Review Article
Volume 5 Issue 1 - 2023
New Avenues Explored for Metabolite Driven Clinical Drug Development
1M. Pharm (Pharmaceutical Analysis), Jamia Hamdard New Delhi-110062, India
2R&D-CPP, SPIL Gurgaon, Clinical Pharmacology and Pharmacokinetics Sun Pharmaceutical Industries Limited, Vill. Sarhaul, Sector-18, Gurugram - 122015, Haryana, India
2R&D-CPP, SPIL Gurgaon, Clinical Pharmacology and Pharmacokinetics Sun Pharmaceutical Industries Limited, Vill. Sarhaul, Sector-18, Gurugram - 122015, Haryana, India
*Corresponding Author: Vanapalli Rohith, M. Pharm (Pharmaceutical Analysis), Jamia Hamdard New Delhi-110062, India.
Received: February 16, 2023; Published: March 10, 2023
Abstract
Efficacy and safety for drug formulation is dependent on pharmacokinetic parameters. Metabolism of drug plays a crucial role to understand response of dosage form while treating any disease. Metabolic studies are helpful in assessing safety and efficacy of drug, estimation of metabolic clearance, analyzing the inter-individual variability and toxicity profile of drugs during drug discovery and development. This article gives a brief review on approaches for metabolite identification such as in-vitro, in-vivo, in-silico models. Invitro models range from sub cellular to organ systems, which are now applied for qualitative, quantitative evaluation in pre-clinical drug development, post approval routines and determination of drug-drug, drug-herb, drug-food interactions. In-vitro studies include the subcellular and cellular fractions of the liver like human liver microsomes, hepatocytes, S9 fractions, cell lines, baculosomes etc. In-vivo animal and human models give an appropriate information degrading the drug in the matrix which is helpful for estimation of metabolic profile, drug clearance. In-silico methods, different softwares (METEOR, SyGMa) were developed to support accurate and fast examination of data generated by several analytical techniques for metabolite identification. Various analytical strategies are in use for metabolite identification, of which LC-MS/MS is widely used due to its good specificity, selectivity, speed gives accurate data which is further analyzed by in-silico methods.
Keywords: Metabolism; Human Liver Microsomes (HLM); Metabolic Profile; METEOR; LC-MS/MS
Abbreviations : DMPK: Drug Metabolism and Pharmacokinetics; DDI’s: Drug-Drug Interactions; CYP: Cytochrome P450; FDA: Food and Drug Administration; ADME: Absorption, Distribution, Metabolism and Excretion; PK/PD: Pharmacokinetic and Pharmacodynamics; SPE: Solid Phase Extraction; QSAR: Quantitative Structure-Activity Relationship; DEREK: Deductive Estimation of Risk from Existing Knowledge (software); SyGMa: Systematic Generation of Potential Metabolites (software); LC: Liquid Chromatography; MS: Mass Spectrometry; LC-MS/MS: Liquid Chromatography Coupled with Tandem Mass; HLM: Human Liver Microsomes; RP-UHPLC: Reverse Phase Ultra-High Performance Liquid Chromatography; HR-MS: High Resolution Mass Spectrometry; MPP: Mass Profiler Professional (software); PCA: Principal Component Analysis; T.E.S.T: Toxicity Estimation Software Tool; ACD: Advanced Chemistry Development; IVIVC: In Vitro-In Vivo Correlation;
Introduction
