Research Article
Volume 4 Issue 1 - 2022
Investigation and Prevalence of Gastrointestinal Parasites of Equestrian clubs Horses in Misurata, Libya
1Zoology Department, Faculty of Science, Misurata University
2Department of Parasitology, Faculty of Veterinary Medicine, Kafrelsheikh University, 33516, Egypt
3Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, 51452 Qassim, Saudi Arabia
4Department of Parasitology, Faculty of Veterinary Medicine, South Valley University, Qena, 83523 Egypt
2Department of Parasitology, Faculty of Veterinary Medicine, Kafrelsheikh University, 33516, Egypt
3Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, 51452 Qassim, Saudi Arabia
4Department of Parasitology, Faculty of Veterinary Medicine, South Valley University, Qena, 83523 Egypt
*Corresponding Author: Elmajdoub Layla O, Zoology Department, Faculty of Science, Misurata University.
Received: August 20, 2022; Published: September 19, 2022
Abstract
Background and Objective: The horse has been a loyal friend and trusted partner of human being. Horses are prone to infestation with both internal and external parasites. A cross-sectional study was performed to estimate the gastrointestinal parasite infecting horses in equestrian clubs.
Materials and Method: For the study, faecal samples were obtained from 50 randomly chosen horses of varying sexes and ages and analyzed using sedimentation techniques.
Results: The overall prevalence of egg/larva or cyst in the study area was 98.0% (49/50). Moreover, the mixed infection was higher than that single infection; there were significant differences between Protozoa and helminths (P ≤ 0.01). The types of helminth parasites (egg/larva) detected were Anoplocephala spp. (17%), Parascaris equorum (17%), and Moniezia spp (20%) in increasing with the age of horses had a significant effect with ages above one year, moreover, there reported the oocysts of Cryptosporidium parvum (33%).
Conclusion: Hence, the present study's findings indicated a high prevalence of parasites compromising horses' health and welfare in the study area. Thus, proper screening and monitoring of the horses should be carried out regularly; regular and strategic anti -parasites programmers, should be carried out.
Keywords: Horses; Intestinal parasites; Helminths; Protozoa; Anoplocephala spp; Parascaris equorum and Moniezia spp
Introduction
The digestive system of horses provides a target site for many intestinal parasites species; gastrointestinal parasites are responsible for severe pathological conditions, sometimes fatal, and reduced performance and physical illness, (Love et al., 1999). Also provides the unique type of digestive system compared to other higher mammals. The stomach have four compartments. Among four compartments, rumen is the largest part in the rumen partially chewed grass is stored and broken down into balls of cud, (Love et al., 1999).
Horses was belonged to the equine group. It is found mainly in temperate, semi-arid or highland areas, Horses is herd animal and will happily live in groups with other animals of a different species such as, sheep and goats. Horse is very friendly animals and enjoy the company of humans. Today its importance has scarcely diminished in parts of South America, Asia, Africa and Eastern Europe, (Addis et al., 2017; Jajere et al., 2016). Equids play crucial role in both urban and rural areas, providing agricultural energy and transport, and in many cases, the sole means of income generating for their resource-limited owners, (Getachew et al., 2014).
Equines were infected with a wide range of intestinal parasites over 100 species, half of these species belonged to strongyles parasites (Lichtenfels et al., 2008). In contrast, while cestodes and trematodes are less occurring, Eimeria and Isospora species can also be encountered (Bowman, 2003). In the presence of a high infection rate of nematodes, chronicle catarrhal gastritis develops (Umue and Acici, 2009). Gastrointestinal parasites are one of the greatest limiting factors to successful horse rising throughout the world, (Jajere et al., 2016). They are worldwide problem for both small and large-scale farmer with a greatest impact due to the availability of a wide range of agro-ecological factors suitable for diversified hosts and parasitic species, (Jajere et al., 2016). The development and survival of helminths egg of larvae with faeces on pasture are depending on temperature and moisture, thus forming suitable environment for development of larvae of nematode and trematoda to infected stage, (AlAnazi and Alyousif, 2011).
