Research Article
Volume 1 Issue 1 - 2019
Oogenesis, Oocyte Atresia, Ovarian Development and Reproductive Senescence in the Dusky Flathead Platycephalus fuscus (Teleostei)
Sunfish Queensland, Brisbane, Australia
*Corresponding Author: BR. Pollock, Sunfish Queensland, Brisbane, Australia.
Received: July 02, 2019; Published: July 15, 2019
Abstract
The aim of the present study is to examine developmental changes of oocytes and ovaries of a wild population of dusky flathead Platycephalus fuscus (Cuvier, 1829). This fish is endemic to the east coast of Australia where it inhabits estuaries and coastal waters. It is extensively fished throughout its range. It is a serial spawning teleost, capable of producing vast numbers of externally fertilised eggs in batches over a protracted annual spawning period. Successful egg production, as indicated by the presence of hydrated oocytes and post ovulatory follicles, is commonly observed in small and mid-size females (35cm–65cm Total Length; 2-6 years old) which numerically dominate the female component of the spawning aggregation. Oocyte atresia, at various levels, commences at the vitellogenic oocyte stage, and occurs in all mature fish during the spawning period. Mass oocyte atresia and degenerate ovaries were commonly observed in large fish (>70 cm Total Length and 7 years old), indicating that reproductive senescence occurs after females reach this size.
Keywords: Mass atresia; Ovulation; Reproductive senescence; Serial spawning; Teleost fish
Introduction
Reproduction in teleost fishes has been the subject of numerous studies which have revealed extremely diverse strategies (Wootton & Smith, 2014). One common form of reproduction in teleosts, including dusky flathead Platycephalus fuscus (Cuvier, 1829), involves external fertilization following the release of very large numbers of eggs, an absence of parental care, and successive annual spawnings by individual fish. In relation to this reproductive strategy several authors (Barneche., et al. 2018; Birkeland & Dayton, 2005; Gwinn., et al. 2015; Hixon., et al. 2014) have stressed the importance of large, highly fecund females to the population. The occurrence of mass oocyte atresia, the resorption of oocytes within the ovary, is another common feature of this reproductive strategy (Rideout & Tomkiewicz, 2011). Mass oocyte atresia occurs in long-lived teleosts in both young and old age classes, and it has been associated with poor nutrition, stress and unfavourable environmental conditions (Rideout., et al. 2005). There are few reports of reproductive senescence in teleosts. The review of senescence in natural populations by Nussey., et al. (2013) states that studies of fish were limited to Pacific salmon which show rapid senescence during and after spawning, and guppies (Poecilia reticulate) which are live bearers, not mass spawners, but whichexhibit reproductive senescence and extended post-reproductive lifespans (Reznick., et al. 2006). More recently Benoit., et al. (2018) report evidence of reproductive senescence in the Atlantic herring (Clupea harengus), a marine teleost with a reproductive strategy similar to that of dusky flathead.
The present study was initiated following observations by fishers that many of the large female dusky flathead caught during the peak spawning period commonly possessed “mushy ovaries” rather than gravid ovaries prior to ovulation. Dusky flathead is a serial spawner producing eggs in batches over a protracted annual spawning period. Their fecundity is indeterminate. They are able to continually produce new oocytes during the annual spawning period after spawning has commenced (Gray & Barnes, 2015; Hicks., et al. 2015). In a previous study, Pollock (2014) confirmed the presence of degenerate ovaries with mass oocyte atresia in large dusky flathead, but the developmental processes of oocytes and ovaries were not examined in detail. The present study examines oogenesis and ovarian development in a wild population of dusky flathead. Particular attention is given to the ontogenetic pathways to ovulation and oocyte atresia, and to the hypothesis that reproductive senescence occurs in large female dusky flathead.
Female and male dusky flathead are similar in appearance but there is a marked sexual dimorphism in relation to size. Males are much smaller and mature at a smaller size than females. Both sexes commence maturing as 2 year olds and can live to about 12 years of age with females reaching up to 100 cm Total Length (TL) (Gray., et al. 2002). Previous studies show that sex-related differences in the size of dusky flathead are not associated with protantrous sex inversion (Gray & Barnes, 2008; Pollock, 2014). The dusky flathead is endemic to estuaries and inshore waters on the east coast of Australia, and they are the subject of fisheries throughout their range. They form annual spawning aggregations from October to March at locations where estuaries meet the Pacific Ocean (Gray & Barnes, 2008). Hicks., et al. (2015) estimate that individual dusky flathead potentially produce up to 4.8 million eggs annually. Tagging studies (Gray & Barnes, 2008; O’Neill, 2000) show that the majority of juvenile and adult dusky flathead remain within a given estuary system, with small proportions of the population moving along the coast to other estuaries.