The drug development process is complex and vast in which pharmacokinetic parameters such as Absorption, distribution, Metabolism and Excretion are helpful for assessing their safety and efficacy of drug. Drug formulation is first absorbed into the systemic circulation, followed by distribution and metabolism. The metabolic fate of drugs is an essential and important part of the drug development process. In drug evaluation, the study of drug metabolism is of great importance, particularly when the metabolites are pharmacologically active or toxic or when a drug is extensively metabolised. Inter-individual differences in drug metabolism also led to the search for factors affecting drug metabolism [1]. In early stages of discovery, drug metabolism inputs provide the basis for selecting chemical structures and leads with desired drug metabolism and pharmacokinetics (DMPK) or a safety profile. In addition to traditional drug metabolism research focusing on absorption, distribution, metabolism, and excretion from in vitro and in vivo studies, knowledge of pharmacogenomics, pharmacology and transporters has brought many advances in drug metabolism research [2]. In order to be able to successfully monitor drug metabolism, suitable bio-analytical methods must be developed and validated. Studies of drug metabolic fate in living systems can be divided into three areas that include elucidation of bioconversion pathways, determination of parent drug pharmacokinetics and/ or its major metabolites and chemically identified the reactive metabolites that play an important role in drug-induced toxicity. [3]
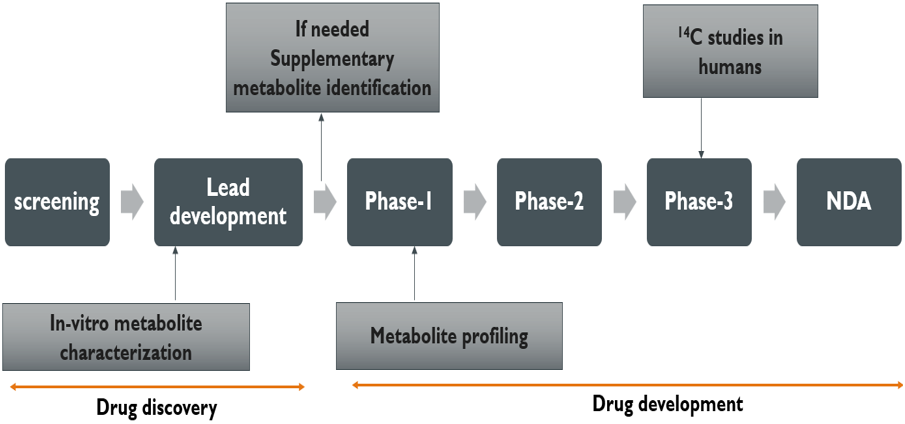
The metabolites are identified initially from the compound selection period of drug development using microsomes, hepatocytes or alternative metabolising systems in vitro, as well as the examination of in vivo samples from suitable animal species. The majority of metabolite identification work has traditionally been done late in drug discovery and late in drug development, mainly because structural elucidation is hard and requires a lot of time and resources. To understand the metabolic identification process during drug discovery and development [4], it is illustrated in Figure (1).
Bio-transformation
Metabolism is a biological process where a drug is changed into another chemical form by enzymatic reactions. Basically, metabolism increases the hydrophilicity of drugs and reduces the toxicity and activity of most drugs. On the other hand, biotransformation reactions can lead to bioactivation of the drug, in which case metabolite is more toxic and/or more active than the parent drug (reactive metabolite formation). The bioactivation mechanism of drugs can be classified into the following categories: a) bioconversion to stable but toxic metabolites b) bioconversion to electrolytes c) bioconversion to radicals free and formed reactive oxygen metabolites d) Furthermore, bio stimulation is also the transformation of a precursor, promoter, or precursor biocatalyst into a more efficient metabolite [1].
Metabolism is a biological process where a drug is changed into another chemical form by enzymatic reactions. Basically, metabolism increases the hydrophilicity of drugs and reduces the toxicity and activity of most drugs. On the other hand, biotransformation reactions can lead to bioactivation of the drug, in which case metabolite is more toxic and/or more active than the parent drug (reactive metabolite formation). The bioactivation mechanism of drugs can be classified into the following categories: a) bioconversion to stable but toxic metabolites b) bioconversion to electrolytes c) bioconversion to radicals free and formed reactive oxygen metabolites d) Furthermore, bio stimulation is also the transformation of a precursor, promoter, or precursor biocatalyst into a more efficient metabolite [1].