Transmission of parasitic helminths is via the faecal-oral route, either indirectly within contact. In addition, transmission can occur from person-to-person, from animal-to-person, animal-to-animal, moreover the diagnosis of gastrointestinal parasites through stool analysis is quite challenging even in severe infections. Moreover, the effects of gastrointestinal parasites are more evident in young and under nourished horses. Small numbers causes minimal damage, but large number pose a risk for colic and other symptoms. As a rule, older horses appear to develop immunity against the common gastrointestinal parasites and tend not be affected by parasite related problems as commonly as younger horses, (Jajere et al., 2016).
Several studies have been conducted on gastrointestinal parasites from horses in many countries, such as Saudi Arabia in 20118, in Turkey (Negash et al., 2021), in Western Australia (Boxell et al., 2004), no gastrointestinal parasite fauna of horse in Libya is to date. According to estimates by the Libyan Ministry of Agriculture, Libya, the number of horses in Libya in 2016 exceeded 45,000 (Ministry of Agriculture, 2017). Parasitic helminths are more prevalent in foals, and young horses (Relf et al., 2013), that is explained by an age acquired immunity. The males were shown to have higher infection compared to females (Buzatu et al., 2017). The present study was planned to diagnosis the gastrointestinal parasite fauna in some equines grazed in different Equestrian clubs in Misurata, Libya.
Materials and Methods
Study area
The present study was carried out from March to the end of November 2018 of some Equines from the Equestrian clubs in Misurata, Libya. Misurata is the third-largest city in Libya and occupies around 4000 km2 of Libya's northwestern part (long. 15°6` E and lat. 32°23` N). The population in Misurata is estimated to be about 419,192 (Ministry of Agriculture, 2017).
The present study was carried out from March to the end of November 2018 of some Equines from the Equestrian clubs in Misurata, Libya. Misurata is the third-largest city in Libya and occupies around 4000 km2 of Libya's northwestern part (long. 15°6` E and lat. 32°23` N). The population in Misurata is estimated to be about 419,192 (Ministry of Agriculture, 2017).
Collection of samples
A total of 50faecal samples were collected and examined for equids from the Equestrian clubs for gastrointestinal helminth parasites (33 males and 17 from females). About 10 gram fresh faecal samples were collected directly from rectum using disposable polythene gloves, kept in plastic sachets. The necessary information was noted, such as faecal samples collection date, sex of horse, and preserved at four ?C in the Zoology department laboratory.
A total of 50faecal samples were collected and examined for equids from the Equestrian clubs for gastrointestinal helminth parasites (33 males and 17 from females). About 10 gram fresh faecal samples were collected directly from rectum using disposable polythene gloves, kept in plastic sachets. The necessary information was noted, such as faecal samples collection date, sex of horse, and preserved at four ?C in the Zoology department laboratory.
Faecal examination
The collected faecal samples were taken in clean Petri plates and thoroughly examined for colour, consistency, presence of blood, mucus, tapeworm segments and dead worms which sometimes, provided an important clue about the parasitic infection. The samples were examined microscopically using sedimentation method to detect parasitic infection (Solusby, 1982). One drop from the mixture was taken to prepare on the slide. The specimen was stained with Iodine wet mount solution and examined at 10X and 40X objectives. In this way, two slides were designed from each sample were examined at 10X and 40X objectives of a microscope to detect eggs of helminths, protozoan's trophozoites or cysts of gastrointestinal parasites. For detection, the cryptosporidium cysts were made a smear on the slide and were air-dried; stained by modi?ed Ziehl–Neelsen (Majewska et al., 2004) examined using an oil immersion objective.
The collected faecal samples were taken in clean Petri plates and thoroughly examined for colour, consistency, presence of blood, mucus, tapeworm segments and dead worms which sometimes, provided an important clue about the parasitic infection. The samples were examined microscopically using sedimentation method to detect parasitic infection (Solusby, 1982). One drop from the mixture was taken to prepare on the slide. The specimen was stained with Iodine wet mount solution and examined at 10X and 40X objectives. In this way, two slides were designed from each sample were examined at 10X and 40X objectives of a microscope to detect eggs of helminths, protozoan's trophozoites or cysts of gastrointestinal parasites. For detection, the cryptosporidium cysts were made a smear on the slide and were air-dried; stained by modi?ed Ziehl–Neelsen (Majewska et al., 2004) examined using an oil immersion objective.
