mediates the recruitment of neutrophils and macrophage to sites of infection by stimulating endothelial cells to produce adhesion molecules and by producing chemokine’s which are called chemotactic cytokines. TNF- α also acts on the hypothalamus to produce fever and it promotes the production of acute phase proteins (Wideraetal, 2006).
Interleukin 1 (IL-1)
There are two biologically distinct form of interleukin IL-1 α and IL-1β. Each is encoded by separate gens. Most of IL-1 produced by a wide variety of cells, but macrophages is the most important source because of their location and their ability to synthesize in large amounts. IL-1 production by mononuclear phagocytes is induced by bacterial products such as LPS and by other cytokines such as TNF. IL-1 produced spontaneously by macrophages in small quantities and in much larger amount when the macrophages are activated. T cells may induce high level of IL-1 synthesis by direct contact between a helper T cell and a macrophage, or IL-1 synthesis may be stimulated by release of proteins such as tumor necrosis factor from the T cell. Three macrophage proteins called CD58, CD44, and CD45 found on macrophage surface may be the physiologic triggers for IL-1 release. These three molecules are cell adhesion proteins so that when they bind to by their ligands IL-1 may release (Tizard, 1991).
IL-1 is an essential initiation molecule that acts on cells that participate in immunological or inflammatory responses. It acts on T cell to cause their activation and promote lymphokine release and it acts as cofactor with IL-2 to stimulate B cell proliferation and differentiation. It regulates T and B cell development through regulation of maturation B and T cell precursors.IL-1 also act on bone marrow stromal cells to induce the synthesis of colony stimulatory factors such as GM-CSF,G-CSF and IL-6 by its action on non-lymphoid cells such as mast cells, fibroblasts, hepatocytes, neural cells, and endothelial cells. IL-1 plays a central role in innate immune response to tissue damage (inflammation) (Tizard, 1998).
Interleukin 6 (IL-6)
IL-6 is a cytokine that functions in both innate and adaptive immunity. It is synthesized by mononuclear phagocytes, vascular endothelial cells, fibroblasts, and other cells in response to microbes and to other cytokines, notably IL-1 and TNF. It is also made by some activated T cells. The functional form of IL-6 is a homodimer, with each subunit forming a four-a-helical globular domain. The receptor for IL-6 consists of a cytokine-binding protein and a signal-transducing subunit, both of which belong to the type I cytokine receptor family. The 130-kD signal-transducing subunit is called gp130; it activates a JAK/STAT signaling pathway and is also the signaling component of other cytokine receptors. IL-6 has several diverse actions. In innate immunity, it stimulates the synthesis of acute-phase proteins by hepatocytes and thus contributes to the systemic effects of inflammation; the so-called acute-phase response.IL-6 stimulates production of neutrophils from bone marrow progenitors, usually acting in concert with colony-stimulating factors. In adaptive immunity, IL 6 stimulates the growth of B lymphocytes that have differentiated into antibody producers. IL6 similarly acts as a growth factor for neoplastic plasma cells (myelomas), and many myeloma cells that grow autonomously secrete IL-6 as an autocrinc growth factor. Moreover, IL-6 can promote the growth of monoclonal antibody-producing hybridomas, which are derived from myelomas (Abbas and Lichtman, 2003).
Interleukin 12 (IL-12)
IL-12 exists as a disulfide-linked heterodimer of 35-KD (p35) and 40-kD (p40) subunits. The p35 subunit has a four-a-helical globular domain structure. The p40 subunit is homologous not to other cytokines but to the receptor for IL-6, containing both an Ig-like domain and motifs characteristic of type I cytokine receptors. The principal sources of IL-12 are activated mononuclear phagocytes and dendritic cells. Many cells appear to synthesize the p35 subunit, but only these antigen-presenting cells (APCs) produce the p40 component and therefore the biologically active cytokine. During innate immune reactions to microbes, IL-12 is produced in response to many microbial stimuli (including LPS), infection by intracellular bacteria (such as Listem'a and mycobacteria), and virus infections. In addition, antigen-stimulated helper T cells induce the production of IL-12 from macrophages and dendritic cells, mainly by CD40 ligand on the T cells engaging CD40 on the macrophages and dendritic cells. IFN-I produced by NK cells or T cells also stimulates IL-12 production. Thus, IL-12 is produced by APCs when they present antigens to T cells, during the induction and effector phases of cell-mediated immune responses (Abbas and Lichtman, 2003).
