Case Report
Volume 2 Issue 1 - 2020
Parry Romberg Syndrome. An unusual case with dental features.
1Orthodontic Department, Leeds Dental Institute, Leeds, UK
2Oral and Maxillofacial Surgery Department, Leeds Dental Institute, Leeds, UK
2Oral and Maxillofacial Surgery Department, Leeds Dental Institute, Leeds, UK
*Corresponding Author: Hamed Safaei, Oral and Maxillofacial Surgery Department, Leeds Dental Institute, Leeds, UK.
Received: October 21, 2020; Published: October 29, 2020
Abstract
Parry-Romberg syndrome, also known as progressive hemifacial atrophy has been described as having clinical overlap with Morphea en coup de sabre or linear scleroderma. The aetiology of this rare disorder is believed to be of auto-immune origin and presents with both intra-oral and extra-oral deformities in the craniofacial region.
There is currently little information available in the literature discussing the dental features and orthodontic management of patients with Parry-Romberg syndrome. Here we hope to raise awareness in the dental community regarding the presenting features and management of Parry-Romberg syndrome. We have outlined the treatment and management the patient received from both Maxillofacial and Orthodontic departments over a six year period.
Introduction
Parry-Romberg syndrome was first described by Parry (Parry, 1825) and Romberg (Romberg, 1846). It is also known as progressive hemifacial atrophy, progressive orofacial hemiatrophy, and has been described as having clinical overlap with Morphea en coup de sabre or linear scleroderma (Toussaint et al., 2010; Maletic et al., 2010; Tollefson and Witman, 2007; De Somer et al., 2015).
The aetiology of this rare disorder is unknown but thought to be of autoimmune nature due to favourable response to immunosuppressive drugs (Korkmaz et al., 2005). The presence of the disease in one of two twins reduces the likelihood of a genetic aetiology (Hulzebos et al., 2004) although some familial association has been reported in two first cousins, whose fathers were non-identical twins and mothers were sisters (Anderson et al., 2005). The disease is self-limiting and although a skin and subcutaneous disease, will progress to the underlying muscles, cartilage and hard tissues. Parry-Romberg Syndrome typically presents in children or adolescents and has a slow, progressive nature until it stabilises(Rogers, 1964).
Following initial clinical features it may become apparent that there are areas of hyper or hypopigmented skin which may be present on the ipsilateral or contralateral side of the facial deformity. Another early clinical feature may be the presence of a depressed vertical scar along the centre of the forehead known as an “en coup de sabre”. (Stone, 2006; Cory et al., 1997)
The facial deformity as a result of the syndrome can vary in severity, dependant on when the disease begins. If during early childhood, during rapid growth, the asymmetry can be marked and severe. The atrophy can result in restriction of growth of hard tissues in all three dimensions. The mandible and maxilla are commonly affected, but up to 20% of individuals suffering with Parry Romberg Syndrome have disorders of other limbs or the contralateral craniofacial area (Jain et al., 2016). There may be vascular abnormalities in the brain resulting in epilepsy or other neurological conditions and eye malformations on the ipsilateral side (Hoppe et al., 2009; Zahlane and Kissani, 2013; English et al., 2018; Gambichler et al., 2001; Hulzebos et al., 2004; Vix et al., 2015). Migraines and Neuropathic facial pain such as trigeminal neuralgia are common neurological features of up to 45% of this patient group (Stone, 2006).Intra-orally there have been reports of atrophy affecting the tongue, upper lip and soft palate on the affected side (Stone, 2006; O'Flynn and Kinirons, 2006). There are few reports in the literature regarding the dental manifestations of progressive hemifacial atrophy or Parry Romberg Syndrome. There is often delayed eruption or hypodontia. Idiopathic resorption (Fayad and Steffensen, 1994) or dilaceration of teeth (O'Flynn and Kinirons, 2006) on the affected side have been reported sporadically but not in separate teeth on the affected side. We have documented the main clinical features in table 1.