Material and Methods
Animals
The female dusky flathead sample in the present study (n = 41) was taken at a spawning aggregation area (the mouth of the Clarence River, New South Wales, Australia: 29.43°S, 153.36°E) at the peak of the spawning period (November 2017 to February 2018). Sampling was not carried out after mid-February to avoid fish with spent ovaries. The specimens for this study were obtained from experienced fishers. The participating fishers held fishing licences and complied with the relevant fisheries legislation. All dusky flathead were humanely treated. At capture, each fish was percussion stunned followed by brain destruction in accordance with standards for the ethical treatment of fish given by the relevant Australian code of practice, and specified by the responsible government agency (NSW Department of Primary Industries Animal Welfare, 2017). After capture each fish was immediately placed into an ice-slurry, and within two hours each was given to the author for processing. The sizes of fish in the sample ranged from 36 cm Total Length (TL) to 87 cm TL (Figure 1).
The female dusky flathead sample in the present study (n = 41) was taken at a spawning aggregation area (the mouth of the Clarence River, New South Wales, Australia: 29.43°S, 153.36°E) at the peak of the spawning period (November 2017 to February 2018). Sampling was not carried out after mid-February to avoid fish with spent ovaries. The specimens for this study were obtained from experienced fishers. The participating fishers held fishing licences and complied with the relevant fisheries legislation. All dusky flathead were humanely treated. At capture, each fish was percussion stunned followed by brain destruction in accordance with standards for the ethical treatment of fish given by the relevant Australian code of practice, and specified by the responsible government agency (NSW Department of Primary Industries Animal Welfare, 2017). After capture each fish was immediately placed into an ice-slurry, and within two hours each was given to the author for processing. The sizes of fish in the sample ranged from 36 cm Total Length (TL) to 87 cm TL (Figure 1).
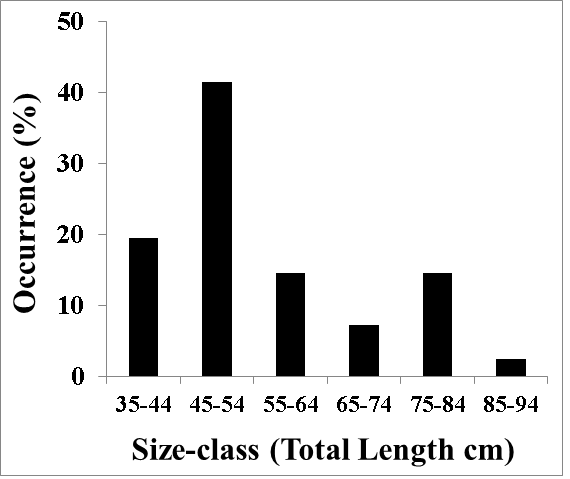
Figure 1: Length frequency composition of female dusky flathead taken from a spawning aggregation site (mouth of the Clarence River, Australia) during the peak spawning period, November 2017 to February 2018 (n = 41).
All fish were measured to the nearest 1 cm and ovaries were dissected and photographed under natural light (Canon IXUS 105 digital camera). Ovarian tissue samples, taken from the mid-section of one lobe of the ovary, were obtained from all fish greater than 70 cm TL (n=9), and randomly from fish in the range 36 cm - 70 cm TL (n = 13).
Fixation and tissue processing
The tissues samples were immediately fixed in 10% formalin solution, neutral buffered (Sigma-Aldrich). After five days the fixed tissues were rinsed in running water (15 minutes) and then transferred to 70% alcohol. The fixed tissue samples were washed in 70% ethanol and then dehydrated in an ascending alcohol series (45 minutes each) through 90%, 95% and 100% ethanol (two changes), cleared in xylene (three changes), and impregnated in high quality paraffin (Paraplast Plus, Sigma-Aldrich). The processing was made using an automatic tissue processor (Leica ASP300S1020, Germany). The samples were then embedded onto plastic cassettes using a modular tissue embedding centre (Medite TES Valida, Germany). The final blocks with the entire tissue samples were cut at 8 μm thick sections using a semiautomatic rotary microtome (Leica RM2245, Germany). Sections were placed on Superfrost Plus slides. Three sections were placed on each slide. Staining was done with Mayer’s haematoxylin and eosin (Sigma-Aldrich). Slides were mounted with DPX (Thermo Fisher Scientific, Australia). High resolution images (2560×1920 pixels) were taken with a light microscope (Nikon Eclipse 50i, Japan ) with attached digital camera (Nikon DS Fi1, Japan ) using NIS-Elements BR 4.0.
The tissues samples were immediately fixed in 10% formalin solution, neutral buffered (Sigma-Aldrich). After five days the fixed tissues were rinsed in running water (15 minutes) and then transferred to 70% alcohol. The fixed tissue samples were washed in 70% ethanol and then dehydrated in an ascending alcohol series (45 minutes each) through 90%, 95% and 100% ethanol (two changes), cleared in xylene (three changes), and impregnated in high quality paraffin (Paraplast Plus, Sigma-Aldrich). The processing was made using an automatic tissue processor (Leica ASP300S1020, Germany). The samples were then embedded onto plastic cassettes using a modular tissue embedding centre (Medite TES Valida, Germany). The final blocks with the entire tissue samples were cut at 8 μm thick sections using a semiautomatic rotary microtome (Leica RM2245, Germany). Sections were placed on Superfrost Plus slides. Three sections were placed on each slide. Staining was done with Mayer’s haematoxylin and eosin (Sigma-Aldrich). Slides were mounted with DPX (Thermo Fisher Scientific, Australia). High resolution images (2560×1920 pixels) were taken with a light microscope (Nikon Eclipse 50i, Japan ) with attached digital camera (Nikon DS Fi1, Japan ) using NIS-Elements BR 4.0.
