Opinion Article
Volume 1 Issue 1 - 2018
NDDS Product and Innovation of Packaging Material and Device
Post Graduate in Packaging Technology and Polymer Science (First Class). India AutoCAD for Injectable device design BSC(Math), University of Calcutta
*Corresponding Author: Anupam Chanda, Post Graduate in Packaging Technology and Polymer Science (First Class). India AutoCAD for Injectable device design BSC(Math), University of Calcutta.
Received: November 29, 2018; Published: December 24, 2018
Biography
Anupam completed his Post Graduate in Packaging from “Indian Institute of Packaging, Mumbai, India and worked for 25 years in different Pharmaceutical companies in Packaging Development department in India and abroad. Widely traveled throughout the world. Gain and share his thoughts with scientist from various institution and industry professionals. Author’s Technical book “ Packaging Technology An advance Practical approach “Published from Germany and Amazon is distributing to 77 countries. Book is available in www.amazon.de, www.amazon.com and www.morebooks.de. He is attached with various charitable institutions and research institute. He has more than 15 publications in national and International Journals. He currently works mainly in packaging device innovation for oncology and no oncology products for Regulated market, zero and low gravity environment for Mars and Moon.
Introduction
Novel drug delivery system plays a role for improve its performance in terms of patient compliance, safety and efficacy. In the form of a Novel Drug Delivery System an existing drug molecule can get a new life. Design need to plan well in advance that Novel Drug Delivery System can be a major advance for solving the problems related towards the release of the drug at specific site with specific rate. Effective drug delivery is most important to patients. Most of Pharmaceutical companies are avoiding side effect of drug delivery system by using latest technology which is involve efficiency of R&D, packaging development and production.
Purpose and Special Role
- Packaging material device need to manufacture according to new drugs, Formulation, Nano Particle Technology, Biodegradable Implant/Injection Technology. There are many latest technology available like: “Dry Powder Inhalation Technology, Swollen Micelle Microemulsilon Technology”, Gel Free Reservoir Technology and wrap matrix controlled Release system.
Technology are using for NDDS (Novel Drug Delivery System)
- “Controlled release Formulation”
- Using “Nano Particle Technology” which is able to penetrate drugs in specific area to kill the most wanted cells.
- “Biodegradable Implant/Injection” Technology is applicable where “Drug has to release for long duration”
- “Dry Powder Inhalation Technology”
- “Swollen Micelle Microemulsilon “ Technology
- “Gel Free Reservoir Technology”
- “Wrap matrix controlled Release System”
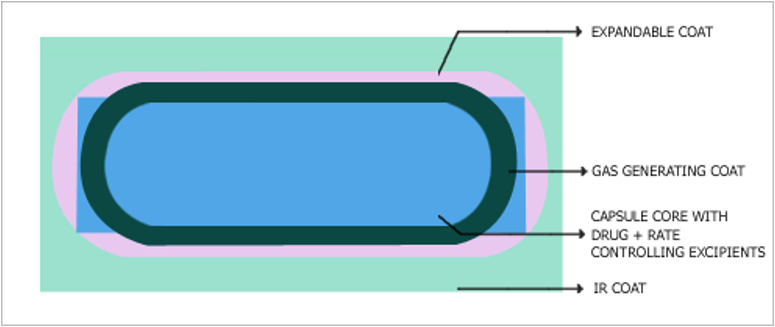
1. Controlled release Formulation
Controller drug delivery is one which delivers the drug at a predetermined rate for locally and systematically for a specified period of time.
Controller drug delivery is one which delivers the drug at a predetermined rate for locally and systematically for a specified period of time.
Technology and Advantages
- Gas generating and expandable coating
- This is an ideal once-a-day delivery system
- Absorbed only from the stomach or intestine.
- Improves drug absorption in the stomach.
- The product can be designed to offer a combination of instant and sustained drug release profiles, and since it is once-a-day.
- Improves patient compliance.
Selection of Primary Packaging material (precaution to take)
- Don’t follow 100% innovator’s primary packaging material of construction. Since this Technology is quite different from innovator so during stability study need to pack product in different “material of construction” and observe which combination is best suited for this product.
- Select the best combination.