| Main clinical features |
| Hemifacial atrophy of skin, fat, connective tissue, muscle and or bone |
| Intra-oral abnormalities, including delayed dental eruption, root abnormalities and hypodontia |
| Migraine / facial pain such as trigeminal neuralgia |
| Ocular abnormalities |
| Midline facial deformity; “en coup de sabre” |
| Hyper or hypopigmented facial lesion |
| Epilepsy or other neurological disorders |
| Atrophy of contralateral or ipsilateral arm, trunk, leg |
Table 1: List of main clinical features associated with Parry-Romberg syndrome.
The following case report documents Parry Romberg Syndrome/Progressive hemifacial atrophy in a growing female with the unusual presentation of dilaceration of tooth roots, ectopic eruption of teeth, idiopathic resorption and microdontism.
Case Report History
A 10-year-old female was referred to the paediatric department in the Leeds Dental Institute, in 2014 by her Paediatric Rheumatologist regarding investigation of her dental asymmetry. She had a history of onset of linear scleroderma, which was diagnosed at the age of five years old. This was subsequently treated with various immunosuppressive drugs attempting to combat the progression of the scleroderma and was under regular review in the scleroderma clinic at Leeds General Infirmary (Table 2).
| Date | Drug |
| March – Sept 2010 | Cyclosporin |
| October 2010 – April 2011 | Methotrexate |
| November 2011 | IV Methylprenisolone (3 pulses then five monthly pulses) |
| November 2011 – July 2016 | Subcutaneous Methorexate 15mg |
| September 2015 – present | Levetiracetum 250mg BD rising to 1500mg BD |
Table 2: Summary of medications used in treatment of Hemifacial atrophy and epilepsy.
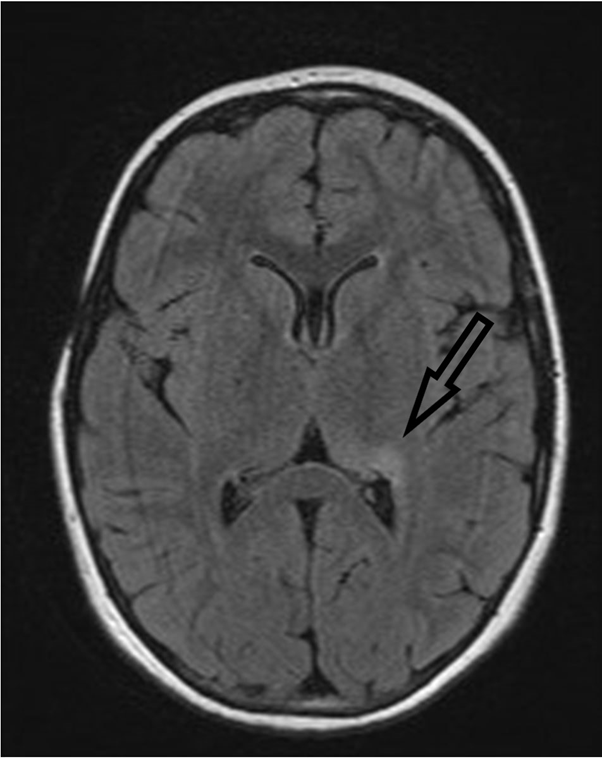
An MRI of the head was obtained in 2012 with no abnormality noted and a reduction of skin pigmentation was noted clinically whilst being maintained on the methotrexate (Figure 1). In mid 2015 the patient presented with an episode of encephalopathy, which was followed by generalised tonic/clonic seizures consistent with epilepsy. Investigation with MRI showed persistent abnormality in the left thalamus and posterior medial hippocampal regions and EEG found changes in the left temporal lobe (Figure 2). Levetiracetum was prescribed in September 2015 at a dose of 250mg BD, but failed to control seizures. Dosage was increased steadily until the maximum dose of 1500mg BD was reached in October 2018, with seizures still persisting on a bi-weekly basis.