Approaches For Metabolite Identification
In-Vitro Approach
In drug discovery and development, in vitro methods are helpful for determination of metabolic stability, producing large quantities of metabolites, developing metabolites for bio-analytical assays, comparing metabolite profiles, and evaluating species differences in metabolism. The sensible selection of animal species for toxicity studies benefits greatly from in vitro approaches. They also support a drug's clinical development, especially in terms of anticipating pharmacokinetic diversity among patients and drug-drug interactions (DDIs) [5]. During the past 15-20 years, in-vitro methods were developed most extensively with the cytochrome P450 (P450, or CYP) enzymes. During this period there occurred a shift of emphasis from in-vivo experiments with animal models to in-vitro studies with human enzymes [6].
In-Vitro Approach
In drug discovery and development, in vitro methods are helpful for determination of metabolic stability, producing large quantities of metabolites, developing metabolites for bio-analytical assays, comparing metabolite profiles, and evaluating species differences in metabolism. The sensible selection of animal species for toxicity studies benefits greatly from in vitro approaches. They also support a drug's clinical development, especially in terms of anticipating pharmacokinetic diversity among patients and drug-drug interactions (DDIs) [5]. During the past 15-20 years, in-vitro methods were developed most extensively with the cytochrome P450 (P450, or CYP) enzymes. During this period there occurred a shift of emphasis from in-vivo experiments with animal models to in-vitro studies with human enzymes [6].
In the end, invitro findings support in vivo animal testing to comprehend human drug metabolism and possible DDI prior to clinical trials. As a result, human models are now more frequently accepted and used than ever before. In-vitro methods also clarify the species variations in metabolism. Some of the in-vitro models (Human liver microsomes, Cytosolic fractions, S9 fractions, Hepatocytes, Cell lines, Recombinant enzymes) used to study metabolism are mentioned in detail Table (1). It discusses more about the underlying biological mechanisms and enzymes involved in metabolism.
| Approach | Source | Enzymes | Advantages | Disadvantages |
| Human liver microsomes (HLM) | Hepatocyte vesicles formed by liver homogenates centrifugation | CyP450, FMO, Carboxylesterase, Epoxide, Hydrolase, UGT. | In-vitro drug metabolism estimation | HLM is inadequate for assessing in vivo drug changes since it is deficient in cofactors and enzymes like NAT, GST, and SULT. |
| CYTOSOLIC FRACTION | centrifugation(differential)- liver homogenate | NAT, GST, SULT, Carboxylesterase, Soluble epoxide hydrolase, Diamine oxidase, Xanthine oxidase, Alcohol dehydrogenase |
greater prevalence of the enzymes than in liver S9 fraction | glucuronidation cannot be studied by this model. |
| S9 FRACTIONS | Includes both microsome and cytosolic fractions. | CYP, FMO, Carboxylesterase, epoxide hydrolase, UGT, SULT, Methyltransferase, acetyltransferase, GST | Complete metabolic profile of drug can be estimated | Due to lower enzyme activity some metabolites cannot be detected |
| CELL LINES | Tumours of liver parenchyma (Hep G2, SNU398, BC2, PLC / PRE / 5, C3A, SKHep1) | easy to culture and has stable enzyme concentration | Major Metabolic enzymes absence | |
| HEPATOCYTES | Hepatic Cells | Strong resemblance of in vivo situation | Less viability during incubation period(2-4hrs) | |
| LIVER SLICES | cutting of liver into slices of uniform thickness(<250µm) | Intact structure and normal spatial arrangement | short-term decline in CYP activity can be observed. | |
| Supersomes and baculosomes | transfection of insect cells with human CYP and UGT cDNA by help of baculovirus | CYP enzymes and UGT enzymes | Individual metabolic enzymes and their contribution to the pathway can be evaluated. | Due to the active site of UGT being hidden behind a hydrophobic barrier, incubation of glucuronidation experiences significant problems. |
Table 1: In-vitro models for metabolite identification.