Examination of oocytes and ovaries
Oocytes-types were identified by microscopic examination, and all ovaries (n = 41) were classified, based on macroscopic appearance and oocyte-types present. Frequencies of occurrence of vitellogenic, hydrated and atretic oocytes in individual ovaries were obtained from counts in randomised grids in the microscope slides (n=85 oocytes in each slide), viewed with a binocular microscope (Olympus BH2, Japan).
Oocytes-types were identified by microscopic examination, and all ovaries (n = 41) were classified, based on macroscopic appearance and oocyte-types present. Frequencies of occurrence of vitellogenic, hydrated and atretic oocytes in individual ovaries were obtained from counts in randomised grids in the microscope slides (n=85 oocytes in each slide), viewed with a binocular microscope (Olympus BH2, Japan).
Results
Oocyte-types
The ovaries of dusky flathead contain oocytes at all stages of maturation as well as empty oocyte follicles following ovulation, and atretic oocytes at all stages of resorption. Details of these are as follows:
The ovaries of dusky flathead contain oocytes at all stages of maturation as well as empty oocyte follicles following ovulation, and atretic oocytes at all stages of resorption. Details of these are as follows:
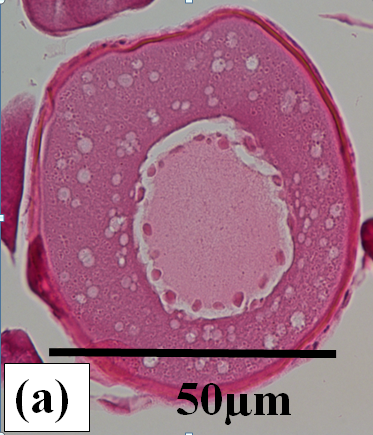
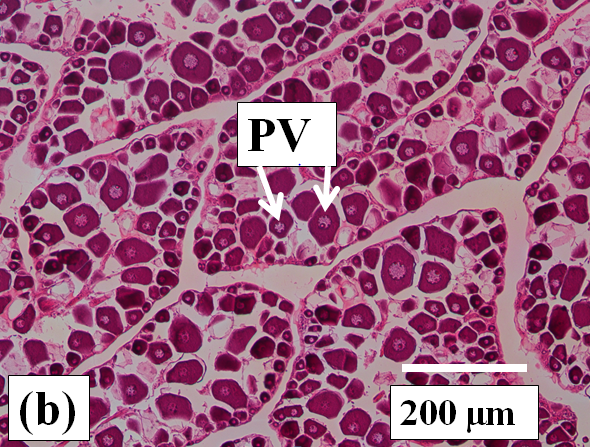
Previtellogenic oocytes: These are very small oocytes (< 50µm diameter) at an early stage of development with a relatively thin oocyte follicle and zona radiata. Thick cytoplasm surrounds a central nucleus which has few to many peripheral nucleoli (Figure 2a). These oocytes are present in all ovaries examined (Figure 2b and c).
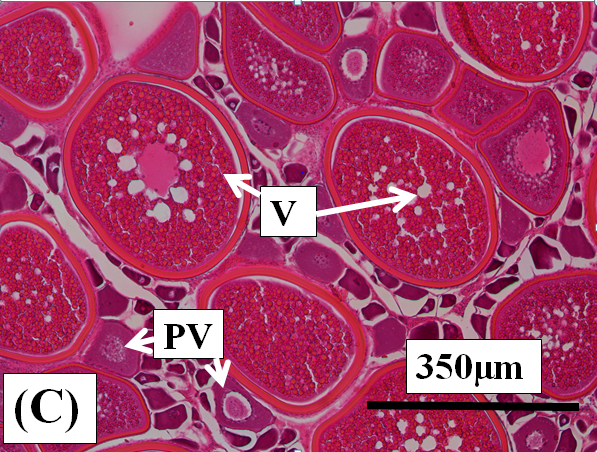
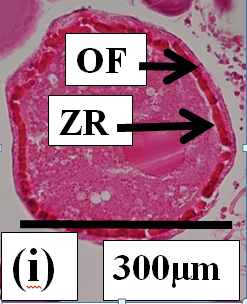
Vitellogenic oocytes: These are medium to large oocytes (150µm to 350µm in diameter) with the oocyte follicle and zona radiata being well defined. Yolk granules and vesicles are abundant in the cytoplasm (Figure 2c and d).
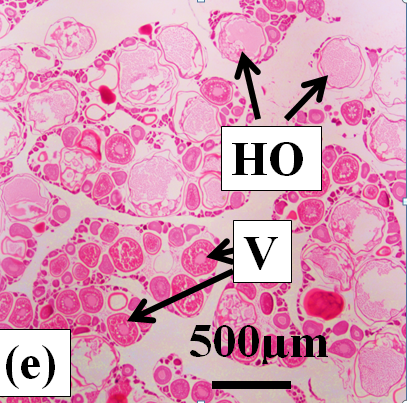
Hydrated oocytes: These are the largest oocytes (400µm diameter) with the oocyte follicle and zona radiata being well defined. Yolk granules and vesicles have been fused into a clear mass enclosed by the zona radiata (Figure 2e).