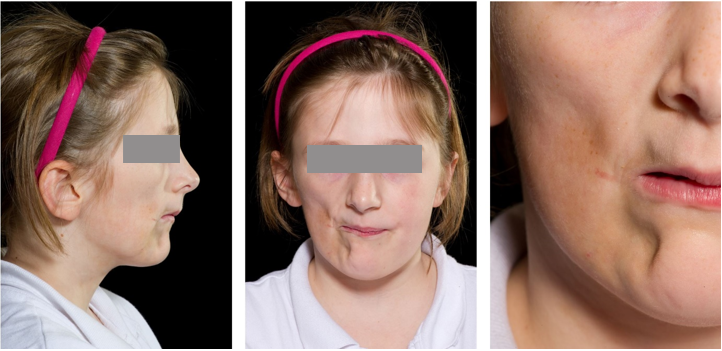
A Plastic Surgery review in 2012 noted complete atrophy of the right sided subcutaneous fat in the Trigeminal (V2 and V3) dermatomes (Figure 3) and consideration was given to the Coleman Technique for structural fat grafting in the affected facial region (Pu et al., 2008). Whilst under regular review of the paediatric rheumatology team little progression of the scleroderma/morphea was noted and the disease was felt to be relatively stable. Thermography was undertaken in 2013 and revealed some small differences in skin temperatures between the affected and unaffected tissues of the face. Methotrexate was continued for a period of 5 years and discontinued in 2017 following a prolonged period of disease remission.
Initial Orthodontic assessment
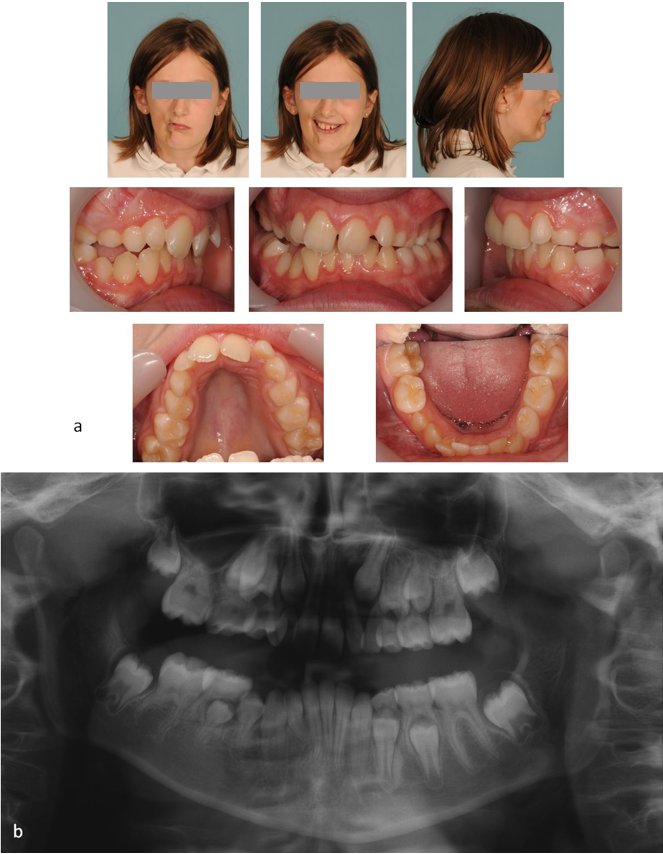
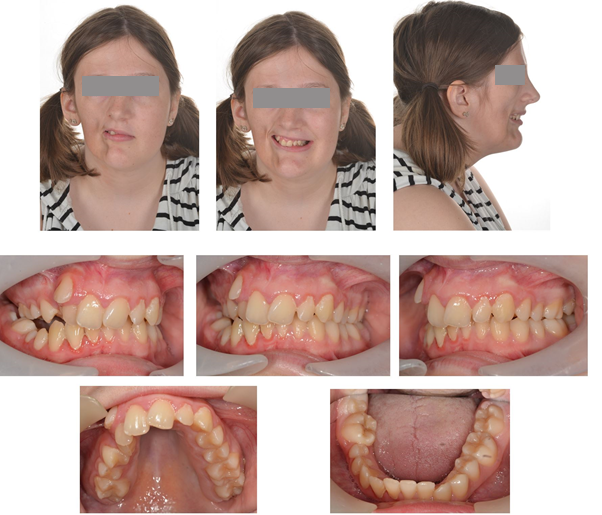
At the paediatric dentistry review in 2014 it was noted that the patient was 10 years old in the late mixed dentition with a mild right hand sided unilateral crossbite and a mild cant of the maxillary teeth with associated centreline shift to the right (Figure 4a). The decision was made to refer the patient to the combined orthodontic and maxillofacial distraction clinic at the Leeds Dental Institute.
