Research Article
Volume 2 Issue 2 - 2020
Recombinant Human Epidermal Growth Factor: Medical Experience of Use in Patients with Advanced Diabetic Foot Ulcers in Mexico
From the Hospital Regional “Gral. Ignacio Zaragoza”, Calzada Ignacio Zaragoza, Nº 1711, Col. Ejército Constitucionalista, CP 09220, Iztapalapa (F.E.V., O.C.C); Hospital Regional “1º de Octubre”', Av. Instituto Politécnico Nacional N° 1669. Magdalena de las Salinas. Gustavo A. Madero, Cd. Mx. C.P. 07760 (J.M.E.A., L.E.M.); Hospital Regional 'Monterrey' Av. Adolfo López Mateos Nº 122, Calle Burócratas Federales, CP 64980, Monterrey, Nuevo León (S.G.M.E., S.H.M.L.); Hospital Regional: Dr. “Valentín Gómez Farías”, ISSSTE, Zapopan, Guadalajara (J.O.G., A.G.O.R); National Institute for Angiology and Vascular Surgery, Havana, Cuba (J.I.F.M.); Clinical Research Direction, Center for Genetic Engineering and Biotechnology, Havana, Cuba (R.M.G., E.R.C., J.E. C., E.L.M., J.A.B., A.D.R.M., J.E.S.C., V.L.M.G., A.D.T.I., M.A.V., J.E.B.H.)
*Corresponding Author: MS. José A. Buxadó, Center for Genetic Engineering and Biotechnology, Ave. 186 esq. Ave. 31, Cubanacan, Playa, P.O. Box 6162, Habana 6, Cuba.
Received: September 14, 2020; Published: September 21, 2020
Abstract
Background: Diabetic foot ulcer (DFU) is one of the most frequent severe complications of Diabetes mellitus, and timely diagnostic and intervention is a significant driver of reduction of major amputation rate, long-term prognosis, and patients’ survival.
Methods: Implementation and deploy of advanced DFU treatment with intralesional infiltration of recombinant human epidermal growth factor (rhEGF) was performed in four hospitals of the Mexican Institute of Health and Social Insurance of State Workers (ISSSTE) from November 2018 to March 2019. Fifty-six in-hospital patients with neuropathic or ischemic ulcers, classified as grades 3 and 4, according to Wagner’s scale, were included in a non-controlled pilot study. Demographic and clinical variables, such as total granulation, wound closure, healing time, and adverse reactions, were evaluated.
Results: Total granulation of DFU was obtained in 100.0% of patients. Wound closure was observed in 90.0% of patients, mostly in neuropathic DFUs, and average healing time was 6.4 weeks in these patients. Treatment with rhEGF was effectivein both neuropathic and ischemic patients.
Conclusions: In this trial, percent of granulation, wound closure, and healing time were significantly high, regardless of wound location. Perilesional and intralesional injection of rhEGF is recommended in this report for treatment of advanced DFU, based on the efficacy and safety profile demonstrated. (Funded by Laboratorios Pisa S.A. de C.V.; gob.mx/cofepris number, 15CI09012060.)
Keywords: Diabetes mellitus; Diabetic foot ulcer; Wound, EGF; Healing.
Introduction
Diabetes mellitus (DM) remains as a major challenge for health systems, governments, and societies, [1,2] and probably the most concerning complication is the diabetic foot ulcer (DFU). [3,4] Timely diagnostic and intervention is a significant driver of reduction of major amputation rate, long-term prognosis, and survival of DFU patients. [5,6]
Prevalence of DFU in Mexico, and amputation rate were 9.1% of diabetics and 5.5% of DFU patients, respectively, in 2016. Both variables have kept a persistent growing trend from 2006, and attention to DFU accounts for 20.0% of total expense in DM. The cost of DFU treatment in Mexico has been reported in the [7] range between 2 806 and 5 361 USD/patient, depending on wound severity. [7,8]
Safety and efficacy of DFU treatment by intralesional and perilesional injection of recombinant human epidermal growth factor (rhEGF) have been demonstrated in clinical trials. [7-10] Post-marketing information from 2 702 patients confirmed results obtained in clinical trials. [7,8] This was taken into account to implement and deploy EGF treatment, and evaluating results in patients with DFU grades 3 and 4, according to Wagner’s classification, [7] in four hospitals.
Methods
Trial Oversight
Four hospitals participated in a multicenter prospective implementation and deploy of the treatment. The trial was designed and overseen by a steering committee and was supported by Laboratorios Pisa S.A. de C.V., which had no influence on the design or conduct of the trial, and was not involved in data collection or analyses, in the writing of the manuscript or in the decision to submit it for publication. The trial protocol was approved by National Commission for Protection against Health Risks (COFEPRIS). The trial was performed in accordance the principles of the Declaration of Helsinki. [7] The authors assume responsibility for the accuracy and completeness of the data and analyses, as well as for the fidelity of the trial and this report to the protocol.