Data analysis
Data were statistically analyzed using Pearson's Chi-square test with Yates continuity correction, performed by "R", In all cases 95% confidence interval (CI) and P<0.05 was considered for a statistically significant difference.
Data were statistically analyzed using Pearson's Chi-square test with Yates continuity correction, performed by "R", In all cases 95% confidence interval (CI) and P<0.05 was considered for a statistically significant difference.
Results
The overall prevalence of gastrointestinal parasites in horses
From Table (1), out of 50 samples, 49 (98%) were positive, 32 (97%) of males and 17 (100%) of females for the parasitic egg and cyst. The horse age was ranged from below one year to above ten years. The age ranged 1-4 years was the highest preva-lence rate (36.7%) followed by 5-9 years (34.7%), based on statistical differences, was showed non-significant different be-tween the prevalence rates according to sex and ages (P>0.05).
From Table (1), out of 50 samples, 49 (98%) were positive, 32 (97%) of males and 17 (100%) of females for the parasitic egg and cyst. The horse age was ranged from below one year to above ten years. The age ranged 1-4 years was the highest preva-lence rate (36.7%) followed by 5-9 years (34.7%), based on statistical differences, was showed non-significant different be-tween the prevalence rates according to sex and ages (P>0.05).
| Ages | Examined Males | Examined Females | Number of Male infected horses | Number of infected female horses | Total infection rate |
| Below one year | 6 | 0 | 6 (18.7%) | 0 (0%) | 6 (12.24%) |
| 1 – 4 years | 10 | 8 | 10 (31.3%) | 8 (47.1%) | 18 (36.7%) |
| 5 – 9 years | 10 | 7 | 10 (31.3%) | 7 (41.2%) | 17 (34.7%) |
| Above ten years | 7 | 2 | 6 (18.7%) | 2 (11.8%) | 8 (16.3%) |
| Total | 33 | 17 | 32 (97%) | 17 (100%) | 49 (98%) |
Table 1: Overall prevalence rates of gastrointestinal parasites in horses.
The prevalence of protozoan and helminthic parasites in horses
Overall, horses were infected with protozoan and helminthic parasites. The mixed infection was the highest prevalence rate (63.3%) followed by an independent infection with protozoan parasites (36.7%), whereas, did not find any separate infection with helminths as illustrated in Table (2). Based on horse sex, the mixed infection in female horses was higher than those in males (70.6% and 57.6%), respectively. In contrast, male horses' independent infection was higher than those in females (39.4% and 29.4%), respectively, as Table (2). Statistical analysis found the highly significant difference between the proto-zoan and helminthic infection rates (P≤0.01). The highest prevalence rate with protozoan parasites at age range 5 – 9 years (36.6%) followed by aged ranged 1-4 years (34.4%). While the highest prevalence rate with helminth parasites at aged, 1-4 years (35.6%) followed by age 5-9 years (30.5%) with significant differences at (P≤0.01) as in Table (3).
Overall, horses were infected with protozoan and helminthic parasites. The mixed infection was the highest prevalence rate (63.3%) followed by an independent infection with protozoan parasites (36.7%), whereas, did not find any separate infection with helminths as illustrated in Table (2). Based on horse sex, the mixed infection in female horses was higher than those in males (70.6% and 57.6%), respectively. In contrast, male horses' independent infection was higher than those in females (39.4% and 29.4%), respectively, as Table (2). Statistical analysis found the highly significant difference between the proto-zoan and helminthic infection rates (P≤0.01). The highest prevalence rate with protozoan parasites at age range 5 – 9 years (36.6%) followed by aged ranged 1-4 years (34.4%). While the highest prevalence rate with helminth parasites at aged, 1-4 years (35.6%) followed by age 5-9 years (30.5%) with significant differences at (P≤0.01) as in Table (3).
| Protozoan infection Rate | Helminthic infection Rate | Mixed infection Rate | |
| Males | 13 (39.4%) | 0% | 19 (57.6%) |
| Females | 5 (39.4%) | 0% | 12 (70.6%) |
| Total | 18 (36.7%) | 0% | 31(63.3%) |
| Mean ± S.E | 5.17 ± 1.31 | 0% | 1.7 ± 0.35 |
| Sign | ** | ** |
**Significant different (P≤ 0.01) S.E. Standard Error
Table 2: Overall prevalence rates with Mean ± S.E of protozoan and helminthic parasites in horses.