At the paediatric dentistry review in 2014 it was noted that the patient was 10 years old in the late mixed dentition with a mild right hand sided unilateral crossbite and a mild cant of the maxillary teeth with associated centreline shift to the right (Figure 4a). The decision was made to refer the patient to the combined orthodontic and maxillofacial distraction clinic at the Leeds Dental Institute.
On presentation to the distraction team there was poor plaque control and upper and lower centreline shift to the right and an increased overjet of 8mm, measured to the left lateral incisor. A crossbite without displacement was noted on the right first permanent molars. The patient was clinically reviewed by the distraction team in 2018 at age 15 after awaiting further growth.
Orthodontic Assessment
Extra-oral assessment
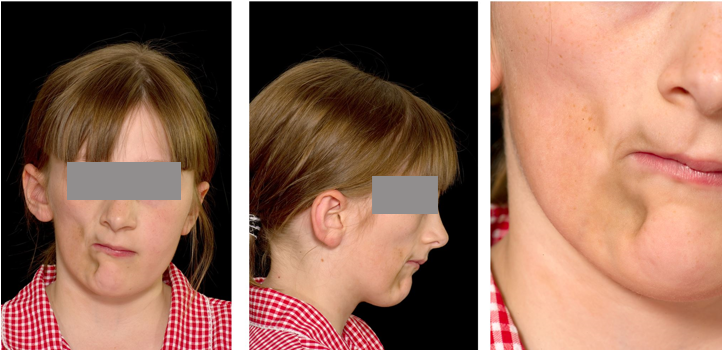
The patient presented with a mild Class II skeletal pattern with slightly increased vertical proportions and right sided facial atrophy affecting both soft and hard tissues of the maxilla and mandible, with associated asymmetry and deviation to the right of the entire midface. The lips were incompetent at rest
The patient presented with a mild Class II skeletal pattern with slightly increased vertical proportions and right sided facial atrophy affecting both soft and hard tissues of the maxilla and mandible, with associated asymmetry and deviation to the right of the entire midface. The lips were incompetent at rest
Intra-oral assessment
Intra-orally, the patient presented with a Class II/1 malocclusion in the permanent dentition complicated by:
Intra-orally, the patient presented with a Class II/1 malocclusion in the permanent dentition complicated by:
- 5mm overjet to UL2
- Increased overbite
- Right lateral open bite
- 7mm upper centreline shift to the right
- 6mm Lower centreline shift to the right
- Unilateral posterior crossbite without displacement
- Mild lower arch and moderate upper arch crowding
- Retained URC
Radiographic assessment
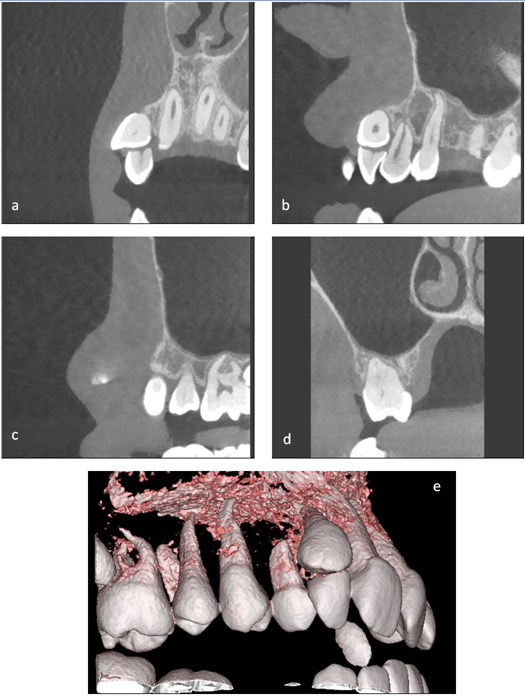
At presentation to the combined orthodontic and maxillofacial distraction clinic in 2014 bony changes were evident on an orthopantomogram (OPT) radiograph, with the right ramus being shorter than the left. Dentally there was impression of poorly malformed roots affecting the LR6, LR5 and UR6. The LR2 appeared to have a slightly dilacerated root morphology (Figure 4b).
At presentation to the combined orthodontic and maxillofacial distraction clinic in 2014 bony changes were evident on an orthopantomogram (OPT) radiograph, with the right ramus being shorter than the left. Dentally there was impression of poorly malformed roots affecting the LR6, LR5 and UR6. The LR2 appeared to have a slightly dilacerated root morphology (Figure 4b).