Four hospitals participated in a multicenter prospective implementation and deploy of the treatment. The trial was designed and overseen by a steering committee and was supported by Laboratorios Pisa S.A. de C.V., which had no influence on the design or conduct of the trial, and was not involved in data collection or analyses, in the writing of the manuscript or in the decision to submit it for publication. The trial protocol was approved by National Commission for Protection against Health Risks (COFEPRIS). The trial was performed in accordance the principles of the Declaration of Helsinki. [7] The authors assume responsibility for the accuracy and completeness of the data and analyses, as well as for the fidelity of the trial and this report to the protocol.
Patients
Fifty-six type 1 or 2 diabetes patients of both sex, ≥ 20 years old, Wagner’s grade 3 or 4 DFU, wound size > 1 cm2, more than 4 weeks of wound evolution, and signed informed consent to participate were eligible for enrollment (Tables 1 and 2). Patients who required revascularization, hemoglobin < 100 g/l, uncompensated chronic diseases, diabetic coma or ketoacidosis and renal failure, malignancies, neurological diseases, immunosuppressor drugs or corticosteroid use, pregnancy, and nursing were excluded.
Fifty-six type 1 or 2 diabetes patients of both sex, ≥ 20 years old, Wagner’s grade 3 or 4 DFU, wound size > 1 cm2, more than 4 weeks of wound evolution, and signed informed consent to participate were eligible for enrollment (Tables 1 and 2). Patients who required revascularization, hemoglobin < 100 g/l, uncompensated chronic diseases, diabetic coma or ketoacidosis and renal failure, malignancies, neurological diseases, immunosuppressor drugs or corticosteroid use, pregnancy, and nursing were excluded.
Trials Procedures
Participants received rhEGF 75 μg intralesional and perilesional, three times per week on alternate days, during a maximum of eight weeks, and good wound care (GWC). Lyophilized rhEGF was dissolved with 5 ml of water for injection, and injected using a standard disposable syringe with a 27G x 0.5” insulin needle (5 – 10 injections of 0.5 – 1 ml), first into the dermo-epidermal junction at equidistant points all over the lesion contours and then deeply downward into the wound bottom in circles and centripetally to ensure a uniform distribution. Wounds were dressed with sterile gauze, and all patients were seen at follow-up visits three times per week until the end of the trial.
Participants received rhEGF 75 μg intralesional and perilesional, three times per week on alternate days, during a maximum of eight weeks, and good wound care (GWC). Lyophilized rhEGF was dissolved with 5 ml of water for injection, and injected using a standard disposable syringe with a 27G x 0.5” insulin needle (5 – 10 injections of 0.5 – 1 ml), first into the dermo-epidermal junction at equidistant points all over the lesion contours and then deeply downward into the wound bottom in circles and centripetally to ensure a uniform distribution. Wounds were dressed with sterile gauze, and all patients were seen at follow-up visits three times per week until the end of the trial.
Outcomes
The primary outcome was wound closure. The secondary outcomes were per cent of granulation, time to appear granulation tissue, wound area, and healing time. Ankle-brachial index was measured at baseline, and end of treatment to evaluate vascular haemodynamic. Laboratory tests included blood cell counts, haemoglobin, haematocrit, globular sedimentation rate, glycohaemoglobin (HbA1C), creatinine, and aspartate aminotransferase. Blood glucose was measured for patients’ metabolic control. Bacterial infection was monitored by wound cultures, before and during therapy.
The primary outcome was wound closure. The secondary outcomes were per cent of granulation, time to appear granulation tissue, wound area, and healing time. Ankle-brachial index was measured at baseline, and end of treatment to evaluate vascular haemodynamic. Laboratory tests included blood cell counts, haemoglobin, haematocrit, globular sedimentation rate, glycohaemoglobin (HbA1C), creatinine, and aspartate aminotransferase. Blood glucose was measured for patients’ metabolic control. Bacterial infection was monitored by wound cultures, before and during therapy.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (25th and 75th percentiles). Categorical variables were given as absolute values and percentages. Absolute frequency, percent of granulation, and wound closure were estimated in categories: satisfactory and unsatisfactory. Normality (QQPlot), and fitness tests (Shapiro Wilk and Kolmogorov-Smirnov) were performed to verify if data were uncorrelated with one another, come from a normal distribution, and random component had fixed variation. A cross tabulation was performed including control variables and the response variable wound closure. Evaluation of safety was performed from frequency of patients with adverse events and frequency of adverse events. Two-sided P values of 0.05 or less were considered to indicate statistical significance. The Bayes Factor test was used for benefit-risk ratio analysis, considering wound closure as benefit criterion, and amputation and interruption due to adverse events as risk criteria. [7,8] Data were double entered and validated on the free software OpenClínica [1] and imported to SPSS software version 15 (IBM SPSS Statistics, IBM, New York.) [8]