- Range of primary packaging material options are available like strip pack, blister pack, Polymeric bottle pack, glass bottle pack.
2. Nano Particle Technology
Nano Particles are sub-nanosized colloidal structures composed of synthetic or semi-synthetic Polymers. Particulate systems like nanoparticles have been used as a physical approach to alter and improve the pharmacokinetic and pharmacodynamic properties of various types of drug molecules. They have been used in vivo to protect the drug entity in the systemic circulation; various polymers have been used in the formulation of nanoparticles for drug delivery research to increase therapeutic benefit, while minimizing side effects.
Nano Particles are sub-nanosized colloidal structures composed of synthetic or semi-synthetic Polymers. Particulate systems like nanoparticles have been used as a physical approach to alter and improve the pharmacokinetic and pharmacodynamic properties of various types of drug molecules. They have been used in vivo to protect the drug entity in the systemic circulation; various polymers have been used in the formulation of nanoparticles for drug delivery research to increase therapeutic benefit, while minimizing side effects.
Technology and Advantages (For oncology product)
- Toxic surfactants often have to be used to solubilise the drug.
- Such drugs not only reach the tumour tissues but also reach and penetrate healthy tissues in the body.
- Higher drug localization to the cancer cells
- This Technology do not need any pre-administration preparation
Selection of Primary Packaging material (precaution to take)
- If we find possibility of delamination in glass vial and prefilled syringe then we can go for COC/COP vial and syringe.
- Since this Technology is quite different from innovator so during stability study need to pack product in different “material of construction” and observe which combination is best suited for this product.
- This is advisable to procure primary packaging materials from “reputed vendors ”only to avoid delamination and un wanted extractable and leachable
3. Biodegradable Implant/Injection Technology
This technology is most useful where requires long term maintenance of drug levels in the body, over several months or weeks This may require daily or frequent injections, which is most painful and critical for the patient. One solution involves use of a depot or reservoir from which drug is released over a long period. Now a days few drugs are developed in the field of cancer and cardiovascular.
This technology is most useful where requires long term maintenance of drug levels in the body, over several months or weeks This may require daily or frequent injections, which is most painful and critical for the patient. One solution involves use of a depot or reservoir from which drug is released over a long period. Now a days few drugs are developed in the field of cancer and cardiovascular.
Technology and Advantages (For oncology product)
- Applicable where “Drug has to systematic release for long duration
- In this delivery system drug is encapsulated within microspheres from where it is gradually released.
- Rapid onset and prolonged release over months
Selection of Primary Packaging material (precaution to take)
- Don’t follow 100% innovator’s primary packaging material of construction. Since this Technology is quite different from innovator so during stability study need to pack product in different “material of construction” and observe which combination is best suited for this product.
- Select the best combination.
- Range of primary packaging material options are available like strip pack, blister pack, Polymeric bottle pack, glass bottle pack.
4. Dry Powder Inhalation Technology
These formulations also pose patient compliance and good shelf life. Earlier DPI formulations were intended for local effect in lung (asthma and chronic obstructive pulmonary diseases), whereas 21(st) century witnessed formulations intended for systemic effect too. A better understanding of physiology of lung and fluidics of air flow helped in targeting alveoli using DPI technology. Modern technology also accelerated the research pace. In addition to the synthetic molecules, DPI also was proved to be a better system for delivering biological molecules including vaccines. This review includes the mechanisms of drug deposition, advancements in the fields of DPI devices, various characterization tools and particle engineering. Inhaled short and long-acting beta agonists and corticosteroids are fundamental to the treatment of asthma
These formulations also pose patient compliance and good shelf life. Earlier DPI formulations were intended for local effect in lung (asthma and chronic obstructive pulmonary diseases), whereas 21(st) century witnessed formulations intended for systemic effect too. A better understanding of physiology of lung and fluidics of air flow helped in targeting alveoli using DPI technology. Modern technology also accelerated the research pace. In addition to the synthetic molecules, DPI also was proved to be a better system for delivering biological molecules including vaccines. This review includes the mechanisms of drug deposition, advancements in the fields of DPI devices, various characterization tools and particle engineering. Inhaled short and long-acting beta agonists and corticosteroids are fundamental to the treatment of asthma
Technology and Advantages (For Asthama)
- Device is small, convenient and easy to carry.