After clinical review by the distraction team in 2018 a new set of radiographs (Figure 5) were taken as the patient was in the late mixed dentition and was concerned regarding the position of the UR3 (Figure 6). These revealed idiopathic resorption and shortening of the UR6 LR6 UR2 and severe dilaceration of the LR2. The LR5 was microdont with abnormal root morphology and the UR7 was ectopically placed also. The development of the teeth on the left hand side was uneventful.
A cone beam computed tomograph (CBCT) was performed to fully assess the root morphology of the affected teeth and the potential for orthodontic alignment of the malpositioned UR3 (Figure 7a-e). The imaging revealed that the UR2 root was almost entirely resorbed and the UR6 had shortened root morphology.
Treatment
Initial Treatment plan
Initially at presentation in 2014 to the distraction team consideration was given to the provision of a hybrid functional appliance to attempt to ameliorate the maxillary cant and increased overjet and attempt growth modification but it was decided to await further growth.
Initially at presentation in 2014 to the distraction team consideration was given to the provision of a hybrid functional appliance to attempt to ameliorate the maxillary cant and increased overjet and attempt growth modification but it was decided to await further growth.
Definitive treatment plan
During the review by the distraction team in 2018, the patient stated that her greatest concern was the position of the UR3. With this information and previous assessment, the following treatment plan was devised:
During the review by the distraction team in 2018, the patient stated that her greatest concern was the position of the UR3. With this information and previous assessment, the following treatment plan was devised:
- Extraction UR2
- Upper fixed appliance to align UR3 only
- Maintain the URC
Treatment Progress
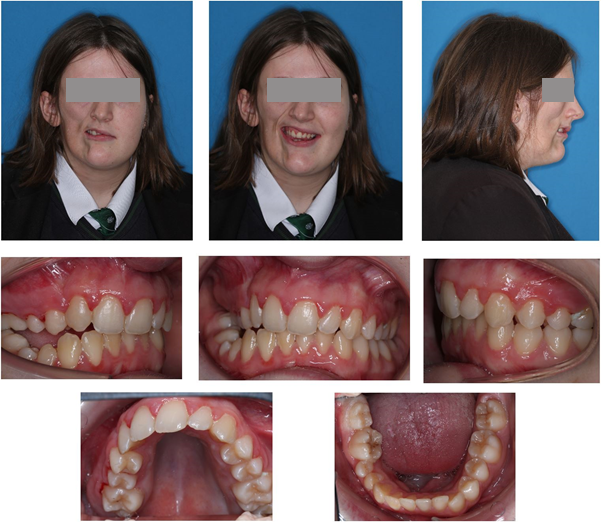
Following extraction of the UR2, 0.022” Preadjusted Edgewise brackets (American Orthodontics, MBT prescription) brackets were placed in the upper arch with light aligning archwires. The URC prevented alignment of the UR3 and therefore it was agreed to arrange extraction of the URC. Space closure was completed on round Stainless Steel archwires to minimise torqueing forces on upper incisor roots and minimise the risk of root resorption.Orthodontic treatment was completed in 12 months and removable vacuum formed retainers were provided for night time wear (figure 8).