In-Vivo Approach
The identity of metabolites present in the animal or human matrix provides important information about the bio transformation pathways involved in drug clearance. If the metabolite profile of the parent drug is qualitative and quantitatively similar between animals and humans, the potential clinical risk of the parent drug and metabolites is adequately assessed in non-clinical studies. If there is a difference between in-vitro and in-vivo findings, the in-vivo results should always take precedence over in-vitro studies. The FDA defines that if steady-state systemic exposure to metabolite in humans exceeds 10% of exposure to the parent drug (unbalanced metabolite), the metabolite requires additional safety assessment. In a contracted in-vivo ADME study, metabolite profiling and identification is performed on urine, faeces and bile collected during mass balance and bile excretion studies using plasma collected during the PK study. The samples are analysed and identified using a variety of analytical techniques. The biological matrix includes plasma, urine, faecal homogenate, and bile as study samples.
The identity of metabolites present in the animal or human matrix provides important information about the bio transformation pathways involved in drug clearance. If the metabolite profile of the parent drug is qualitative and quantitatively similar between animals and humans, the potential clinical risk of the parent drug and metabolites is adequately assessed in non-clinical studies. If there is a difference between in-vitro and in-vivo findings, the in-vivo results should always take precedence over in-vitro studies. The FDA defines that if steady-state systemic exposure to metabolite in humans exceeds 10% of exposure to the parent drug (unbalanced metabolite), the metabolite requires additional safety assessment. In a contracted in-vivo ADME study, metabolite profiling and identification is performed on urine, faeces and bile collected during mass balance and bile excretion studies using plasma collected during the PK study. The samples are analysed and identified using a variety of analytical techniques. The biological matrix includes plasma, urine, faecal homogenate, and bile as study samples.
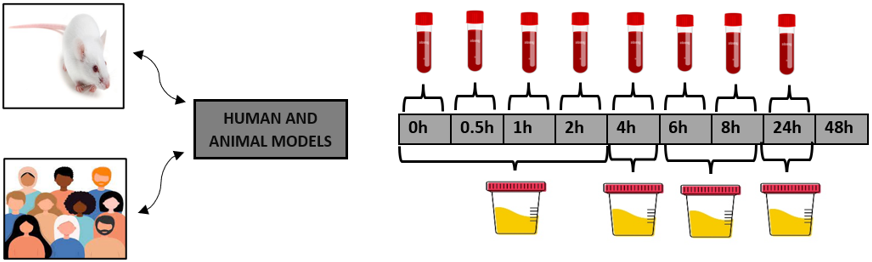
Although other biological fluids (including saliva) or tissues have also been gathered and analysed, plasma, urine, and faeces samples are the most frequently employed as matrices. The major samples to demonstrate that substances pass the intestinal barrier and establish the pharmacokinetics curves are plasma or serum samples. One of the key elements that must be properly designed is the time intervals at which the samples are gathered. Although there are variations amongst the research, the most typical times for blood sample collection are at 0 h, 0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h, and 24 h. [Figure 2]
Excreted metabolites seen in urine samples may reveal metabolic changes made to the extract's original components. There are studies that only collect samples throughout a single and multiple intervals. In order to properly monitor the excretion of metabolites, urine samples are often obtained after several time intervals have been established. Urine is typically collected in human models at the following intervals: (0-2), (2-5), (5-8), and (8-24) h [Figure 2]. They are often collected at the conclusion of animal test experiments. For extraction from biological materials, some researches have used extraction methods such solid-phase extraction (SPE). This method has the benefits of improving analytical selectivity and minimising potential matrix effects and interferences. [7]
In-Silico Approaches
Simultaneous increase in both drug metabolism knowledge base and general computing power over the last 40 years Inevitably led to the development, evaluation, and implementation of in-silico models as well as metabolite data processing and interpretation. The software was developed to support accurate and fast deconvolution of the abundant data generated by various analytical techniques used to identify metabolites.