Post ovulatory follicles: These are the collapsed oocyte capsules following ovulation (Figure 2f).
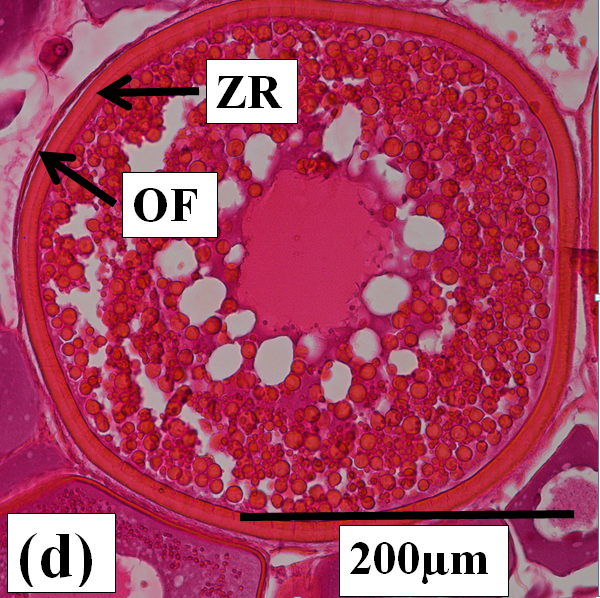
Atretic oocytes: These are oocytes under resorption. Oocyte atresia commences at the late vitellogenic stage. Atresia was not observed in previtellogenic or early vitellogenic oocytes. Two types of oocyte atresia (Ramadan & EL-Halfawy, 2007) are present in dusky flathead. The most common form by far is where the outer wall of the oocyte, comprising the follicular envelope and the zona radiata, stays intact (non-bursting atresia). The yolk granules and other inclusions surrounded by the zona radiata progressively collapse (Figure 2g). The oocyte becomes increasingly smaller, and an envelope of phagocytic cells develops between the follicle and the oocyte (Figure 2h). The vitellogenic oocytes at the onset of atresia are large (250µm diameter) (Figure 2g). As atresia progresses the oocyte becomes small, the phagocytic envelope is distinct, and the contents within the zona radiata are replaced by clear a cytoplasm (Figure 2h). At the completion of atresia nothing remains of the oocyte structure within the ovary. The second, less common form of atresia (Figure 2i) involves the fragmentation of the zona radiata (bursting atresia) (Miranda., et al. 1999; Ramadan & EL-Halfawy, 2007). In dusky flathead a single ovary may contain oocytes at different stages of atresia, and in the extreme case of mass atresia oocytes were observed at all stages of resorption (Figure 2j).

Figure 2j: Photomicrographs of oocytes and ovarian tissue sections (haematoxylin and eosin stained) from dusky flathead taken during the peak of the spawning period: AO – atretic oocyte; EF – empty (post ovulatory) follicle; HO – hydrated oocyte; OF – oocyte follicle; P – phagocytic envelope; PV – previtellogenic oocyte; V – vitellogenic oocyte; ZR – zona radiata. (a) Oocyte at early stage of development (previtellogenic). (b) Juvenile ovary section with abundance of previtellogenic oocytes. (c) Ovary section with abundance of vitellogenic oocytes (ripening maturing). (d) Vitellogenic oocyte. (e) Ovary section with abundance of hydrated oocytes (running ripe). (f) Ovary section with abundance of empty (post ovulatory) follicles. (g) Early- stage atretic oocyte, zona radiata intact (non-bursting type). (h) Late-stage atretic oocyte, zona radiata intact (non-bursting type). (i) Atretic oocyte with fragmented zona radiata (bursting type). (j) Ovary section with abundance of atretic oocytes at different stages of atresia (degenerate ovary with mass atresia).
Ovary-types
The stages of ovarian development in the present study are based on the macroscopic appearance of the ovary and the occurrence of vitellogenic or hydrated oocytes observed visually through the ovary wall. The stages are also based on microscopic details of the oocytes. The resulting descriptions of ovarian stages provide further details to the classifications of platycephalid ovaries given by Bani., et al. (2009) and Pollock (2014). The features of each ovarian stage are as follows:
The stages of ovarian development in the present study are based on the macroscopic appearance of the ovary and the occurrence of vitellogenic or hydrated oocytes observed visually through the ovary wall. The stages are also based on microscopic details of the oocytes. The resulting descriptions of ovarian stages provide further details to the classifications of platycephalid ovaries given by Bani., et al. (2009) and Pollock (2014). The features of each ovarian stage are as follows:
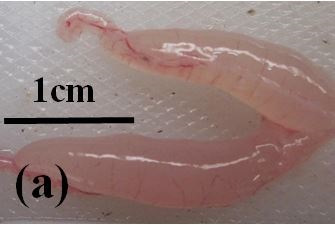
Juvenile: Juvenile (immature) fish have very small ovaries which are clear and almost colourless (Figure 3a). There is an abundance of previtellogenic oocytes, but no vitellogenic or atretic oocytes are present (Figure 2b).