Following extraction of the UR2, 0.022” Preadjusted Edgewise brackets (American Orthodontics, MBT prescription) brackets were placed in the upper arch with light aligning archwires. The URC prevented alignment of the UR3 and therefore it was agreed to arrange extraction of the URC. Space closure was completed on round Stainless Steel archwires to minimise torqueing forces on upper incisor roots and minimise the risk of root resorption.Orthodontic treatment was completed in 12 months and removable vacuum formed retainers were provided for night time wear (figure 8).
Should the patient become more concerned with facial asymmetry in the future, Orthognathic treatment would be considered at that point with any adjunctive orthodontic treatment if indicated.
Discussion
The orthodontic management of Parry Romberg Syndrome can be complex. In the present case the patient’s concerns with the alignment of her teeth were directly addressed, whilst balancing the risks and benefits of orthodontic treatment. It has been reported that the use of hybrid appliance(Vig and Vig, 1986) can potentially affect growth and ameliorate the developing asymmetry or occlusal cant, as described by You et al.(You and Baik, 2011).
The Distraction team considered such appliances when the patient first presented aged 10. However as the patient’s asymmetry was mild and she was unconcerned with her malocclusion, it was agreed not to provide any intervention until a later date. A Hybrid appliance was considered, and may have encouraged favourable growth, but would have required use for an extended period of time(Lee et al., 2014), potentially burning compliance that would be required in the future.
Consideration must also be given to the other treatment that the patient is receiving. Medications such as cyclosporin are often associated with gingival hyperplasia, and fixed or removable orthodontic treatment should preferably be delayed (Daley et al., 1991). The short-term administration of corticosteroids has not been shown to have an effect on orthodontic tooth movement, but prolonged steroid treatment increases the rate of tooth movement and relapse potential (Kalia et al., 2004).
The onset of uncontrolled epilepsy at a similar time as treatment in an adolescent orthodontic patient, contraindicated the use of removable appliances (Patel et al., 2009; Krishnan and Davidovitch, 2006). The orthodontic team should be up-to-date with the management of seizures within the dental setting also (Appleton et al., 2012; Sheller, 2004). This, in addition to, the increased burden of care that starting orthodontics would have placed on the patient and family further supports the distraction team’s decision to delay orthodontic treatment.
Neurological abnormalities including, seizures, focal neurological deficits, trigeminal neuralgia, Cranial nerve palsies, migraines, masticatory spasms and cerebellar hemiatrophy have been documented in the literature as being associated with Parry-Romberg syndrome(Tollefson and Witman, 2007; Amaral et al., 2013). It is rare for the neurological symptoms to be related to brain abnormalities on the contralateral side to the facial atrophy such as in this patient and usually present on the ipsilateral side (Stone, 2006).