Continuous variables were expressed as mean ± standard deviation (SD) or median (25th and 75th percentiles). Categorical variables were given as absolute values and percentages. Absolute frequency, percent of granulation, and wound closure were estimated in categories: satisfactory and unsatisfactory. Normality (QQPlot), and fitness tests (Shapiro Wilk and Kolmogorov-Smirnov) were performed to verify if data were uncorrelated with one another, come from a normal distribution, and random component had fixed variation. A cross tabulation was performed including control variables and the response variable wound closure. Evaluation of safety was performed from frequency of patients with adverse events and frequency of adverse events. Two-sided P values of 0.05 or less were considered to indicate statistical significance. The Bayes Factor test was used for benefit-risk ratio analysis, considering wound closure as benefit criterion, and amputation and interruption due to adverse events as risk criteria. [7,8] Data were double entered and validated on the free software OpenClínica [1] and imported to SPSS software version 15 (IBM SPSS Statistics, IBM, New York.) [8]
Results
Characteristics of the Patients
From November 2018 to February 2019, a total of 56 patients with advanced DFU were enrolled at four centers. Most patients suffered type 2 DM (94.6%), and received insulin treatment (51.8%), followed by Metformin (46.4%) and Glibenclamid (25.0%). Patients were predominantly males (66.0%), 61 years old on average, 33 (59.0%) with neuropathic, and 23 (41.0%) with ischemic DFU (Table 1). The average time with DM was 16 years. Ulcer size was in the range between 2 and 148 cm2, and most of them in fingers (Table 2). DFUs were classified as grade 3 (60.7%), and grade 4 (39.2%), according to Wagner’s scale, and 60.7% of them located in the left foot.
From November 2018 to February 2019, a total of 56 patients with advanced DFU were enrolled at four centers. Most patients suffered type 2 DM (94.6%), and received insulin treatment (51.8%), followed by Metformin (46.4%) and Glibenclamid (25.0%). Patients were predominantly males (66.0%), 61 years old on average, 33 (59.0%) with neuropathic, and 23 (41.0%) with ischemic DFU (Table 1). The average time with DM was 16 years. Ulcer size was in the range between 2 and 148 cm2, and most of them in fingers (Table 2). DFUs were classified as grade 3 (60.7%), and grade 4 (39.2%), according to Wagner’s scale, and 60.7% of them located in the left foot.
| Characteristic | Neuropathics | Ischemics | Total | |
| Patients (%) | 33 (59.0) | 23 (41.0) | 56 (100.0) | |
| Gender (%) | males | 22 (66.7) | 15 (65.2) | 37 (66.1) |
| females | 11 (33.3) | 8 (34.8) | 19 (33.9) | |
| Age | mean± SD | 58 ± 13 | 66 ± 10 | 61 ± 12 |
| (minimum; maximum) | (28; 88) | (47; 82) | (28; 88) | |
| Diabetes type (%) | 1 | 1 (3.0) | 2 (8.7) | 3 (5.4) |
| 2 | 32 (97.0) | 21 (91.3) | 53 (94.6) | |
| Diabetes evolution time (years) | media ± SD | 15 ± 9 | 17 ± 11 | 16 ± 10 |
| (minimum; maximum) | (3; 45) | (3; 48) | (3; 48) | |
| Glucose control treatment (%) | Insulin | 19 (57.6) | 10 (43.5) | 29 (51.8) |
| Metformin | 15 (45.4) | 11 (47.8) | 26 (46.4) | |
| Glibenclamid | 8 (24.2) | 6 (26.1) | 14 (25.0) | |
Table 1: Demographic and baseline characteristics of patients. SD: standard deviation.
| Patients (%) | Neuropathics | Ischemics | Total | |
| 33 (59.0) | 23 (41.0) | 56 (100.0) | ||
| Wagner’s classification grade (%) | 3 | 31 (93.9) | 3 (13.0) | 34 (60.7) |
| 4 | 2 (6.1) | 20 (87.0) | 22 (39.2) | |
| Affected lower limb (%) | left | 21 (63.6) | 13 (56.5) | 34 (60.7) |
| right | 12 (36.4) | 10 (43.5) | 22 (39.3) | |
| Wound location (%) | finger | 17 (51.5) | 11 (47.8) | 28 (50.0) |
| sole | 14 (42.4) | 3 (13.0) | 17 (30.3) | |
| dorsum | 7 (21.2) | 5 (21.7) | 12 (21.4) | |
| internal edge | 2 (6.1) | 4 (17.4) | 6 (10.7) | |
| calcaneus | 1 (3.0) | 4 (17.4) | 5 (8.9) | |
| extreme edge | 3 (9.1) | 1 (4.3) | 4 (7.1) | |
| transmetatarsal | 0 (0.0) | 3 (13.0) | 3 (5.3) | |
| stump | 0 (0.0) | 1 (4.3) | 1 (1.8) | |
| Wound evolution time (days) | median± QR | 97 ± 169 | 120 ± 168 | 110 ± 152 |
| (minimum; maximum) | (3; 730) | (28; 395) | (3; 730) | |
| Wound area (cm2) | median ± QR | 5.0 ± 15.0 | 17.0 ± 31.0 | 6.5 ± 22.5 |
| (minimum; maximum) | (2.0; 105.0) | (3.0; 65.0) | (2.0; 105.0) | |
| Indication of minor surgical procedures (%) | yes | 15 (45.4) | 8 (34.8) | 23 (41.1) |
| toilette | 12 (80.0) | 7 (87.5) | 19 (82.6) | |
| disarticulation | 4 (26.7) | 2 (25.0) | 6 (26.1) | |
Table 2: Wound classification and data. QR: quartile range.