Table 2: Overall prevalence rates with Mean ± S.E of protozoan and helminthic parasites in horses.
| Age | Protozoan infection rate | Helminthic infection rate |
| Below 1 year | 8 (8.6%) | 5 (8.5%) |
| 1-4 years | 32 (34.4%) | 21 (35.6%) |
| 5–9 years | 34 (36.6%) | 18 (30.5%) |
| Above 10 years | 19 (20.4%) | 14 (23.7%) |
| Sign | ** | |
** Significant different (P≤ 0.01).
Table 3: Overall prevalence rates of protozoan and helminthic parasites in horses.
Table 3: Overall prevalence rates of protozoan and helminthic parasites in horses.
The intensity infection rate of protozoan and helminthic parasites in horses
The intensity infection was ranged between rarely to heavy density with eggs and cysts, which showed the intensity infection with protozoa was higher than that with helminths. Table (4) illustrated the significant differences (P ≤ 0.01) between protozoa and helminths' intensity rates.
The intensity infection was ranged between rarely to heavy density with eggs and cysts, which showed the intensity infection with protozoa was higher than that with helminths. Table (4) illustrated the significant differences (P ≤ 0.01) between protozoa and helminths' intensity rates.
| Protozoan infection | Helminthic infection | |||||
| Intensity description | Rare | Moderate | Heavy | Rare | Moderate | Heavy |
| Mean ± S.E | 6.8 ± 2.2** | 3.67 ± 1.7 | 5 ± 3 | 3 ± 0.71** | 1.3± 0.45** | 0.82 ± 0.44** |
**Significant different (P≤ 0.01) S.E. Standard Error
Table 4: Significant differences with Mean ± S.E of the intensity of protozoan and helminthic parasites in horses.
Table 4: Significant differences with Mean ± S.E of the intensity of protozoan and helminthic parasites in horses.
Overall protozoan and helminthic parasites in horses
Overall, horses were found to have been infected with protozoan parasites belonging to the three groups. The Sporozoa class (22.4 percent) saw the highest prevalence, and the Sarcodina class (7.32 percent) saw the lowest prevalence. The helminth parasites belonging to three groups, on the other hand, were isolated. The Nematode class was the highest prevalence rate (50 percent), and the Trematode class was the lowest prevalence rate (5.4 percent) in Table 5. The statistical analysis found highly significant differences between the three protozoa classes (P≤0.01), whereas non-significant differences between the three classes of helminths (P>0.05).
Overall, horses were found to have been infected with protozoan parasites belonging to the three groups. The Sporozoa class (22.4 percent) saw the highest prevalence, and the Sarcodina class (7.32 percent) saw the lowest prevalence. The helminth parasites belonging to three groups, on the other hand, were isolated. The Nematode class was the highest prevalence rate (50 percent), and the Trematode class was the lowest prevalence rate (5.4 percent) in Table 5. The statistical analysis found highly significant differences between the three protozoa classes (P≤0.01), whereas non-significant differences between the three classes of helminths (P>0.05).
| Protozoan classes | Helminthic classes | |||||
| Sporozoa | Sarcodina | Ciliophora | Nematoda | Cestoda | Trematoda | |
| Prevalence rate | 22.4% | 7.32% | 15.6% | 50% | 44.6% | 5.4% |
| Mean ± S.E | 4.1 ± 2.1 | 10.7 ± 2.7** | 5 ± 2.52** | 2.8 ± 0.33 | 1.3 ± 0.73 | 1 ± 0.44NS |
** Significant different (P≤ 0.01) NS non-Significant different (P> 0.05) S.E. Standard Error
Table 5: Prevalence rate and significant differences with Mean ± S.E of classes of protozoan and helminthic parasites in horses.
Table 5: Prevalence rate and significant differences with Mean ± S.E of classes of protozoan and helminthic parasites in horses.