IL-12 stimulates the production of ZFN-y by NK cells and T lymphocytes. Macrophages produce IL-12 in response to many microbes. Secreted IL-12 stimulates NK cells and T cells to produce IFN-y, which then activates the macrophages to kill the phagocytosed microbes (Charles et al., 2001). Large amounts of IL-12 are produced in severe gram negative species, resulting in the production of IFNy, which synergizes with bacterial LPS to stimulate macrophage production of TNF, the principal mediator of septic shock. IL12 antagonists prevent lethalityin experimental a model of LPS-induced septic shock.IL-12 stimulates the differentiation of CD4 helper T lymphocytes into IFN-y- Producing THl cells. TheTH1 subset of helper T cells activates phagocytes in cell-mediated immunity (Tizard, 1998).
Interleukin 13 (IL-18)
IL-18is structurally homologous to IL-1 and signals by a similar receptor-associated kinase, but it has a different function than IL-1. IL-18 is produced by macrophages in response to LPS and other microbial products. It stimulates the production of IFN-I by NK cells and T cells and synergizes with IL-12 in this response. Thus, IL-18 is an inducer of cell-mediated immunity, especially in combination with IL-12. Knockout mice lacking IL-18 are deficient in IFN-y production, and mice lacking both IL-12 and IL-18 are (Abbas and Lichtman, 2003).
Type I interferon (IFN α and β)
Interferon type one are a family of glycoprotein secreted by virus infected cells that play an important role in the first line of defense against viral infections. They are part of the innate immune system and are induced at an early stage in viral infection before the specific immune system has had time to respond. Interferon’s are made by cells in response to an appropriate stimulus, and are released into the surrounding medium; they then bind to receptors on target cells and induce transcription of approximately 20-30 genes in the target cells, and this results in an anti-viral state in the target cells (Margaret, 2006).
The actions of type I IFNs protect against viral infections and promote cell-mediated immunity against intracellular microbes. Type I IFN causes cells to synthesize a number of enzymes, such as 2', 5’oligoadenylatesynthetase, that interfere with transcription of viral RNA or DNA and viral replication. The antiviral action of type I IFN is primarily aparacrine action, in that a virally infected cell secretes IFN to protect neighboring cells that are not yet infected. A cell that has responded to IFN and is resistant to viral infection is said to be in an "antiviral state." IFN secreted by an infected cell may also act in an autocrine fashion to inhibit viral replication in that cell. Type I IFN increases expression of class I MHC molecules. Because CD8' CTLs recognize foreign antigens bound to class I MHC molecules, type I IFN enhances the recognition of class I-associated viral antigens on infected cells and therefore the efficiency of CTL mediated killing of these cells. Type I IFN stimulates the development of TH1 cells in humans. This effect is mainly due to the ability of type I IFN to promote in T cells the expression of functional receptors for the major TH1-inducingcytokine IL-12. Type I IFN may also increase the Cytolytic activity of NK cells (Playfair and Chain, 2004).
Chemokines
Chemokine’s are chemotactic cytokines produced by many kinds of leukocytes and other cell types. They represent a large family of molecules that function to recruit leukocytes to sites of infection and play a role in lymphocyte trafficking by determining which cells will cross the epithelium and where they are directed to go (Margaret, 2006).
All Chemokine’s are 8- to 12-kD polypeptides that contain two internal disulfide loops. The Chemokines are classified into families on the basis of the number and location of N terminal cysteine residues. The two major families are the CC Chemokines, in which the cysteine residues are adjacent, and the CXC family, in which these residues are separated by one amino acid. These differences correlate with organization of the subfamilies into separate gene clusters. In inflammation, the CXC Chernokines act mainly on neutrophils, and the CC chemokines act mainly on monocytes, lymphocytes, and eosinophils (Craig and Adam, 2000).
Leukocyte recruitment is a result of several sequential actions of Chemokines on these cells. Chemokines that are expressed on endothelial cells bound to heparan act on leukocytes rolling on the endothelium and increase the affinity of leukocyte integrins for their ligands. This step of integrin activation is critical for the firm adherence of the leukocytes to the endothelium, as a prelude to subsequent migration in to extravascular tissue. Recall that TNF and IL-1 stimulate expression of integrin ligands on endothelium and these two cytokines and Chemokines act cooperatively in the process of leukocyte migration. Chemokines induce movement of leukocytes and their migration toward the chemical gradient of the cytokine by stimulating alternating polymerization and de-polymerization of actin filaments. Different chemokines act on different cells and thus control the nature of the inflammatory infiltrate. For instance, the CXC chemokine IL-8 recruits neutrophils preferentially, and the CC chemokine eotaxin recruits eosinophils (Abbas and Lichtman, 2003).