The aetiology for Parry–Romberg syndrome isn’t clear, several factors point to a possible autoimmune origin. The positive effect of immunosuppressive treatment highlights this, with methotrexate and systemic corticosteroids being outlined as the favoured treatment modality (Wong et al., 2015; Kreuter, 2012). The presence of anti-nuclear antibodies is a common finding in these patients(Kreuter, 2012). A possible theory regarding the hemifacial atrophy has been attributed to an inflammation of the superior cervical ganglion resulting in hyperactivity of the sympathetic nervous system, who’s distribution in conjunction with the internal carotid nerve plexus provide an anatomic basis to account for the unilateral facial and intracranial presentation(Cory et al., 1997). Trophic facial changes are mediated by the sensory root of the trigeminal nerve, which is derived from the sympathetic trunk.(Monobe et al., 2012)
When the patient re-presented at age 15 to the distraction team, she was concerned only by the position of the UR3 and the appropriate investigations (CBCT) were undertaken to fully assess the root morphology of teeth that would be moved orthodontically.
Surgical correction of the facial and occlusal asymmetry can potentially be addressed in the future using distraction osteogenesis or osteotomy, however there is limited scope within the occlusion to advance the affected side of the mandible, without creating a reversed overjet. As mentioned earlier, the soft tissue asymmetry is more challenging to manage and would involve Coleman fat transfer procedures.
Conclusions
In this case report, we have discussed the medical and orthodontic management of a young female with Parry Romberg Syndrome. There were interesting dental and medical features that can arise associated with the Syndrome and clinicians should be mindful of these. Also referral to Rheumatology should be considered for confirmation of diagnosis and systemic treatment if the first presentation of hemifacial atrophy is to the orthodontic team.
References
- Amaral TN, Peres FA, Lapa AT, et al. (2013). Neurologic involvement in scleroderma: a systematic review. Seminars in arthritis and rheumatism. Elsevier, 335-347.
- Anderson PJ, Molony D, Haan E, et al. (2005). Familial Parry-Romberg disease. Int J Pediatr Otorhinolaryngol 69: 705-708.
- Appleton RE, Freeman A and Cross JH. (2012). Diagnosis and management of the epilepsies in children: a summary of the partial update of the 2012 NICE epilepsy guideline. Arch Dis Child 97: 1073-1076.
- Cory RC, Clayman DA, Faillace WJ, et al. (1997). Clinical and radiologic findings in progressive facial hemiatrophy (Parry-Romberg syndrome). American Journal of Neuroradiology 18: 751-757.
- Daley TD, Wysocki GP and Mamandras AH. (1991). Orthodontic therapy in the patient treated with cyclosporine. Am J Orthod Dentofacial Orthop 100: 537-541.
- De Somer L, Morren MA, Muller PCEH, et al. (2015). Overlap between linear scleroderma, progressive facial hemiatrophy and immune-inflammatory encephalitis in a paediatric cohort. European Journal of Pediatrics 174: 1247-1254.
- English SW, Ho ML, Tollefson MM, et al. (2018). Focal Epilepsy in a Teenager With Facial Atrophy and Hair Loss. Seminars in Pediatric Neurology 26: 68-73.
- Fayad S and Steffensen B. (1994). Root resorptions in a patient with hemifacial atrophy. Journal of endodontics 20: 299-303.
- Gambichler T, Kreuter A, Hoffmann K, et al. (2001). Bilateral linear scleroderma "en coup de sabre" associated with facial atrophy and neurological complications. BMC Dermatology 1 (no pagination).
- Hoppe M, Aufenberg C, Schulz R, et al. (2009). Chronic epilepsies resulting from Parry-Romberg Syndrome. Epilepsia 6): 12.
- Hulzebos CV, de Vries TW, Armbrust W, et al. (2004). Progressive facial hemiatrophy: a complex disorder not only affecting the face. A report in a monozygotic male twin pair. Acta Paediatr 93: 1665-1669.
- Jain RS, Kumar S and Srivastava T. (2016). Lower limb onset Parry–Romberg syndrome: an unusual presentation of a rare disease. Oxford Medical Case Reports 2016: omw031.