Simultaneous increase in both drug metabolism knowledge base and general computing power over the last 40 years Inevitably led to the development, evaluation, and implementation of in-silico models as well as metabolite data processing and interpretation. The software was developed to support accurate and fast deconvolution of the abundant data generated by various analytical techniques used to identify metabolites.
Bio-print programme consists of biological and in-vitro ADME assays so called biological fingerprint of many marketed drugs along with reference drugs by using neighbourhood relationships and QSAR models the possible outcome of the new compound and possible interactions of compound with metabolic enzymes can be estimated. Identification of metabolites is usually done by fragmenting a given molecule and passing through collection of transformation rules. For the above-mentioned task there are several databases available such as the Accelrys Metabolism Database or the MDL Metabolite database. Examples of some software which are based on the above-mentioned databases are METEOR, META, MetabolExpert, Meta Drug which provide a list of most metabolites. [8]
Meteor: This computational system utilizes base of reactions and rules related to biotransformation and structures to predict the metabolic fate of a given chemical entity. It has a reasoning engine which consists of two rules absolute and relative, where in absolute evaluation of biotransformation takes place basis on 5 levels (Probable, Plausible, Equivocal, Doubted, and Improbable) and in relative priorities will be assigned to competing reactions and equal priority is given when there is no preference. Further by usage of non-numerical logical argumentation for formulating arguments for and against hypothesis and evaluate the possibility of a reaction taking place in the chemical entity.
The system accepts Molfile imports or graphical input. Individual metabolites can be chosen and processed at deeper levels within the application to evaluate the results, or they can be transferred to DEREK for a toxicological assessment. You can search the metabolic tree using a molecular mass, molecular formula, or molecular structure. A knowledge-based editor is also included with the system so that users can create their own biotransformation and regulations. [9]
SyGMa: By the expert knowledge and empirical analysis of original data of any particular drug an approach called SyGMa (systematic generation of potential metabolites) an approach was developed on a basis of MDL metabolite database which describes metabolic rules. These rules are important factors which were formed from different metabolic reactions of a drug in the original data. This application SyGMa supports experimental detection of metabolites. [10]
Analytical Strategies
Gas chromatography coupled with MS (GC-MS) has been widely used for the quantification and identification of minor components in complex sample mixtures of various compounds. It has some major disadvantages like derivatization of analytes prior to analysis, less sensitivity and low sample throughput hence liquid chromatography coupled with Tandem MS (LC-MS/MS) has become a preferred choice for the identification of metabolites. Liquid that enters the MS from LC must be converted from liquid phase to gaseous phase is major important consideration in LC-MS and it depends on the ion source. Most widely used ionisation sources include: Electrospray ionization (ESI), Atmospheric Pressure Chemical Ionization (APCI), Atmospheric Pressure Photoionization (APPI). Most widely used tandem mass spectrometers include triple-stage quadrupole (TSQ), ion Trap, and quadrupole time-of-flight (Q-TOF). [11]
Gas chromatography coupled with MS (GC-MS) has been widely used for the quantification and identification of minor components in complex sample mixtures of various compounds. It has some major disadvantages like derivatization of analytes prior to analysis, less sensitivity and low sample throughput hence liquid chromatography coupled with Tandem MS (LC-MS/MS) has become a preferred choice for the identification of metabolites. Liquid that enters the MS from LC must be converted from liquid phase to gaseous phase is major important consideration in LC-MS and it depends on the ion source. Most widely used ionisation sources include: Electrospray ionization (ESI), Atmospheric Pressure Chemical Ionization (APCI), Atmospheric Pressure Photoionization (APPI). Most widely used tandem mass spectrometers include triple-stage quadrupole (TSQ), ion Trap, and quadrupole time-of-flight (Q-TOF). [11]