Overall protozoan and helminthic species in horses
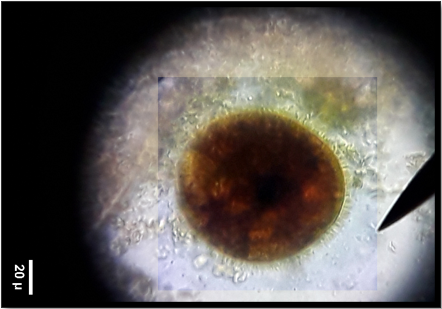
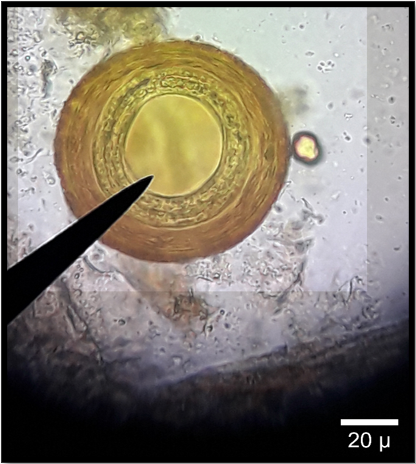
Among Protozoa parasites, Blantidum coli showed the highest prevalence (34.4%) as Figure (1) followed by Cryptosporidium sp. (33.3%), Entamoeba coli (16.1%), Eimeria sp. (13.9%), and the lowest prevalence rate Isosopra spp (2.15%), (Table 6). The study showed an effect of sex on the prevalence of protozoan infection. In contrast, that indicates a higher prevalence in male horses. On the other hand, Moniezia sp. Showed the highest prevalence (20.4%) followed by Parascaris equorum as Figure (2), Anoplocephala sp. Trichostongyliode sp. (16.9%), and the lowest prevalence showed by Capllaira sp. and Gongulonema pulcurum (3.4%). The study showed no effect of sex on the prevalence of helminth infection (Table 7). The statistical analysis showed the significant differences among the different species of protozoa and helminthic in both males and females (P≤0.01) in Figure (3).
Among Protozoa parasites, Blantidum coli showed the highest prevalence (34.4%) as Figure (1) followed by Cryptosporidium sp. (33.3%), Entamoeba coli (16.1%), Eimeria sp. (13.9%), and the lowest prevalence rate Isosopra spp (2.15%), (Table 6). The study showed an effect of sex on the prevalence of protozoan infection. In contrast, that indicates a higher prevalence in male horses. On the other hand, Moniezia sp. Showed the highest prevalence (20.4%) followed by Parascaris equorum as Figure (2), Anoplocephala sp. Trichostongyliode sp. (16.9%), and the lowest prevalence showed by Capllaira sp. and Gongulonema pulcurum (3.4%). The study showed no effect of sex on the prevalence of helminth infection (Table 7). The statistical analysis showed the significant differences among the different species of protozoa and helminthic in both males and females (P≤0.01) in Figure (3).
| Total prevalence rate | Male prevalence rate | Female prevalence rate | |
| Blantidum coli | 32 (34.4%) | 22 (68.8%) | 10 (31.3%) |
| Entamoeba coli | 15 (16.1%) | 11 (73.3%) | 4 (26.7%) |
| Cryptosporidium parvum | 31 (33.3%) | 18 (58.1%) | 13 (41.9%) |
| Eimeria Spp. | 13 (13.9%) | 10 (76.9%) | 3 (23.1%) |
| Isosopra spp. | 2 (2.15%) | 1(50%) | 1 (50%) |
Table 6: Prevalence rate of protozoan species in horses based on sex.
| Total prevalence rate | Male prevalence rate | Female prevalence rate | |
| Parascaris equorum | 10 (16.9%) | 4 (40%) | 6 (60%) |
| Anoplocephalasp | 10 (16.9%) | 6 (60%) | 4 (40%) |
| Paranoplocephala mamillana | 3 (5.1%) | 2 (66.7%) | 1 (33.3%) |
| Gongulonema pulcurum | 2 (3.4%) | 1 (50%) | 1 (50%) |
| Trichostongyliode sp. | 10 (16.9%) | 5(50%) | 5(50%) |
| Strongyliodes westri | 6 (10.2%) | 4 (66.7%) | 2 (33.3%) |
| Paragonimus westermani | 3 (5.1%) | 1 (33.3%) | 2 (66.7%) |
| Capllaira spp. | 2 (3.4%) | 2 (100%) | 0 |
| Moniezia sp. | 12 (20.4%) | 7 (58.3%) | 5 (41.7%) |
Table 7: Prevalence rate of helminth species in horses based on sex.