- Device delivers uniform dose independent of breathing flow rate.
- Unique design to avoid double dosing
- A glow-in-the-dark feature ensures easy night-time use.
Selection of Primary Packaging material (precaution to take)
- Don’t follow 100% innovator’s primary packaging material of construction. Always possible to develop more patient friendly device design.
- Select the device after successful completion of clinical studies.
- Range of primary packaging material options are available. Final selection of primary packaging material depend on extractable and leachable value.
5. “Swollen Micelle Microemulsion” Technology
Micelles made of surfactant molecules with oil phase present inside them. Oil/surfactant ratio defines the size of a micelle. When water and surfactant are present without any oil added (oil/surfactant ratio = 0.0), there will be empty micelles. The size of a micelle keeps on increasing (for a given micelle shape) with the addition of oil. In other words, with increase in ratio of oil to surfactant, the micelles swell. All microemulsions are made of swollen micelles with oil/water inside them.
Micelles made of surfactant molecules with oil phase present inside them. Oil/surfactant ratio defines the size of a micelle. When water and surfactant are present without any oil added (oil/surfactant ratio = 0.0), there will be empty micelles. The size of a micelle keeps on increasing (for a given micelle shape) with the addition of oil. In other words, with increase in ratio of oil to surfactant, the micelles swell. All microemulsions are made of swollen micelles with oil/water inside them.
Technology and Advantages (For Glucoma)
- Product is preservative free.
- Product does not need any special refrigeration for storage/ transport.
- Device delivers uniform dose independent of breathing flow rate.
- Unique design to avoid double dosing
- A glow-in-the-dark feature ensures easy night-time use.
Selection of Primary Packaging material (precaution to take)
- Don’t follow 100% innovator’s primary packaging material of construction. Always possible to develop more patient friendly bottle, nozzle and cap.
- Select the primary packaging material after successful completion of trial and stability study.
6. Gel Free Reservoir Technology (for ophthalmic product)
This technology uses a unique polymer ratio that does not decrease visual clarity and has desired flow property. The physical properties of our product are similar to natural tears. The product has the characteristics of an ideal eye drop- clear colourless solution, bio adhesive yet non sticky.
This technology uses a unique polymer ratio that does not decrease visual clarity and has desired flow property. The physical properties of our product are similar to natural tears. The product has the characteristics of an ideal eye drop- clear colourless solution, bio adhesive yet non sticky.
Technology and Advantages
- Increased product flow property.
- Non sticky bio adhesive
Selection of Primary Packaging material (precaution to take)
- Don’t follow 100% innovator’s primary packaging material of construction. Always possible to develop more patient friendly bottle, nozzle and cap.
- Select the primary packaging material after successful completion of trial and stability study.
7. Wrap matrix controlled Release System
Diffusion controlled systems also known as matrix systems are very popular for sustained release formulations. This can be divided up into different types of mechanisms by which they prolong drug release, these includes reservoir matrix systems, monolithic matrix systems and osmotic pump systems. Here we have given one example for reservoir matrix system.
Diffusion controlled systems also known as matrix systems are very popular for sustained release formulations. This can be divided up into different types of mechanisms by which they prolong drug release, these includes reservoir matrix systems, monolithic matrix systems and osmotic pump systems. Here we have given one example for reservoir matrix system.
Technology and Advantages
- Multi-layered matrix-based tablet of such drugs offers controlled release.
Selection of Primary Packaging material (precaution to take)
- Don’t follow 100% innovator’s primary packaging material of construction. Since this Technology is quite different from innovator so during stability study need to pack product in different “material of construction” and observe which combination is best suited for this product.
- Select the best combination.
- Range of primary packaging material options are available like strip pack, blister pack, Polymeric bottle pack, glass bottle pack.
References
- www.pharmtech.com/packaging-
- https://packagingeurope.com/innovative-opening-aid-for-pharmaceutical-products
- www.freedoniagroup.com
Citation: Anupam Chanda. (2018). NDDS Product and Innovation of Packaging Material and Device. Journal of Pharmacy and Drug Development 1(1).
Copyright: © 2018 Anupam Chanda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.