Ripening maturing: These ovaries are relatively large and pale yellow in colour (Figure 3b). Oocytes are too small to be seen macroscopically through the ovary wall. Previtellogenic and early vitellogenic oocytes are present (Figure 2c).
Ripe: These ovaries are relatively large. Their colour varies from pale to bright yellow. Advanced vitellogenic oocytes may be seen through the ovary wall (Figure 3c). Under microscopic examination early and advanced vitellogenic oocytes are abundant. Atretic oocytes are present at low frequencies.
Running ripe: These ovaries are relatively large and bright yellow to orange in colour. Hydrated and advanced vitellogenic oocytes are clearly visible through ovary wall (Figure 3d). Under microscopic examination advanced vitellogenic oocytes are abundant. Post ovulatory (empty) follicles are present in some ovaries (Figure 2e and f). Atretic oocytes are present at low frequencies.
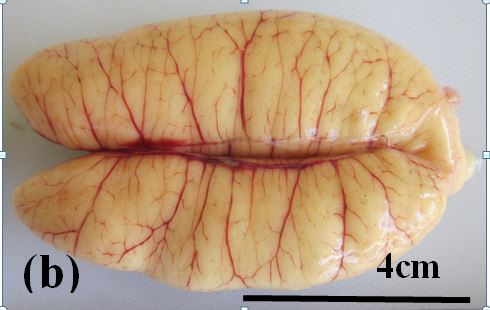
Degenerate: These ovaries are large but flaccid and pale yellow to grey-pink in colour (Figure 3e). Developed oocytes are present up to the advanced vitellogenic stage. Atretic oocytes at all stages of resorption are common to abundant (Figure 2j).
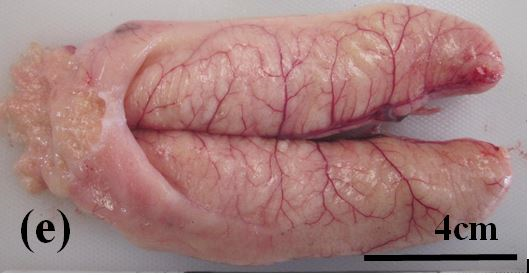
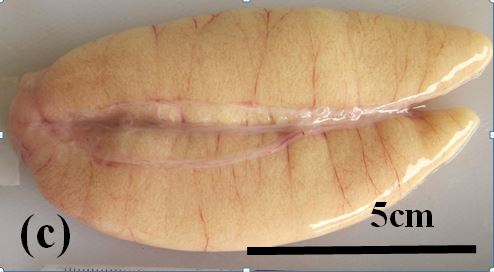
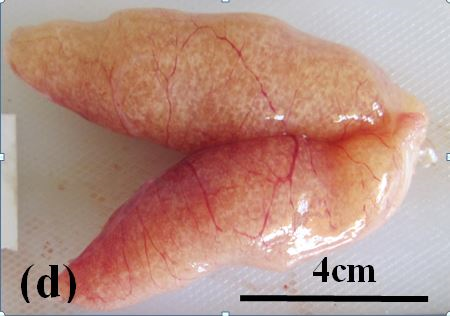
Figure 3e: Photographs of the ovaries of dusky flathead taken from a spawning aggregation site during the peak spawning period. (a) Ovary of juvenile fish, 36 cm TL, taken in February 2018. (b) Ripening maturing ovary of 48 cm TL fish taken in December 2017, no developing oocytes apparent through the ovary wall. (c) Ripe ovary of 55 cm TL fish taken in December 2017, vitellogenic oocytes apparent through the ovary wall. (d) Running ripe ovary of 45 cm TL fish taken in December 2017, hydrated oocytes apparent through the ovary wall. (e) Degenerate ovary of 78 cm TL fish taken in November 2017.
Relative abundance of oocyte-types in different ovaries
The frequencies of occurrence of vitellogenic, hydrated and atretic oocytes within ripe, running ripe and degenerate ovaries are given in Table 1. Vitellogenic oocytes occur in all three ovary-types, and they dominate in ripe ovaries. In running ripe ovaries vitellogenic oocytes are replaced by hydrated oocytes (Table 1 and Figure 2e). Empty follicles are present in some running ripe ovaries indicating recent ovulation (Figure 2f). In degenerate ovaries the vitellogenic oocytes become atretic. Mass oocyte atresia is indicated by the abundance of atretic oocytes in these ovaries (Table 1 and Figure 3j).