Chemokines regulate the traffic of lymphocytes and other leukocytes through Peripheral lymphoid tissues. Some of the most intriguing and surprising discoveries about chemokines have been their roles in the normal migration of immune cells into lymphoid organs. Different chemokines promoting the migration of T cells, B cells, and dendritic cells to different regions of peripheral lymphoid organs. Various chemokines also promote migration of previously activated effector and memory T cells to non-lymphoid tissues, including mucosal organs and skin. The selectivity of different cell types for different tissues depends to a large extent on which chemokines are produced in the tissues and which chemokine receptors are expressed on the cell types. Chemokines are also involved in the development of diverse non lymphoid organs (Kanazawa, 2007).
Cytokines Involved in of Adaptive Immune Response
The cytokines of adaptive immunity are critical for the development of immune responses and for the activation of effector cells that serve to eliminate microbes and other antigens, that responses are specialized to eliminate different types of microbes. Much of this specialization of adaptive immunity is due to the actions of cytokines, which may be produced by subpopulations of helper T cells. Different types of microbes stimulate naive CD4+ T cells to differentiate into effector cells that produce distinct sets of cytokines and perform distinct functions. The best defined of these subsets are the THl and TH2 cells, Many intracellular microbes (bacteria and viruses) induce the development of TH1 cells, which produce IFN, the cytokine that activates phagocytes to destroy intracellular microbes and stimulates the production of opsonizing antibodies that promote more phagocytosis. Helminthic parasites, in contrast, stimulate the development of TH2 cells, which produce IL-4 and IL-5. IL-4 enhances production of helminth-specific IgE antibodies, which coat the parasites, and IL-5 activates eosinophils, which bind to the IgE-coated parasites and destroy them. Thus, cytokines that are essential for the development and effectiveness of adaptive immune system includes: - IL-2, IL-4, IL-5, TGFβ, IL-10 and IFN-γ (Abbas and Lichtman, 2003).
Interleukin 2(IL-2)
IL-2 is a growth factor for antigen-stimulated T lymphocytes and is responsible for T cell clonal expansion after antigen recognition. For this reason, IL-2 was originally called T cell growth factor (Abbas and Lichtman, 2003).Interleukin 2 is produced by TH cells, although it can also be produced by CTC to a lesser extent. It is the major growth factor for T cells. It also promotes the growth of B cells and can activate NK cells and monocytes. IL-2 acts on T cells in an autocrine fashion. Activation of T cells results in expression of IL-2 receptor and the production of IL-2. The IL-2 binds to the IL-receptor and promotes cell division.IL-2 activates helper and cytotoxic T cells, B cells, NK cells. To be responsive to IL-2, a T cell must first be activated by antigen and IL-12.IL-2 triggers the T cell to proliferate and is a key component of the immune response. IL-2 also acts on B cells, promoting their growth and stimulating limited immunoglobulin synthesis. Acting on TH1 cells and NK cells.IL-2 induces production of IFN-y and IL-5 and regulates TNF-α receptor expression.IL-2 can activate macrophages, stimulating their secretion of cytokines and enhancing their cytotoxic activity against cancer cells and intracellular bacteria (Smith, 2006).
Interleukin4 (IL-4)
IL-4 produced primary by activated TH2 cells and acts on B cells, T cells, macrophages, endothelial cells, fibroblasts and mast cells.IL-4 stimulates the growth and differentiates of B cells. And it induces B cell to switch to IgE production and is therefore, of major importance in development of allergic reaction. IL-4 enhances the development of cytotoxic T cells from resting T cells, it can makes helper T cells grow in the absence of IL-2 and stimulates fetal thymocyte, granulocyte, and mast cell growth.IL-4 also has complex effect on macrophages, it down regulates IL-1, IL-6, and TNF-α secretion by monocytes on the other hand, it increase their MHC class II expression, their antigen presenting ability, and their cytotoxic activity (Tizard.R, 1998).