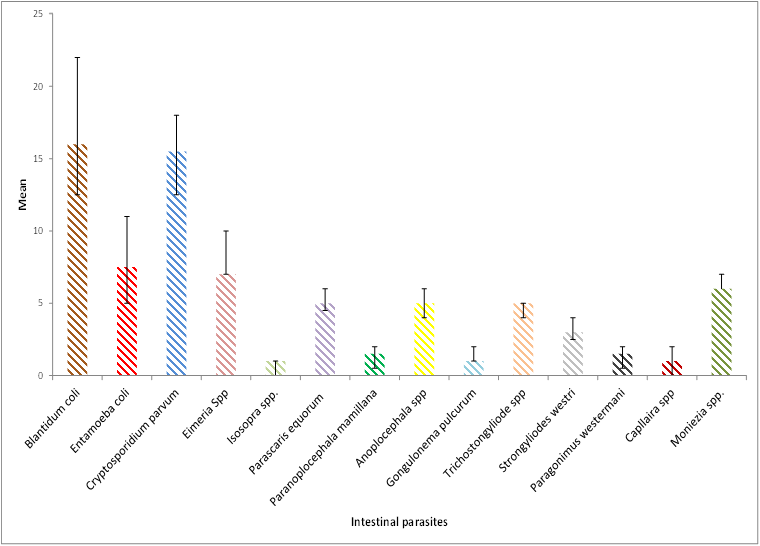
Figure 3: Significant differences with Mean ± S.E of protozoan and helminthic species in horses based on sex, showing the highly signif-icant differences with Blantidium coli and Cryptosporidium parvum more than those other intestinal parasites infection.
Discussion
Gastrointestinal parasites in horses are widespread and affect virtually all grazing animals, and many studies have been carried out regarding the gastrointestinal parasites of domestic livestock. Veterinarians are always interested in research about domestic animals, but very few researches have been carried out to equine populations than other domestic animals. In the present study, the prevalence of horses' gastrointestinal parasites has been carried out for the first time in Misurata, Libya. The current study revealed a high prevalence of gastrointestinal parasites in horses. The overall positive prevalence rate was found to be 98% for different gastrointestinal parasites. The present ?nding almost is in agreement in Iraq (Wannans et al., 2012; Matto et al., 2015), in Ethiopia (Majewska et al., 2004), who reported 100%, 97.9% and 100% respectively. Moreover, it was higher than that in Mumbai (20.6%) (Tilahun et al., 2014), in Karnataka (84%) (Adepp0a, 2014), and in Ethiopia around (92.7%) (Ravi et al., 2021), which the high prevalence rate of the present study due to also poor hygiene and care of the study area.
Among identified parasites, the Blantidum coli showed the highest infection rate (34.4%), followed by Cryptosporidium parvum (33.3%), and Moniezia sp. (20.4%), the present result is higher than work in Nepal (Oli, 2016) that recorded 9.52% in Blantidum sp. The high prevalence of Moniezia sp. in the present study demonstrates that Moniezia sp. infections are high in the study area. The climate could explain the high faecal eggs prevalence, geo-graphical area suitable for development, survival and transmission of pre-parasitic stages on pasture during most of the year and by the absence of anti-parasitic treatments.