Follow-Up and Outcomes
Follow-up of data for all outcomes were available through March 2019. Complete treatment compliance was reported in 52 (93.0%) patients. Interruption was reported in one patient due to renal failure, other two had extensive lesions, and amputation was necessary in three patients. Complete granulation response was achieved in all patients, including abandoners, at a mean time of 26.7 days. Wound closure was obtained in 50 patients (89.2%). Mean time to complete closure was in the range between 6.4 and 7.0 weeks (Table 3). Wound closure was observed in 18 out of 23 ischemic patients (78.0%).
Follow-up of data for all outcomes were available through March 2019. Complete treatment compliance was reported in 52 (93.0%) patients. Interruption was reported in one patient due to renal failure, other two had extensive lesions, and amputation was necessary in three patients. Complete granulation response was achieved in all patients, including abandoners, at a mean time of 26.7 days. Wound closure was obtained in 50 patients (89.2%). Mean time to complete closure was in the range between 6.4 and 7.0 weeks (Table 3). Wound closure was observed in 18 out of 23 ischemic patients (78.0%).
| Patients (%) | Neuropathics | Ischemics | Total | |
| 33 (59.0) | 23 (41.0) | 56 (100.0) | ||
| rhEGF applications | Mean ± QR (minimum; maximum) | 10 ± 5 (4; 24) | 14 ± 13 (3; 24) | 12 ± 8 (3; 24) |
| Complete granulation (%) | yes | 33 (100.0) | 23 (100.0) | 56 (100.0) |
| Wound closure (%) | yes | 32 (97.0) | 18 (78.2) | 50 (89.2) |
| no | 1 (3.0) | 5 (21.8) | 6 (10.8) | |
| Major amputation (%) | yes | 1 (3.0) | 2 (8.6) | 3 (5.3) |
| no | 32 (97.0) | 21 (91.4) | 53 (94.7) | |
| Wound closure time, mean± SE (weeks) | 6.4 ± 0.6 | 7.0 ± 1.0 | 6.6 ± 0.5 | |
| CI (95%) | (5.3; 7.5) | (5.1; 8.9) | (5.6; 7.6) | |
| Log Rank (χ2 = 1.025; p = 0.311) | ||||
Table 3: Granulation response to treatment with intralesional rhEGF, and final outcomes. QR: quartile range, and SE: standard error.
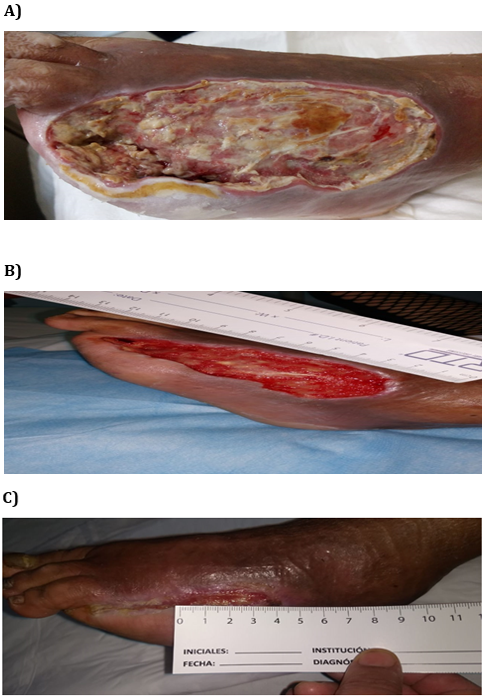
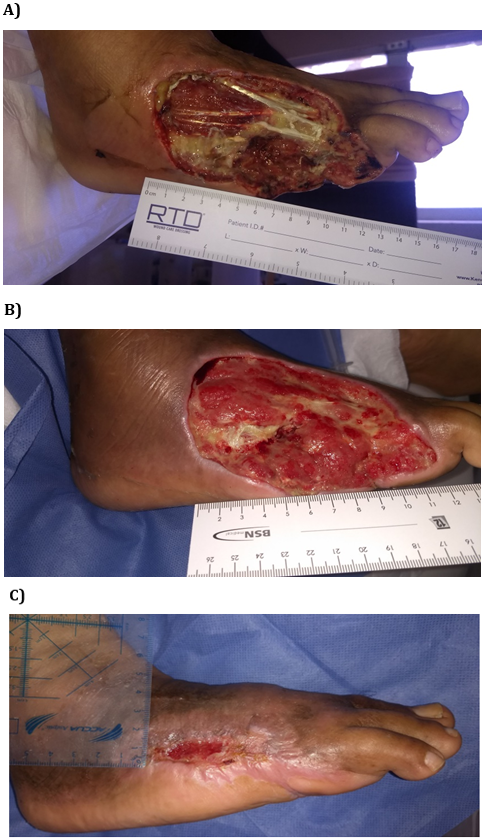
Figure 1 shows two examples of wounds’ clinical aspects. Photo A of the case 1 shows the ulcer of a 54 year old male with diabetes for 4 years and an extensive ischemic wound (area 57 cm2) on the dorsum. Wound evolution was 60 days, and after revascularization, amputation had been indicated. After 2 weeks of rhEGF treatment with 6 interventions, complete granulation was observed. Twenty interventions with rhEGF were necessary to obtain wound closure after 7 weeks. Photo A of the case 2 shows