The frequencies of occurrence of vitellogenic, hydrated and atretic oocytes within ripe, running ripe and degenerate ovaries are given in Table 1. Vitellogenic oocytes occur in all three ovary-types, and they dominate in ripe ovaries. In running ripe ovaries vitellogenic oocytes are replaced by hydrated oocytes (Table 1 and Figure 2e). Empty follicles are present in some running ripe ovaries indicating recent ovulation (Figure 2f). In degenerate ovaries the vitellogenic oocytes become atretic. Mass oocyte atresia is indicated by the abundance of atretic oocytes in these ovaries (Table 1 and Figure 3j).
| Ovary type | Vitellogenic oocytes (% range) | Fully hydrated oocytes (% range) | Atretic oocytes (% range) | Fish in sample |
| Ripe | 89-99 | 0 | 1-11 | 7 |
| Running ripe | 37-98 | 2-60 | 2-8 | 9 |
| Degenerate | 6-87 | 0 | 13-94 | 5 |
Table 1: Occurrence of vitellogenic, fully hydrated and atretic oocytes in ripe, running ripe and degenerate ovaries of dusky flathead at the spawning aggregation site, Clarence River, Australia.
Ovary-types in fish of different sizes
There are differences in the types of ovaries occurring in the various size-classes of the dusky flathead (Figure 4). The smallest size-class, 30 cm - 39 cm TL, is dominated by juveniles with immature ovaries. The next size-class, 40 cm – 49 cm TL has a decreased proportion of juveniles, and is dominated by fish with ripening maturing, ripe and running ripe ovaries. The 50 cm – 59 cm TL and 60 cm – 69 cm TL size-classes are dominated by fish with ripe and running ripe ovaries. In the 70 cm – 79 cm TL size-class degenerate ovaries are common, but some individuals have ripe and running ripe ovaries. In the largest size-class, 70 cm – 89 cm TL, both ripe and degenerate ovaries are present, but and no running ripe ovaries were obsreved.
There are differences in the types of ovaries occurring in the various size-classes of the dusky flathead (Figure 4). The smallest size-class, 30 cm - 39 cm TL, is dominated by juveniles with immature ovaries. The next size-class, 40 cm – 49 cm TL has a decreased proportion of juveniles, and is dominated by fish with ripening maturing, ripe and running ripe ovaries. The 50 cm – 59 cm TL and 60 cm – 69 cm TL size-classes are dominated by fish with ripe and running ripe ovaries. In the 70 cm – 79 cm TL size-class degenerate ovaries are common, but some individuals have ripe and running ripe ovaries. In the largest size-class, 70 cm – 89 cm TL, both ripe and degenerate ovaries are present, but and no running ripe ovaries were obsreved.
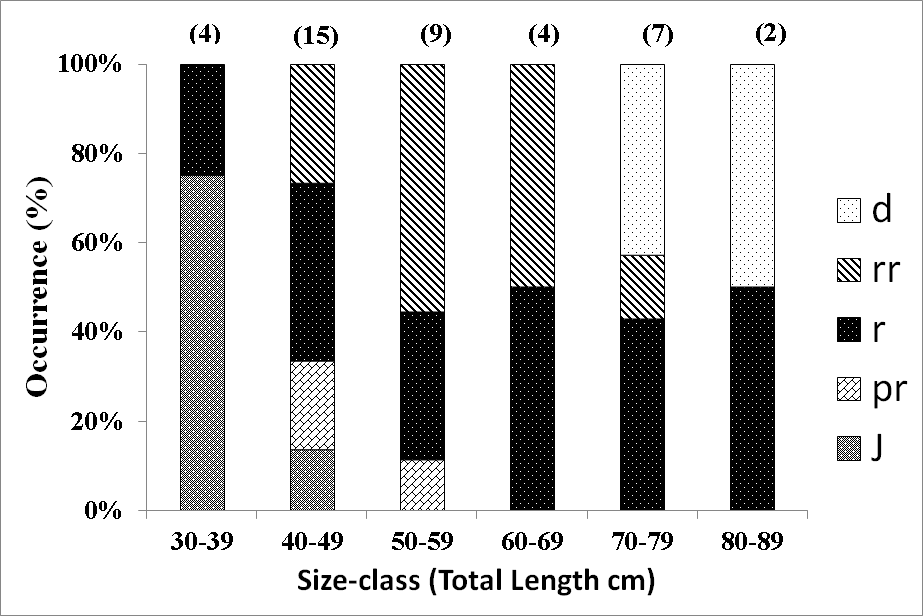
Figure 4: Occurrence of ovary-types in size-classes of dusky flathead taken from the spawning aggregation location. j – juvenile, pr – ripening maturing, r – ripe, rr – running ripe, d – degenerate. Sample sizes are shown in parenthesis.
Discussion
The present study examines changes which occur as oocytes and ovaries develop in dusky flathead. The pattern of oocyte development is typical of highly fecund, serial spawning teleosts, commencing with small previtellogenic oocytes which rapidly increase in size and change in composition during the annual reproductive cycle (Wootton & Smith, 2014). In dusky flathead the advanced vitellogenic oocyte is a critical stage. After this stage is reached, the oocyte may either progress to full hydration and ovulation or undergo atresia and be resorbed. This is not uncommon as other studies of oocyte development in fish have also found that atresia occurs after oocytes reach the vitellogenic stage (Kurita., et al. 2003; Miranda., et al. 1999; Witthames & Greer Walker, 1995). The main form of oocyte atresia in dusky flathead involves progressive shrinkage of the oocyte and the collapse of the yolk vesicles and other components of the ooplasm, but the zona radiata remains intact. This non-bursting form of oocyte atresia has been reported in other teleosts (Ramadan & EL-Halfawy, 2007). The other form of oocyte atresia in dusky flathead, involving fragmentation of the zona radiata (bursting atresia), is rare and only observed at an early stage of resorption. This form of oocyte atresia has also been reported in other teleost species (Miranda., et al. 1999; Ramadan & EL-Halfawy, 2007). Causal factors for the two different types of oocyte atresia are unknown, and the presence in dusky flathead of both types in a single ovary is unusual.