The prevalence rate of Parascaris equorum (16.9%) in this study is almost similar in Nepal (10.47%) (Ravi et al., 2021) 13.9% in Romania (Buzati et al., 2015) and 15.5% in Ethiopia (Ravi et al., 2021), but higher than those recorders 7.9% (Kornas et al., 2010) and 6.3% in Nigeria (Umar et al., 2013). On the other hand, it was lower than those in Iraq (40.9%) (Wannans et al., 2012), 43.8% in Ethiopia (Mezgebu et al., 2013) and 55.8% in southern Ethiopia (Tilahun et al., 2022). In the current study, the lower prevalence of Parascaris equorum could be due to collecting faecal samples from adult horses and few only from young horses. In young horses, less than three years occur by Parascaris equorum (Bucknell et al., 1995). In the current study, the prevalence rate in females was more than those in males with non-significant differences and similarities with other studies, (Yadav et al., 2014; Hassan, 2014 ; Chinwe et al., 2019, and suggested frameless with low body immunities and more comfortable to get the parasitic infection. The mixed infection rate was higher than that single infection rate and similar in Turkey (Negash et al., 2021; Chinwe et al., 2019. The high mixed infection rate was the less used of anti-parasitic, poor management system and lack of de-worming.
Conclusions
In the conclusion, the current study's high parasitic prevalence rate could also be related to poor management or poor hus-bandry study area practices. In conclusion, this study confirmed that horses were found to be the most susceptible and infest-ed by various gastro-parasites.
Funding: “This research received no external funding” only from the authors.
Acknowledgments: The authors are grateful to the Dean of the Zoology department, Misurata University, for providing the facilities for carrying out the research.
References
- Addis, H., Gizaw, T.T., Minalu, B. A. and Tefera, Y. (2017). Cross sectional study on the prevalence of equine Strongyle infection Inmecha Woreda, Ethiopia. International Journal of Advanced Research in Biological Sciences, 2017 4(8): 68-77.
- Adeppa, J. and K. J. Ananda. (2016). Incidence of gastro-intestinal parasites in horses of Shimoga region, Karnataka state. J Parasit Dis. 40(3): 919-21
- AL Anazi A.D. and Alyousif M.S. (2011). Prevalence of non-strongyle gastrointestinal parasites of horses in Riyadh region of Saudi Arabia, Saudi Journal of Biological Sciences, 2011, 18: 299–303.
- Bowman D.D. (2003). Georgis' Parasitology for Veterinarians, 8nd edn, Elsevier Science, USA. pp: 215-216.
- Boxell, A.C., Gibson, K.T., Hobbs, R.P., Thompson, R.A.C. (2004). Occurrence of gastrointestinal parasites in horses in metropolitanPerth, Western Australia. Aust. Vet. J. 82 (1–2): 91–95.
- Bucknell, D.G., Gasser, R.B. and Beveridge, I. (1995). The prevalence and epidemiology of gastrointestinal parasites of horses in Victoria, Australia. International Journal for Parasitology, 25(6): 711-724.
- Buzato, M.C., Mitrea, I.L., Mironm L. and Ionita, M., (2015). Efficacy of two Anthelmintic Products on Strongyles in Horses from Stud Farms in Romania. Agriculture and Agricultural Science Procedia, 6: 293–298.
- BUZATU M.C., MITREA I.L., GRUIANU A. and IONI?? M. (2017). Investigating the Strongyle Populations, with Emphasis on Strongylus vulgaris (Nematoda: Strongylidae) in Romanian Horses, Based on Larval Cultures. Bulletin UASVM Veterinary Medicinem, 74(2): 186- 192.
- Chinwe, E.N., Amawulu, E., Francis, G. C. (2019). Survey of Gastrointestinal Parasites and Ectoparasites of Horses (Equine Equine) in Port Harcourt and Abarka Polo Field, South Southern Nigeria. International Journal of Science and Research, 8(8): 1471-1476.
- Getachew, M., Alemayehu, F., Chala, C., Amare, B., Kassa, D., Burden, F., Wernery, R. and Werner, U. A. (2014). Cross sectional sero-survey of some infectious diseases of working equids in Central Ethiopia. Journal of Veterinary Medicine and Animal Health, 2014 6(9): 231-238.
- Hasson, R.H. (2014). A study in the prevalence of horse "s helminthes parasitic infection in Baquba city, Digala.International Journal of Recent Scientific Research. 5(10): 1810-1813.
- Jajere, S.M., Lawal, J.R., Bello, A. M., Wakil, Y., Turaki, U.A and Waziri I. (2016). Risk Factors Associated with the Occurrence of Gastrointestinal Helminths among Indigenous Donkeys (Equus asinus) in Northeastern Nigeria. Scientifica (Cairo).