the most complicated ulcer treated with rhEGF. This patient was a 50 year old male with diabetes for 14 years and an extensive ischemic wound (area 148 cm2) on the dorsum. Infection and osteomyelitis were also present and amputation had been previously indicated as the only alternative. After soft tissue debridement, bone resection within the necrotic area and broad-spectrum antibiotics, rhEGF intervention was thereafter performed. Complete granulation response was achieved in 3 weeks, after seven rhEGF interventions. He was re-evaluated thereafter and complete wound closure was confirmed at week 8, and 21 interventions with rhEGF.
Case 1
Case 2
Safety
Adverse events (164) are listed in Table 4. The most frequent adverse events were local pain, chills, tremors, and nauseas. More than 90.0% of adverse events were classified as mild, and 3.2% as severe. Adverse events in neuropathic patients account for 58.5% of the total, 41.5% in ischemic patients, and none of them were attributable to rhEGF treatment. The benefit/risk ratio is presented in Figure 2. In both neuropathic and ischemic patients, absence of interceptions between probability distribution functions for benefit (wound closure) and risk (amputation and interruption) were evident. Bayes Factor, representing ratio of the likelihood of benefit (red) to the likelihood of risk (blue), was 12.9 for neuropathic and 6.3 for ischemic patients.
Adverse events (164) are listed in Table 4. The most frequent adverse events were local pain, chills, tremors, and nauseas. More than 90.0% of adverse events were classified as mild, and 3.2% as severe. Adverse events in neuropathic patients account for 58.5% of the total, 41.5% in ischemic patients, and none of them were attributable to rhEGF treatment. The benefit/risk ratio is presented in Figure 2. In both neuropathic and ischemic patients, absence of interceptions between probability distribution functions for benefit (wound closure) and risk (amputation and interruption) were evident. Bayes Factor, representing ratio of the likelihood of benefit (red) to the likelihood of risk (blue), was 12.9 for neuropathic and 6.3 for ischemic patients.
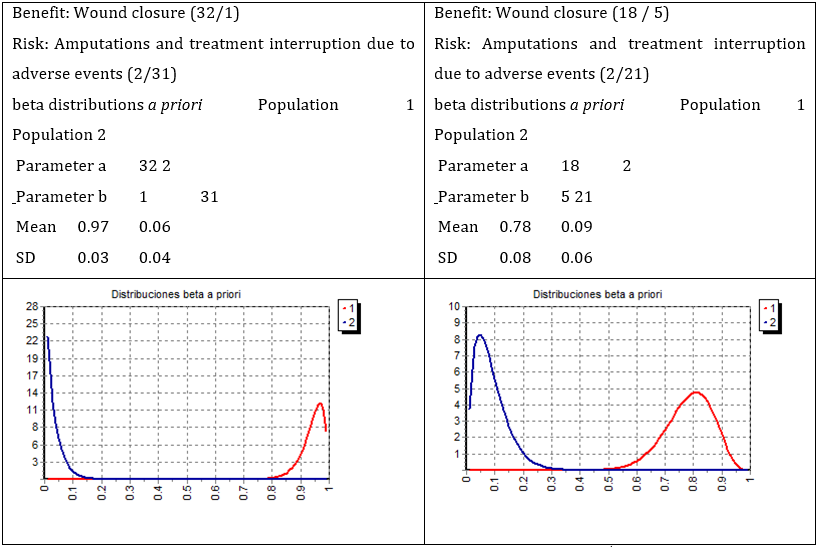
Figure 2: Risk-benefit analysis of neuropathic and ischemic patients. Benefit (red; wound closure) and risk (blue; amputations and treatment interruption due to adverse events) probability distributions of the outcome of DFU patients treated with intralesional rhEGF. Left graph: neuropathic patients, Bayes Factor = 12.9. Right graph: Ischemic patients, Bayes Factor = 6.3.