Interleukin 5 (IL-5)
IL-5 is a homodimer, with each subunit containing a four-a-helical domain. It is produced by the TH2 subset of CD4' T cells and by activated mast cells and it functions to promote the growth and differentiation of B cells and eosinophil’s. It also activates mature eosinophils. The IL-5 receptor is a type I cytokine receptor (Smith, 2006).The major actions of IL-5 are to activate mature eosinophils and stimulate the growth and differentiation of eosinophils. Activated eosinophils are able to kill helminths. Eosinophils express Fc receptors specific for IgE antibodies and are thereby able to bind to IgE-coated microbes, such as helminths. Thus, the two main TH2 cytokines, IL-4 and IgG5, function in concert: IL-4 stimulates production of IgE, which opsonizes helminths and binds eosinophils, and IL-5 activates the eosinophils to destroy the parasites. Knockout mice lacking IL-5 are defective in eosinophil responses and are susceptible to some helminthic infections. IL-5 stimulates the proliferation of B cells and the production of IgA antibodies (Abbas and Lichtman, 2003).
Interleukin 10 (IL-10)
IL-10 (IL-10) is an 18.5 kD acid-sensitive protein that lacks detectable carbohydrate moieties. It is a member of the four alpha-helical bundle cytokine super family and binds to a type I cytokine receptor and appears to function as a homodimer. IL-10 is an inhibitor of activated macrophages and dendritic cells and is thus involved in the control of innate immune reactions and cell-mediated immunity. IL10, in contrast, is an inhibitor of host immune responses, particularly responses involving macrophages. IL-l0 is produced mainly by activated macrophages, and because it inhibits macrophage functions, it is an excellent example of a negative feedback regulator. It is not clear whether different stimuli may act on macrophages to induce the production of a regulatory cytokine like IL-10 and effector cytokines like TNP and IL-12, or whether the same stimuli elicit production of all these cytokines but with different kinetics. T lymphocytes also secrete IL-10, and it is produced by some non-lymphoid cell types (e.g., keratinocytes) (Delves et al., 1998). Like other cytokines interleukin-10 has many effects upon the functions of cells such as lymphocytes, monocytes, natural killer cells, and dendritic cells. Specifically, IL-10 is a cytokine that regulates immune-mediated inflammation. It appears to have two major functions: (1) to inhibit cytokine (TNF, IL-1, chemokine, and IL-12) production by macrophages and (2) to inhibit the accessory functions of macrophages in T cell activation. The biologic effects of IL-l0 result from its ability to inhibit many of the functions of activated macrophages.. IL-l0 acts on the activated macrophages to terminate these responses and return the system to its resting state as the microbial infection is eradicated (Abbas and Lichtman, 2003). IL-10 accomplishes the latter function through the reduced expression of MHC class II molecules and certain co-stimulators. Inhibitory effects on proliferation, survival, and cytokine production of T-cells. For example, direct interaction of IL-10 with the IL-10 receptor on T cells acts to suppress transcription of the gene for IL-2, which inhibits T cell proliferation. IL-10 Inhibits IFN (gamma) synthesis by CD8+ T cells without affecting the cytotoxic function of these CD8+ T cells and also inhibits its own production (IL-10) by monocytes, thus demonstrating the workings of a self-regulatory negative feedback loop (Bulpitt et al, 1999). IL-10 inhibits the production of IL-12 by activated macrophages and dendritic cells. Because IL-12 is a critical stimulus for IFN-y secretion and is an inducer of innate arid cell mediated immune reactions against intracellular microbes, IL-10 functions to down-regulate all such reactions Delves et al., 1998).
Transforming growth factor beta (TGF-β)
TGF- β1 is a homodimeric protein that is synthesized as a precursor and activated by proteolytic cleavage. It is secreted by antigen-stimulated T cells, LPS-activated mononuclear phagocytes, and many other cell types (Geijsenet al, 2001). Some regulatory T cells produce TGF- β, and the same cells may also produce IL-10, which, like TGF- β, has immunosuppressive activities. TGF- β receptors include two high-affinity polypeptide receptors (type I and type II) receptors. TGF- β inhibits the proliferation and differentiation of T cells and the activation of macrophages. TGF- β also acts on other cells, such as neutrophils and endothelial cells, largely to counteract the effects of pro-inflammatory cytokines. By these actions, TGF- β functions to inhibit immune and inflammatory responses. TGF- β stimulates production of IgA antibodies by inducing B cells to switch to this isotype. IgA is the antibody isotype required for mucosal immunity. TGF- β has many diverse actions outside the immune system. It may inhibit proliferation of some cell types and stimulate others. Often, TGF- β can either inhibitor stimulate growth of the same cell type. TGF- β causes synthesis of extracellular matrix proteins such as collagens, of matrix-modifying enzymes such as matrix metallo proteinases, and of cellular receptors for matrix proteins such as integrins. These actions may promote tissue repair after local immune and inflammatory reactions have been controlled (Abbas and Lichtman, 2003).