- Kornas, S., Marta, B., Vitalij, K. and Marta, S. (2010). Infection with large gastrointestinal parasites in slaughtered horses. Bull Veterinary Institute polawy, 54: 577-580.
- Lichtenfels J.R., Kharchenko V.A., Dvojnos G.M. (2008). Illustrated identification keys to strongylid parasites (Strongylidae: Nematoda) of horses, zebras and asses (Equidae). Veterinary Parasitology, 156: p. 4-161.
- Love S., Murphy D., Mellor D. (1999). Pathogenicity of cyathostome infection. Veterinary Parasitolgy, 85: p. 113-122.
- Majewska AC, Solarczyk P, Tamang L, Graczyk TK. (2004). Equine Cryptosporidium parvum infections in western Poland. Parasitol Res. 93(4): 274-8.
- Matto, T. N., Bharkad, G. P. and Bhat, S. A. (2015). Prevalence of gastrointestinal helminth parasites of equids from organized farms of Mumbai and Pune. J Parasit Dis. 39 (2):179–185.
- Mezgebu, T., Tafesi, K. and Tamiru, F. (2013). Prevalence of gastrointestinal parasites of horses and donkeys in and around Gondar town, Ethiopia. Open Journal of Veterinary Medicine, 2: 267-272.
- Ministry of Agriculture. Agriculture Statistical Year Book, 2017, Libya.
- Negash, W., Erdachew, Y. and Dubie T. 2021. Prevalence of Strongyle Infection and Associated Risk Factors in Horses and Donkeys in and around Mekelle City, Northern Part of Ethiopia. Veterinary Medicine International. 7 pages.
- OLI, N. (2016). PREVALENCE OF GASTROINTESTINAL PARASITES OF HORSES (Equuscaballus Linnaeus, 1758) IN SEVEN VDCS OF RUKUM DISTRICT, NEPAL. Master thesis, Tribhuvan University Kirtipur, Kathmandu. Nepal.
- Ravi Prasad D., Janak R.S. and Kopila W. (2021). Prevalence of gastrointestinal parasites in equines of Mustang District, Nepal. B I O D I V E R S I T A S, 22, (9): 3958-3963.
- Relf, V.E., Morgan, E.R., Hodgkinson, J.E., Matthews, J.B. (2013). Helminth egg excretion with regard to age, gender and management practices on UK thoroughbred studs. Parasitology. 140: 641–652.
- Solusby, E.J. L. (1982). Helminths, Arthropods and Protozoa of Domesticated Animals. 7th. ed. Bailliere Tindal. London.
- Tilahun, B., Nuraddis, I., Benti, D. and Tadele, T. (2014). Prevalence of helminth parasites of Horses in and around Hawasa town, southern Ethiopia. College of agriculture and Vet. Med. 5(1): 07-11.
- Umar, Y.A., Maikaje, D.B., Garba, U.M. and Alhassan, M.A.F. (2013). Prevalence of gastrointestinal parasites in horses used for cadets training in Nigeria. Journal of Veterinary Adv. 3(2): 43-48.
- Umur, ? and Aç?c?, M. A. (2009). survey on helminth infections of equines in the Central Black Sea region, Turkey. Turkish Journal of Veterinary and Animal Sciences, 33 (5): 373-378.
- Wannans, H.Y., Dawood, K.A. and Gassem, G.A. (2012). Prevalence of gastro- intestinal parasites of horses and donkeys in Diwaniyah. Qudisiya Journal of Vet. Med. Sci., 11(1): 147-155.
- Yadav, K.S., Shukla, P.C., Gupta, D.V and Mishra, A. (2014). Prevalence of gastrointestinal nematodes in horses of Jabalapur region. Research Journal for Veterinary Practioner. 2(3): 44-48.
Citation: Elmajdoub Layla O, Shimaa Sobhy Sorour, Mosaab A Omar and Alsaghir Om Assaed. (2022). Investigation and Prevalence of Gastrointestinal Parasites of Equestrian clubs Horses in Misurata, Libya. Archives of Veterinary and Animal Sciences 4(1).
Copyright: © 2022 Elmajdoub Layla O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.