| Patients (%) | Neuropathics | Ischemics | Total | ||
| 96 (58.5) | 68 (41.5) | 164 (100.0) | |||
| Events | |||||
| Pain at site of administration (%) | Frequency (%) | 38 (39.6) | 56 (82.4) | 94 (57.3) | |
| Intensity | mild | 37 (97.4) | 54 (96.4) | 91 (96.8) | |
| severe | 1 (2.6) | 2 (3.6) | 3 (3.2) | ||
| Severity | non severe | 38 (100.0) | 56 (100.0) | 94 (100.0) | |
| Shivering (%) | Frequency | 25 (26.0) | 2 (2.9) | 27 (16.5) | |
| Intensity | mild | 23 (92.0) | 2 (100.0) | 25 (92.6) | |
| moderate | 2 (8.0) | 0 (0.0) | 2 (7.4) | ||
| Severity | non severe | 24 (96.0) | 2 (100.0) | 26 (96.3) | |
| hospitalization or extend hospitalization | 1 (4.0) | 0 (0.0) | 1 (3.7) | ||
| Tremor (%) | Frequency (%) | 18 (18.8) | 5 (7.4) | 23 (14.0) | |
| Intensity | mild | 17 (94.4) | 5 (100.0) | 22 (95.7) | |
| severe | 1 (5.6) | 0 (0.0) | 1 (4.3) | ||
| Severity | non severe | 18 (100.0) | 5 (100.0) | 23 (100.0) | |
| Nausea (%) | Frequency (%) | 3 (3.1) | 2 (2.9) | 5 (3.0) | |
| Intensity | mild | 2 (66.7) | 2 (100.0) | 4 (80.0) | |
| severe | 1 (33.3) | 0 (0.0) | 1 (20.0) | ||
| Severity | non severe | 3 (100.0) | 2 (100.0) | 5 (100.0) | |
| Burning at site of administration (%) | Frequency (%) | 4 (4.2) | 0 (0.0) | 4 (2.4) | |
| Intensity | mild | 3 (75.0) | 0 (0.0) | 3 (75.0) | |
| severe | 1 (25.0) | 0 (0.0) | 1 (25.0) | ||
| Severity | non severe | 4 (100.0) | 0 (0.0) | 4 (100.0) | |
| Local infection (%) | Frequency (%) | 3 (3.1) | 1 (2.9) | 4 (3.0) | |
| Intensity | mild | 2 (66.7) | 0 (0.0) | 2 (50.0) | |
| moderate | 1 (33.3) | 0 (0.0) | 1 (25.0) | ||
| severe | 0 (0.0) | 1 (100.0) | 1 (25.0) | ||
| Severity | non severe | 3 (100.0) | 0 (0.0) | 3 (75.0) | |
| Hospitalización o la prolonga | 0 (0.0) | 1 (100.0) | 1 (25.0) | ||
| Headache (%) | Frequency (%) | 2 (2.1) | 1 (1.5) | 3 (1.8) | |
| Intensity | mild | 1 (50.0) | 1 (100.0) | 2 (66.7) | |
| severe | 1 (50.0) | 0 (0.0) | 1 (33.3) | ||
| Severity | non severe | 2 (100.0) | 1 (100.0) | 3 (100.0) | |
| Diarrhoea (%) | Frequency (%) | 2 (2.1) | 1 (1.5) | 3 (1.8) | |
| Intensity | mild | 1 (50.0) | 1 (100.0) | 2 (66.7) | |
| moderate | 1 (50.0) | 0 (0.0) | 1 (33.3) | ||
| Severity | non severe | 2 (100.0) | 1 (100.0) | 3 (100.0) | |
| Pneumonia (%) | Frequency (%) | 1 (1.0) | 0 (0.0) | 1 (0.6) | |
| Intensity | severe | 1 (100.0) | 0 (0.0) | 1 (100.0) | |
| Severity | threat to life | 1 (100.0) | 0 (0.0) | 1 (100.0) | |
Table 4: Adverse events reported in DFU patients treated with intralesional rhEGF.