Metabolite Identification in Nebivolol
Nebivolol is a potent cardio selective β1-adrenergic antagonist which is administered as a racemic mixture of D-nebivolol which is more likely responsible for selective antagonistic action and L-nebivolol for vasodilatory action in equal ratios. It is used for the treatment of hypertension and helpful in the management of heart failure. [12]
Nebivolol is a potent cardio selective β1-adrenergic antagonist which is administered as a racemic mixture of D-nebivolol which is more likely responsible for selective antagonistic action and L-nebivolol for vasodilatory action in equal ratios. It is used for the treatment of hypertension and helpful in the management of heart failure. [12]
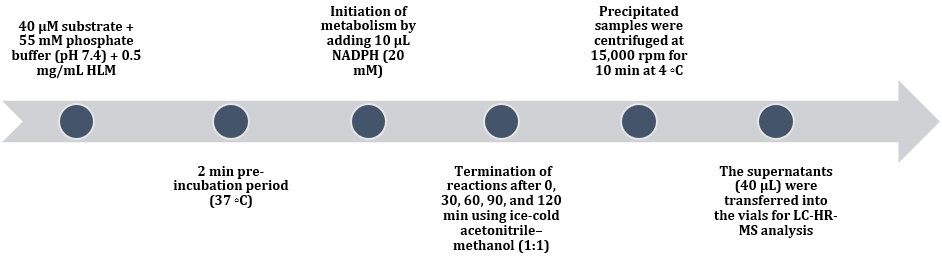
In this study they utilized HLM assay for identification of metabolites [Figure 3], where 60min incubation time was selected for the chemometric and qualitative analysis. For chemometric analysis metabolic profiles were obtained by using RP-UHPLC coupled with HR-MS [Table 2] and these metabolic profiles were filtered by using Mass Profiler Professional (MPP) software and selected 6 metabolites for further chemometric study. Principal component Analysis (PCA) was performed using SIMCA software where one ion with low mass(M6) was found which was previously not reported in any literature. These six metabolites were analysed by HR-MS/MS for structural elucidation and in-silico toxicity of M6 metabolite was studied by utilizing Toxicity Estimation Software Tool (T.E.S.T) and ACD/ Labs Percepta software’s. [13]
| Drug | Approach | Analytical method | Metabolites identified |
m/z value | Metabolic pathway |
| Nebivolol | In vitro (Human liver microsomes) | LC-HR-MS coupled with chemometric analysis | M1 | 422.1761 | Alicyclic hydroxylation of parent compound |
| M2 | 422.1785 | ||||
| M5 | 438.1726 | Further Hydroxylation of M1 or M2 | |||
| M4 | 420.1618 | Alicyclic oxidation of parent compound | |||
| M3 | 422.1761 | Aromatic hydroxylation of parent compound | |||
| M6 | 212.1083 | N-dealkylation of parent compound |
Table 2: Metabolites identified in nebivolol by HLM assay and chemometric analysis
Table abbreviations
HLM: Human liver microsomes; FMO: Flavin-containing monooxygenase; UGT: Uridine diphosphate glucuronosyl transferase; NAT: N-acetyltransferase; GST: Glutathione S-transferase; SULT: Sulfotransferase; CYP: Cytochrome P450; LC-HR-MS: Liquid chromatography–high resolution mass spectrometry; (M1-M6): Metabolites
HLM: Human liver microsomes; FMO: Flavin-containing monooxygenase; UGT: Uridine diphosphate glucuronosyl transferase; NAT: N-acetyltransferase; GST: Glutathione S-transferase; SULT: Sulfotransferase; CYP: Cytochrome P450; LC-HR-MS: Liquid chromatography–high resolution mass spectrometry; (M1-M6): Metabolites
Conclusion
Drug metabolism studies are helpful in predicting the fate of drug in the body and thus, help in determining appropriate drug dosage regimen. Identification and characterisation of metabolites of specific drug and enzymes responsible for metabolism will give an opportunity to assess impact of metabolite on safety and efficacy of the drug and we can structurally assess the metabolic clearance, inter-individual variability, safety of the drug, drug interactions, PK/PD studies, toxicity profile of drug. Metabolite identification can be performed by in-silico, in-vitro, in-vivo approaches. In-silico methods has the advantage of reducing the time of metabolite identification compared to conventional approaches, it can give a glance about the metabolites and their toxicological behaviour according to their structural characterisation using software’s. To better understand drug metabolism, a variety of in-vitro techniques, from subcellular to organ range, and in-vivo approaches are used. The most often used in-vitro models among them for drug interaction and metabolic profile studies are hepatocytes and microsomes. It is necessary to develop and validate appropriate bioanalytical methods for the monitoring of drug metabolism. The most effective analytical technique for identifying and measuring drug metabolites is liquid chromatography combined with mass spectrometry (LC-MS) due to its inherent specificity, sensitivity, and speed. This model can be further extrapolated for predicting IVIVC when formulation dissolution gets conducted in bio- relevant media. Gut model helps in predicting in-vivo behaviour of drug to understand bioavailability of challenging molecules.