The ripe ovary stage in dusky flathead, in which advanced vitellogenic oocytes dominate, is followed by either the running ripe or degenerate ovary stages. In large female dusky flathead (>70 cm TL) ripe ovaries were commonly observed, but running ripe ovaries with fully hydrated oocytes or empty oocyte follicles were uncommon. Running ripe ovaries with hydrated oocytes and empty follicles are most common in small and mid-size dusky flathead, not the large females. This indicates that successful egg production in dusky flathead is closely associated with these size-classes. The low levels of oocyte atresia commonly occurring in small and mid-size dusky flathead during the peak spawning period is possibly a mechanism to resorb and recycle components and energy from the small proportion of poor quality oocytes. The study by Hicks., et al. (2015) found no significant differences in the quality of advanced oocytes across different size-classes of dusky flathead, which reinforces the importance of both small and mid-size fish in egg production.
The hypothesis that reproductive senescence occurs in large dusky flathead is supported by the present study. Firstly, high levels of oocyte atresia (mass atresia) and associated degenerate ovaries occur in the large females (>70 cm TL), and not in the smaller adult size-classes. Secondly, successful egg production, as previously mentioned, mostly occurs in small and mid-size females (
Oocyte and ovarian development have not been examined to date in that component of the adult dusky flathead population which remains in the upper estuaries during the spawning period and hence does not participate in the annual spawning aggregation. The ovaries of this component of the dusky flathead population do not progress to the running ripe stage (Gray & Barnes, 2015). It is probable that all these mature fish undergo mass oocyte atresia regardless of their size as has been reported in other teleost species which fail to participate in annual spawning aggregations (Rideout & Tomkiewicz, 2011).
The ovaries of mature dusky flathead during the spawning period account for 5%-10% of total body weight (Gray & Barnes, 2015; Hicks., et al. 2015; Pollock, 2014). The production of such large ovaries and their maintenance through serial spawning require individual fish to be in good condition (Wootton, 1985). The resorption of advanced oocytes in large dusky flathead is expected to contribute to maintaining overall body condition and consequently could be important for their post reproductive survival. Reproductive senescence in large dusky flathead appears to have no evolutionary advantage. Reznick., et al. (2006) concludes that a post-reproductive lifespan involving reproductive senescence in teleosts seems to be a random add-on at the end of a life history, and this is most likely the case in dusky flathead.
Acknowledgements
The fishers who kindly provided access to their catches for this study are Dan Currey, Jim Gillespie, Kyle Graham, Ken Orme and Bob Pearson. The cooperation of Jason McGilvray with the literature search and with the provision of laboratory space at the Ecosciences Precinct, Brisbane is much appreciated. Darryl Whitehead and Arnault Gauthier, University of Queensland Histology Laboratory provided invaluable assistance with microscope slide preparation and microphotography. Julian Hughes, New South Wales fisheries agency, is thanked for his support and comments on oocyte development and sampling strategies in the Clarence River. Glenys Pollock assisted with the preparation of the figures. Trevor Eiser, Steve Watson and Jeff Sorrell provided logistical support.
The fishers who kindly provided access to their catches for this study are Dan Currey, Jim Gillespie, Kyle Graham, Ken Orme and Bob Pearson. The cooperation of Jason McGilvray with the literature search and with the provision of laboratory space at the Ecosciences Precinct, Brisbane is much appreciated. Darryl Whitehead and Arnault Gauthier, University of Queensland Histology Laboratory provided invaluable assistance with microscope slide preparation and microphotography. Julian Hughes, New South Wales fisheries agency, is thanked for his support and comments on oocyte development and sampling strategies in the Clarence River. Glenys Pollock assisted with the preparation of the figures. Trevor Eiser, Steve Watson and Jeff Sorrell provided logistical support.
References
- Bani, A., Moltschaniwsky, N., & Pankhurst, N. (2009). Reproductive strategy and spawning activity of sand flathead, Platycephalus bassensis (Platycephalidae). Cybium 33, 151-162.
- Barneche, D. R., Robertson, D. R., White, C. R., & Marshall, D. J. (2018). Fish reproductive-energy output increases disproportionately with body size. Science 360, 642-645.
- Benoit, H. P., Swain, D. P., Hutchings, J. A., Knox, D., Doniol-Valcroze, T., & Bourne, C. M. (2018). Evidence for reproductive senescence in a broadly distributed harvested marine fish. Marine Ecology Progress Series 592, 207-224.
- Birkeland, C., & Dayton, P. (2005). The importance in fishery management of leaving the big ones. Trends in Ecology and Evolution 20, 356-358.