Discussion
We found that the most affected anatomic region was finger. Average area of these wounds was 20 cm2, which is a condition with low probability to develop granulation tissue in a time as short as two weeks. The amount of toilettes (19) and disarticulations (6) indicated before rhEGF treatment is evidence of the high complexity of the treated DFUs (Table 2). Data obtained from post-marketing pharmacosurveillance suggested favorable results of healing time (14.0 weeks), compare to GWC alone (21.4 weeks). [17,18]
The high percentage of patients with effective granulation response observed in this study (100.0%) could be explained on the basis of the contribution of rhEGF to the healing process, since this protein is locally reduced in the surface of chronic wounds. [8] rhEGF injections support its ability to activate tissue repair, [9,10] avoiding degradation action of proteases against growth factors and their cellular receptors, [9,10] reducing diffusion barriers, [9] reaching deeper strata of fibroblasts where EGF receptors are more abundant than at wound surface cell layer, [9] and increasing EGF interaction time with cell receptors. [11-14]
Amputation rate was low (5.3%), which shows that was possible avoiding amputation. Ischemia in the affected limb represents a significant hindrance for DFU healing, but the beneficial impact of rhEGF treatment in ischemic patients was significant (closure time = 7.0 weeks). The median healing time of DFU in ischemic patients had previously been reported in the range between 5 and 16 months, irrespective of surgical interventions. [8]
Results of statistical analysis were strong evidence in favor of the benefit of the rhEGF treatment in neuropathic patients (Bayes Factor = 12.9), and moderate evidence in ischemic patients (Bayes Factor = 6.3). The strength of the statistical hypothesis in favor of the benefit of rhEGF treatment was higher than previously reported in clinical trials (Bayes Factor=3.2) and post-marketing pharmacosurveillance (Bayes Factor=5.4). Cases shown in Figure 1 confirmed the finding previously reported on granulation tissue development after 2 weeks as useful predictor of wound healing. [8] In addition, safety and efficacy of this treatment in patients with large ischemic wounds for which endovascular and surgical revascularization did not reduce major amputation rate at middle and long-term was confirmed. [8,9]
The International Working Group of the Diabetic Foot (IWGDF) evaluated results of the clinical trial with rhEGF as promising. [8] This method was compared with the state-of-the-art in a systematic review, [8] and a random effects meta-analysis stratified by the types of administration route has supported the use of rhEGF in the treatment of DFU. [8] In conclusion, in our trial, efficacy and safety of the treatment of advanced DFU with perilesional and intralesional injection of EGF were equally demonstrated in neuropathic and ischemic patients.
Acknowledgments
We thanks to medical doctors, nurses, and administrative personnel at the four hospitals participating in the implementation and deploy of the treatment with rhEGF for their contributions. They also acknowledge the supply of rhEGF to the Center for Genetic Engineering and Biotechnology (Havana, Cuba) and to the Ministry of Public Health for assistance with Angiologists and Vascular Surgeons for medical advice.
We thanks to medical doctors, nurses, and administrative personnel at the four hospitals participating in the implementation and deploy of the treatment with rhEGF for their contributions. They also acknowledge the supply of rhEGF to the Center for Genetic Engineering and Biotechnology (Havana, Cuba) and to the Ministry of Public Health for assistance with Angiologists and Vascular Surgeons for medical advice.
Disclosure of Conflict of Interests
Julio E. Baldomero-Hernández, MD, Marel A. Valdés, MS., and Ángela D. Tuero-Iglesias, MS are employees of the Center for Genetic Engineering and Biotechnology, Havana, owner of the product’s patent and sanitary registrations, where product Heberprot-P® is produced. The rest of the authors have not conflict of interest to declare.
Julio E. Baldomero-Hernández, MD, Marel A. Valdés, MS., and Ángela D. Tuero-Iglesias, MS are employees of the Center for Genetic Engineering and Biotechnology, Havana, owner of the product’s patent and sanitary registrations, where product Heberprot-P® is produced. The rest of the authors have not conflict of interest to declare.
References
- Instituto Nacional de Salud Pública. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016. Informe Final de Resultados. Available at: http://oment.uanl.mx/wp-content/uploads/2016/12/ensanut_mc_2016- 310oct.pdf. Access: December 26th, 2019.
- Montiel-Jarquín AJ, García-Villaseñor A, Castillo-Rodríguez C, et al. (2017). Costes directos de atención médica del pie diabético en el segundo nivel de atención médica. Rev Chil Cir; 69: 118-23.
- Exchange rate from Mexican pesos to US dollar: 1 USD = 15.38 pesos, June 1rst, 2015.
- Berlanga-Acosta J, Savigne W, Valdez C, et al. (2006). Epidermal Growth Factor intralesional can prevent amputation in diabetic patients with advanced foot wounds. Int Wound J 3: 232-9.
- Fernández-Montequín JI, Infante-Cristiá E, Valenzuela-Silva C, et al. (2007). Intralesional injections of Citoprot P® (Recombinant Human Epidermal Growth Factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 4: 333-43.
- Fernández-Montequín JI, Betancourt BY, Leyva-Gonzalez G, et al. (2009). Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in advanced diabetic foot ulcer: Treatment up to complete wound closure. Int Wound J; 6: 67-72.
- Fernández-Montequín JI, Valenzuela-Silva CM, González-Díaz O, et al. (2009). Intralesional injections of recombinant human Epidermal Growth Factor promote granulation and healing in advanced diabetic foot ulcers. Multicenter, randomized, placebo-controlled, double blind study. Int Wound J 6: 432-43.
- López-Saura PA, Yera-Alos IB, Valenzuela-Silva C, et al. (2013). Medical practice confirms clinical trial results of the use of intralesional human recombinant Epidermal Growth Factor in advanced diabetic foot ulcers. Adv Pharmacoepidem Drug Safety 2: 128.