Interferon gamma (INF-γ)
Interferon-gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. IFN-γ, or type II interferon, is a cytokine that is critical for innate and adaptive immunity against viral and intracellular bacterial infections and for tumor control. The importance of IFN-γ in the immune system stems in part from its ability to inhibit viral replication directly and most importantly from its immune-stimulatory and immune-modulatory effects. IFN-γ is produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by CD4 and CD8 cytotoxic T lymphocyte (CTL) effector T cells once antigen-specific immunity develops (Asirvatham,et al, 2008).
Macrophages infected with mycobacteria produce the cytokine interleukin 12 (IL-12, which is a heterodimer of IL-12 p40 plus IL-12 p35). IL-12 stimulates T cells and NK cells via its hetero-dimeric receptor. In response to stimulation with IL-12, activated T cells and NK cells produce IFN-gamma. IFN-gamma binds to its receptor, IFN-gamma-R1, with high affinity, leading to receptor dimerization. This is followed by aggregation of two accessory chains (IFN-gammaR2) with the receptor complex. Trans phosphorylation of JAK1 and JAK2, the Janus kinases that are constitutively associated with IFN-gamma-R1 and IFN-gamma-R2 respectively, occurs next. The subsequent signaling event is the tyrosine phosphorylation of the latent cytosolic transcription factor STAT1, which is followed by homo-dimerization and translocation to the nucleus as a complex. This initiates the transcription of IFN-gamma-regulated genes. One of the results of IFN-gamma activation on phagocytes is the production of tumor necrosis factor alpha (TNF-alpha) and further up regulation of IL-12 production (Al-Muhsen and Casanova, 2008). IFN-γ has antiviral, immune-regulatory, and anti-tumor properties. It alters transcription in up to 30 genes producing a variety of physiological and cellular responses. Among the effects are: Promotes NK cell activity, Increase antigen presentation and lysosome activity of macrophages, Promotes TH1 differentiation by up regulating the transcription factor, ultimately leading to cellular immunity: cytotoxic CD8+ T-cells and macrophage activity - while suppressing TH2 differentiation which would cause a humoral (antibody) response, Cause normal cells to increase expression of class I MHC molecules as well as class II MHC on antigen presenting cells specifically through induction of antigen processing genes, Promotes adhesion and binding required for leukocyte migration (Schoenborn and Wilson , 2007).
Conclusion
An immune system is an intricate collection of organs, tissues, cells, and soluble factors that allow individuals to defend against harmful agents such as viruses, bacteria, fungi, parasitic organisms, and tumor cells. The ultimate goal of this system is to prevent or limit infections or damage by these agents. Generally an immune system categorized into innate and adaptive immunity, both of which are key participants in generating acute and chronic inflammatory responses. The development of an effective immune response involves lymphoid cells, inflammatory cells, and hematopoietic cells. The complex interactions among these cells are mediated by a group of proteins collectively called cytokines, to denote their role in cell-to-cell communication. Cytokines are the corner stone of our immune system because they regulate the intensity and duration of the immune response by stimulating or inhibiting the activation, proliferation, and/ or differentiation of various cells and by regulating the secretion of antibodies or other cytokines. Binding of a given cytokine to responsive target cells generally stimulates increased expression of cytokine receptors and secretion of other cytokines, which affect other target cells in turn. Thus, the cytokines secreted by even a small number of lymphocytes activated by antigen can influence the activity of numerous cells involved in the immune response. For example, cytokines produced by activated TH cells can influence the activity of B cells, T cells, natural killer cells, macrophages, granulocytes, and hematopoietic stem cells, thereby activating an entire network of interacting cells.
References
- Abbas A, and Lichtman A. (2003). Cellular and Molecular Immunology. (5thedn), Department of Pathology, University of California School of Medicine- San Francisco, California.