- Gray C. A., & Barnes, L. M. (2008). Reproduction and growth of dusky flathead (Platycephalus fuscus) in NSW estuaries. NSW Department of Primary Industries Fisheries. Final Report Series No.101. Sydney, Australia.
- Gray, C. A., & Barnes, L. M. (2015). Spawning, maturity, growth and movement of Platycephalus fuscus (Cuvier, 1829) (Platycephalidae): fishery management considerations. Journal of Applied Ichthyology 31, 1–9.
- Gray, C. A., Gale, V. J., Stringfellow, S. L., & Raines, L. P. (2002). Variations in sex, length and age compositions of commercial catches of Platycephalus fuscus (Pisces: Platycephalidae) in New South Wales. Australian Journal of Marine and Freshwater Research 53, 1091–1100.
- Gwinn, D. C., Allen, M. S., Johnston, F. D., Brown, P., Todd, C. R., & Arlinghaus, R. (2015). Rethinking length?based fisheries regulations: the value of protecting old and large fish with harvest slots. Fish and Fisheries 16, 259-281.
- Hicks T., Kopf R.K., & Humphries P. (2015). Fecundity and egg quality of dusky flathead (Platycephalus fuscus) in East Gippsland, Victoria. Institute for Land Water and Society, Charles Sturt University, Australia. Report No. 94.
- Hixon, M. A., Johnson, D. W., & Sogard, S. M. (2014). BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES Journal of Marine Science 71, 2171-2185.
- Kurita, P. R., Greer Walker, M., & Kjesbu, O. S. (2003). Oocyte growth and fecundity regulation by atresia of Atlantic herring (Clupea harengus) in relation to body condition throughout the maturation cycle. Journal of Sea Research 49, 203– 219.
- McGilvray, J., Green, C., & Hall, K. (2016). Dusky flathead Platycephalus fuscus. In Stewardson, C., Andrews, J., Ashby, C., Haddon, M., Hartmann, K., Hone, P., Horvat, P., Mayfield, S., Roelofs, A., Sainsbury, K., Saunders, T., Stewart, J., Stobutzki, I., & Wise, B. (eds.) (2016). Status of Australian fish stocks reports 2016. Fisheries Research and Development Corporation, Canberra. Australia.
- Miranda, A. C. L., Bazzoli, N., Rizzo, E., & Sato, Y. (1999). Ovarian follicular atresia in two teleost
- species: a histological and ultrastructural study. Tissue and Cell 31, 480-488.
- NSW Department of Primary Industries (Animal Welfare) (2017). Humane harvesting of fish and crustaceans. Retrieved from. Last accessed 7 June 2018.
- Nussey, D. H., Froy, H., Lemaitre, J., Gaillard, J., & Austad, S. N. (2013). Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Research Reviews 12, 214-225.
- O’Neill, M. F. (2000). Fishery assessment of the Burnett River, Maroochy River and Pumicestone Passage. Queensland Department of Primary Industries Project Report QO99012.
- Patnaik, B. K., Mahapatro, N., & Jena, B. S. (1994). Ageing in Fishes. Gerontology 40, 113-132.
- Pollock, B. R. (2014). The Annual Spawning Aggregation of Dusky Flathead Platycephalus fuscus at Jumpinpin, Queensland. Proceedings of the Royal Society of Queensland 119, 23-45.
- Ramadan, A. M., & EL-Halfawy, M. M. (2007). Common Forms of Atresia in the Ovary of Some Red Sea Fishes During Reproductive Cycle. Pakistan Journal of Biological Sciences 10, 3120-3125.
- Reznick, D., Bryant, M., & Holmes, D. (2006). The Evolution of Senescence and Post-Reproductive Lifespan in Guppies (Poecilia reticulata). PLoS Biol 4, 7.
- Rideout , R. M., Rose, G. A., & Burton, M. P. M. (2005 ). Skipped spawning in female iteroparous fishes. Fish and Fisheries 6, 50-72.
- Rideout, R. M., & Tomkiewicz, J. (2011). Skipped Spawning in Fishes: More Common than You Might Think. Marine and Coastal Fisheries 3, 176-189.
- Stewart, J., A. Hegarty, Young, C., Fowler, A. M., & Craig, J. (eds.). (2015). Status of Fisheries Resources in NSW 2013- 14. NSW Department of Primary Industries, Mosman, Australia.
- Witthames, P.R., & Greer Walker, M. (1995). Determinacy of fecundity and oocyte atresia in sole (Solea solea) from the Channel, the North Sea and the Irish Sea. Aquatic Living Resources 8, 91– 109.
- Wootton R.J. (1985). Energetics of Reproduction. In Tytler, P., & Calow P. (eds) Fish Energetics. Springer, Dordrecht.
- Wootton, R. J., & Smith, C. (2014). Reproductive Biology of Teleost Fishes. John Wiley & Sons.
Citation: BR. Pollock. (2019). Oogenesis, Oocyte Atresia, Ovarian Development and Reproductive Senescence in the Dusky Flathead Platycephalus fuscus (Teleostei). Archives of Veterinary and Animal Sciences 1(1). DOI: 10.5281/zenodo.3373253
Copyright: © 2019 BR. Pollock. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.