- Yera-Alos IB, Alonso-Carbonell L, Valenzuela-Silva CM, et al. (2013). Active post-marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacol Toxicol 14: 44.
- Wagner FW. Algorithms of diabetic foot care. In Levin ME, O’Neal FW (ed). The Diabetic Foot. St Louis, MO: Mosby 1983; 290-5.
- World Medical Association (WMA). Declaration of Helsinki. Ethical principles for medical research involving human subjects. 64th WMA General Assembly, Fortaleza, Brazil, October 2013.
- Jeffreys H. Theory of probability. 3rd Ed. Oxford, UK: Clarendon Press, 1961.
- Lee MD, Wagenmakers EJ. Bayesian cognitive modeling: A practical course. Cambridge University Press, 2013.
- OpenClínica, LLC. OpenClínica platform for data capture. Available at: https://www.openclinica.com.
- Pardo A, Ruiz MA. SPSS 11. Guía para el análisis de datos. Madrid: McGraw-Hill, 2002.
- Berlanga-Acosta J, Mendoza-Mari Y, García-Ojalvo A, Buxadó JA. (2019). Fernández-Mayola M, Guillén-Nieto G. Epidermal Growth Factor (EGF) intralesional infiltrations: From the bench to the diabetic ulcers cells. Integr Mol Med 6: 1-7.
- Berlanga-Acosta J, Caballero E, Prats P, López-Saura P, Playford RJ. (1999). The role of the epidermal growth factor in cell and tissue protection. Medicina Clínica 113: 222-9.
- Berlanga-Acosta J, Mella-Lizama C. (1998). Some physiological considerations of epidermal growth factor in relation to its pharmacological applications. Biotecnología Aplicada 15: 141-8.
- Gibson D, Schultz GS. (2009). Chronic wound diagnostic for matrix metalloproteinase. Wound Healing Southern Africa 2: 68-70.
- Mathew S, Ravisanker V, Potluri T, Suchithra T. (2015). Delayed diabetic wound healing: A focus on bacterial proteases in chronic wound and foot ulcer. Int J Curr Res Rev 7: 36-43.
- Cross SE, Roberts MS. (1999). Defining a model to predict the distribution of topically applied growth factors and other solutes in excisional full-thickness wounds. J Invest Dermatol 112: 36-41.
- Berlanga-Acosta J. (2011). Diabetic lower extremity wounds: the rationale for growth factors-based infiltration treatment. Int Wound J; 8: 612-20.
- Taylor JM, Cohen S, Mitchell WM. (1970). Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci 67: 164-71.
- Wiley HS, Cunninghan DD. (1981). A steady state model for analyzing the cellular binding, internalization and degradation of polypeptide ligands. Cell 25: 433-40.
- Buckley A, Davidson JM, Kamerath CD, Wolt TB, Woodward SC. (1985). Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci 82: 7340-44.
- Portero-Otín M, Pamplona R, Belmunt MJ, et al. (2002). Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes 51: 1535-42.
- Elgzyri T, Larsson J, Nyberg P, Thörne J, Eriksson KF, Apelqvist J. (2014). Early revascularization after admittance to a diabetic foot center affects the healing probability of ischemic foot ulcer in patients with diabetes. Eur J Vasc Endovasc Surg 48: 440-6.
- Valenzuela-Silva CM, Tuero-Iglesias AD, García-Iglesias E, et al. (2013). Granulation response and partial wound closure predict healing in clinical trials on advanced diabetes foot ulcers treated with recombinant human epidermal growth factor. Diabetes Care 36: 210-15.
- Ertugrul BM, Buke C, Ersoy OS, et al. (2015). Intralesional epidermal growth factor for diabetic foot wounds: the first cases in Turkey. Diab Foot Ankle 6: 28419.
- Kahraman M, Misir A, Kizkapan TB, Ozcamdalli M, Uzun E, Mutlu M. (2019). The long-term outcomes following the application of intralesional epidermal growth factor in patients with diabetic foot ulcers. J Foot Ankle Surg 58: 282-7.
- Game FL, Hinchliffe RJ, Apelqvist J, et al. (2012). A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 28 (Suppl 1): 119-41.
- Martí-Carvajal AJ, Gluud C, Nicola S, et al. (2015). Growth factors for treating diabetic foot ulcers (Review). The Cochrane Library 10: 7-23, 43-47, 106-39.
- Bui TQ, Bui QVP, Németh D, et al. (2019). Epidermal Growth Factor is effective in the treatment of diabetic foot ulcers: meta-analysis and systematic review. Int J Environ Res Public Health 16: 2584.
Citation: José A. Buxadó., et al. (2020). Recombinant Human Epidermal Growth Factor: Medical Experience of Use in Patients with Advanced Diabetic Foot Ulcers in Mexico. Archives of Endocrinology and Diabetes 2(2).
Copyright: © 2020 José A. Buxadó. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.