Acknowledgments
CPP department SPIL Gurgaon for providing adequate facility for research.
CPP department SPIL Gurgaon for providing adequate facility for research.
References
- Robert Roškar, Tina Trdan Lušin. (2012). Analytical Methods for Quantification of Drug Metabolites in Biological Samples, Chromatography the Most Versatile Method of Chemical Analysis. 79-126.
- Yengi LG, Leung L, Kao J. (2007). The evolving role of drug metabolism in drug discovery and development. Pharm Res. 24(5): 842-58.
- Kamel A, Prakash C. (2006). High performance liquid chromatography/atmospheric pressure ionization/tandem mass spectrometry (HPLC/API/MS/MS) in drug metabolism and toxicology. Curr Drug Metab. 7(8): 837-52.
- Nedderman, A.N.R. (2009). Metabolites in safety testing: metabolite identification strategies in discovery and development. Biopharm. Drug Dispos. 30(4): 153-162.
- Sean Ekins et al., (2000). Present and future in vitro approaches for drug metabolism. J Pharmacol Toxicol Methods 44 (1): 313-324.
- D.J. Birkett et al., (1993). In vitro approaches can predict human drug metabolism. TiPS 14(8): 292–294.
- Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Fernández-Moreno, P.; Rojas-García, A.; Arráez-Román, D.; Segura-Carretero, A. (2022). Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds. Molecules 27(3): 777.
- Czodrowski P, Kriegl JM, Scheuerer S, Fox T. (2009). Computational approaches to predict drug metabolism. Expert Opin. Drug Metab. Toxicol. 5(1): 15-27.
- Testa B, Balmat AL, Long A, Judson P. (2005). Predicting drug metabolism--an evaluation of the expert system METEOR. Chem Biodivers. 2(7): 872-85.
- Ridder, L. and Wagener, M. (2008). SyGMa: Combining Expert Knowledge and Empirical Scoring in the Prediction of Metabolites. Chem MedChem. 3(5): 821-832.
- Prakash C, Shaffer CL, Nedderman A. (2007). Analytical strategies for identifying drug metabolites. Mass Spectrom Rev. 26(3): 340-369.
- Dery AS, Hamilton LA, Starr JA. (2011). Nebivolol for the treatment of heart failure. Am J Health Syst Pharm. 68(10):879-86.
- Trawi?ski J, Wro?ski M, Gawlik M, Skibi?ski R. (2022). Identification of the New Metabolite of Nebivolol Using Liquid Chromatography Coupled with High-Resolution Mass Spectrometry and Chemometrics. Molecules. 27(3): 763.
Citation: Vanapalli Rohith, Varikuti Vyshnavi, Dipanjan Goswami, Sanjeev Mishra, Sanjay J Gurule and Arshad Khuroo. (2023). New Avenues Explored for Metabolite Driven Clinical Drug Development. Journal of Pharmacy and Drug Development 5(1).
Copyright: © 2023 Vanapalli Rohith. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.