- Al-Muhsen S, Casanova J (2008) the genetic heterogeneity of mendelian susceptibility to mycobacterial diseases. J Allergy Clinical Immunology; 122:1043.
- Asirvatham J, GregorieJ, MagnerJ, TomasiB (2008) MicroRNA targets in immune genes and the Dicer/Argonaute and ARE machinery components. MolocularImmunology 45: 95-105.
- Bulpitt K, Paulus H, Clements P (1999) Clinical Trials: Summary of a study on interleukin-10 plus methotrexate to treat rheumatoid arthritis.University of Californinical Trials.
- Charles A, Janeway J, Paul T, Mark W, Mark J, Shlomchik, (2001)Immunobiology : the immune system in health and disease (5thedn)Yale University School of Medicine; London.
- Craig M, and Adam F (2000) Chemokine receptors and their role in inflammation and infectious diseases From the Division of Child Health, University of Sheffield, United Kingdom.
- Delves P and Roitt I (1998) Encyclopedia of Immunology. (2ndedn) San Diego: Academic Press.
- Diefenbach A, Schindler H, Rollinghoff M, Yokoyama M, Bogdan C(1999) Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science 284:951–955.
- Don D. (2002)Microbiology and Immunology Notes Department of Medical Microbiology Immunology, Oklahoma University Health Science Center Oklahoma City,
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. (2010) A meta-analysis of cytokines in major depression. Biol. Psychiatry67: 446–457.
- Geijsen N, Koenderman L, CofferJ (2001) Specificity in cytokine signal transduction: lessons learned from the IL-3/IL-5/GM-CSF receptor family. Cytokine Growth Factor Rev, journal of immunology12:19-25.
- Jason J, Archibald L,Nwanyanwu O,Byrd M, Kazembe P,et al.(2001) Comparison of serum and cell-specific cytokines in humans. Clinical Diagnosis of Laboratory Immunology. http://www.ncbi.nim.nih.gov/entrez/query.fcg
- Kanazawa N, Nakamura T,Tashiro K,Fractalkine (2007) macrophage-derived chemokine,T cell-attracting chemokines expressed in T cell area dendritic cells. EurJournal of Immunology.
- Margaret H (2006) interferon response to viral infection Department of bacteriology, virology Pathology, Microbiology and Immunology, school of medicine University of South Carolina: < http://www.med.sc.edu:85/mhunt/ interferon. Htm>
- National Institute of Allergy and Infectious Diseases (NIAID) (2007) Understandingof the Immune System: http://www.niaid.nih.gov/publications/immune/the immune_system.pdf.
- Nguyen D, Khoa M, Carmen M, Allison B,David D, et al. (2001)Inflammatory Cytokines Regulate Function and Expression of Adenosine A2A Receptors in Human Monocytic. Department of Medicine, New York University.7: 4026-4032.
- Playfair J, Chain B,(2004) Immunology at a Glance (7thed) department of Immunology, Royal Free and University College Medical School London.
- Roitt M and Delves J(2004)Roittis Essential Immunology (10thed) Department of Immunology and Molecular Pathology,Universityof London, London.
- Schoenborn J and Wilson C (2007) Regulation of interferon-gamma during innate and adaptive immune responses".Adv.Immunol96:41–101.
- Smith A (2006) structure of IL-2 bound to the three chains of the IL-2 receptor and how signaling occurs, the Division of Immunology, Department of Medicine, Weill Medical College, Cornell University, NewYork,100:21.
- Tizard R (1991) cytokine and the immune system. Veterinary immunology (3thed) department veterinary pathobiology, the Texas veterinary medical center, Texas Aand M University, Texas.
- Tizard R (1998) cytokine and the immune system. Veterinary immunology (5thed) department veterinary pathobiology, the Texas veterinary medical center, Texas A and M University, Texas.
- Wenny, Z. (2007): Cytokines and their receptors and Regulation of Immune reponses, university of Georgia. http://www.wenliang.myweb.uga.edu/mystudy/immunology/science of immunology.
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B (2006) Tumor necrosis factor α triggers proliferation of adult neural stem cells via IKK/NF-κB signaling. University of Witten/ Herdecke, Germany 64:1471-2202.
Citation: Rigbe Abraha. (2020). Review on the role and Biology of Cytokines in Adaptive and Innate Immune System. Archives of Veterinary and Animal Sciences 2(2).
Copyright: © 2020 Rigbe Abraha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