- Kalia S, Melsen B and Verna C. (2004). Tissue reaction to orthodontic tooth movement in acute and chronic corticosteroid treatment. Orthod Craniofac Res 7: 26-34.
- Korkmaz C, Adapinar B and Uysal S. (2005). Beneficial effect of immunosuppressive drugs on Parry-Romberg syndrome: A case report and review of the literature. Southern Medical Journal 98: 940-942.
- Kreuter A. (2012). Localized scleroderma. Dermatologic therapy 25: 135-147.
- Krishnan V and Davidovitch Z. (2006). The effect of drugs on orthodontic tooth movement. Orthod Craniofac Res 9: 163-171.
- Lee RT, Barnes E, DiBiase A, et al. (2014). An extended period of functional appliance therapy: a controlled clinical trial comparing the Twin Block and Dynamax appliances. The European Journal of Orthodontics 36: 512-521.
- Maletic J, Tsirka V, Ioannides P, et al. (2010). Parry-romberg syndrome associated with localized scleroderma. Case Reports in Neurology 2: 57-62.
- Monobe H, Miyano K, Kagoya R, et al. (2012). Case of progressive facial hemiatrophy with cervical sympathetic hyperactivity as underlying aetiology. The Journal of Laryngology & Otology 126: 725-728.
- O'Flynn S and Kinirons M. (2006). Parry-Romberg syndrome: a report of the dental findings in a child followed up for 9 years. Int J Paediatr Dent 16: 297-301.
- Parry CH. (1825). Collections from the Unpublished Medical Writings of the Late Caleb Hillier Parry: Underwoods.
- Patel A, Burden DJ and Sandler J. (2009). Medical disorders and orthodontics. J Orthod 36 Suppl: 1-21.
- Rogers B. (1964). Progressive facial hemiatrophy: Romberg’s disease: a review of 772 cases. Proc 3d Int Cong Plast Surg. Excerpta Medica ICS 66: 681-689.
- Romberg MH. (1846). Klinische Ergebnisse: A. Foerstner.
- Sheller B. (2004). Orthodontic management of patients with seizure disorders. Seminars in Orthodontics 10: 247-251.
- Stone J. (2006). Parry-Romberg syndrome. Practical Neurology 6: 185-188.
- Tollefson MM and Witman PM. (2007). En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol 56: 257-263.
- Toussaint S, Gutierrez D, Gutierrez C, et al. (2010). "En coup de Sabre" Lesions in parry-romberg syndrome. A subtype of linear morphea? American Journal of Dermatopathology 32 (4): 402.
- Vig PS and Vig KW. (1986). Hybrid appliances: a component approach to dentofacial orthopedics. Am J Orthod Dentofacial Orthop 90: 273-285.
- Vix J, Mathis S, Lacoste M, et al. (2015). Neurological manifestations in parry-romberg syndrome: 2 case reports. Medicine (United States) 94 (28) (no pagination).
- Wong M, Phillips CD, Hagiwara M, et al. (2015). Parry romberg syndrome: 7 cases and literature review. American Journal of Neuroradiology 36: 1355-1361.
- You KH and Baik HS. (2011). Orthopedic and orthodontic treatment of Parry-Romberg syndrome. Journal of Craniofacial Surgery 22: 970-973.
- Zahlane S and Kissani N. (2013). Parry-romberg syndrome associated with epilepsy: A case report. Epilepsia 3): 339.
- Pu LL., Coleman, SR., Cui X., Ferguson RE., & Vasconez HC. (2008). Autologous Fat Grafts Harvested and Refined by the Coleman Technique: A Comparative Study. Plastic and Reconstructive Surgery, 122(3), 932-937.
Citation: Tk Mittal, H Safaei, L Carter and Cj Bates. (2020). Parry Romberg Syndrome. An unusual case with dental features. Journal of Oral Care and Dentistry 2(1). DOI: 10.5281/zenodo.4178244
Copyright: © 2020 Hamed Safaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.