Research Article
Volume 2 Issue 2 - 2020
In Vitro Differentiation of Human Mesenchymal Umbilical Cord Stem Cells into Hepatocytes
1Clinical pathology, Mansoura Research Center for Cord Stem Cells (MARC-CSC)-Faculty of Medicine- Mansoura University, Egypt
2Clinical immunology- clinical pathology department, Faculty of Medicine -Mansoura University, Egypt
3Gynecology and Obstetrics department, Faculty of Medicine - Mansoura University, Egypt
4Clinical immunology, clinical pathology department- Director of Mansoura Research Center for Cord Stem Cells (MARC-CSC), Faculty of Medicine - Mansoura University, Egypt
2Clinical immunology- clinical pathology department, Faculty of Medicine -Mansoura University, Egypt
3Gynecology and Obstetrics department, Faculty of Medicine - Mansoura University, Egypt
4Clinical immunology, clinical pathology department- Director of Mansoura Research Center for Cord Stem Cells (MARC-CSC), Faculty of Medicine - Mansoura University, Egypt
*Corresponding Author: Mervat Abd El-Haleem Dawood, Clinical Pathology, Mansoura Research Center for Cord Stem Cells (MARC-CSC)-Faculty of Medicine- Mansoura University, Egypt.
Received: March 13, 2020; Published: March 23, 2020
Back ground: The human umbilical cord (UC) is non-invasive, primitive and abundant sources of mesenchymal stromal cells (MSCs) that have increasingly. Liver disease is a major cause of mortality and morbidity in Egypt. There are many inflammatory liver conditions for which treatments are not effective and often such patients will progress to end-stage liver disease and require liver transplantation. To prevent progression to end-stage liver disease, mesenchymal stromal cells (MSCs) therapies have been considered and shown to have potential in such liver diseases.
Objectives: The aim of our study was to investigate the in vitro differentiation of human umbilical cord Wharton’s jelly (WJ-MSCs) into hepatocytes lineage.
Materials and Methods: human umbilical cord Wharton's jelly (WJ) were separated by mixed explant & enzymatic method by use of trypsin. The time required for the primary culture range from 10-14 days. The isolated cells were characterized for expression of MSC-specific markers such as CD73, CD90, CD146 and CD105 & negative for CD45.Also cells were counted by automated cell counter for stem cells (showing count, viability, cluster cells).After passage 4, the isolated cells induced to differentiate into hepatocyte-like cells by incubation in basal media with cocktail hepatocyte growth factors (HGFs) for 20 days. To confirm the in vitro hepatocyte differentiation process, the differentiated cells were evaluated by Periodic acid Schiff (P.A.S) for glycogen storage and Immunofluorescence analysis for intracellular granules of albumin.
Results: In vitro functional characterization of hepatocyte detectable by PAS staining for glycogen storage and immunofluorescent staining for albumin by anti-human albumin with FITC stain.
Conclusion: WJ-MSC is able to differentiate into functional hepatocyte like cells & serve as a cell source for tissue engineering and cell therapy for hepatic tissues.
Introduction
The liver is a vital organ that controls different essential biological processes in human body. These involve many hormones production, drug and toxin detoxification, glycogen storage, urea metabolism, plasma proteins synthesis and metabolism control. These functions are performed by hepatocytes. Therefore, the loss of hepatocytes results in inability to compensate major hepatic functions [1].
Healthy hepatic tissue has the ability to substitute damaged cells, however severely damaged lost its viability and stop functioning appropriately[2].
End stage liver failure develops owing to viral infection and hepatic carcinoma, which is lethal and represents a major cause of deaths globally. Liver transplants are used to manage a broad varieties of illnesses, such as liver cancers, hepatic cirrhotic, physically damaged, drug or alcohol abused acute liver failure and genetic liver disorders [3].
Regenerative medicine is energizing basic science and has the possibility for dramatic transformation of health care in the future. Although cell-mediated therapies have been used for hematological malignant disorders and different conditions, the potential application of cellular therapy for acute and chronic hepatic disorders has only more recently been discovered[4].
Mesenchymal stromal cells represent an attractive cell type for research and therapy owing to their capability for proliferation, differentiation, modulation of immune reactions and secretion of trophic factors. MSCs present in multiple tissues, such as bone marrow (BM), umbilical cord (UC) and adipose tissues [5].
Liver transplantation (LT) is the most appropriate therapeutic modality for hepatic failure cases. However, it is very restricted due to organ shortages, high expense, graft rejection, and the necessity for long-term immune-suppression [6].
The technology of turning MSCs from adult tissues into hepatocytes has increased the chance of acquiring transplantable hepatocytes without donor livers with subsequent rising of the interest in hepatology field [7].
UCs have the ability to reveal potential as a source of MSCs for several etiologies; they are considered medical wastes, and thus their usage in researches has minor ethical consideration; they have the ability for rapid proliferation in culture and are believed to be immune privileged [8].
Mesenchymal stromal cells harvested from the “young” WJ are considered more proliferative, immune-suppressive, and therapeutically active stem cells than those isolated from older, adult tissue sources including BM and adipose tissue [9].
Wharton's jelly derived MSC have the ability to suppress and avoid the immune system comparable to various MSCs kinds. They express MHC class I (HLA-ABC) at low levels only and co-stimulatory antigens including CD80, CD86 implicated in stimulation of T and B cell responses [10].
The immunoprofile of UC-MSCs as regards the International Society for Cellular Therapy [11] showing MSCs are positive for adhesion markers including CD29 and CD44; mesenchymal markers including CD90, CD73and CD105 and human leukocyte antigen class I (HLA-ABC). MSCs are negative for endothelial cell marker CD31; hematopoietic cell markers including CD34, CD45 and CD117; and human leukocyte differentiation antigen class II (HLA-DR) [12].
The objectives of the current study were to produce functioning hepatocytes like cells from human Wharton's jelly derived MSC in vitro.
This work was done in Mansoura Research Center for Cord Stem Cells (MARC-CSC) - Faculty of Medicine - Mansoura University-Egypt.
Methods
Ethical considerations
This study was approved by Institutional Research Board (IRB) – Mansoura university- Faculty of medicine (Code number: MD/62). All participant mothers in the study signed written informed consent.
This study was approved by Institutional Research Board (IRB) – Mansoura university- Faculty of medicine (Code number: MD/62). All participant mothers in the study signed written informed consent.
Processing of Human Umbilical Cord tissues and Isolating Cells:
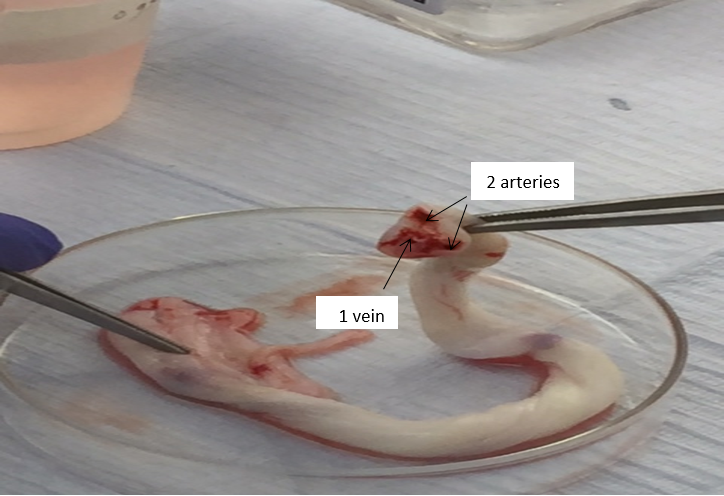
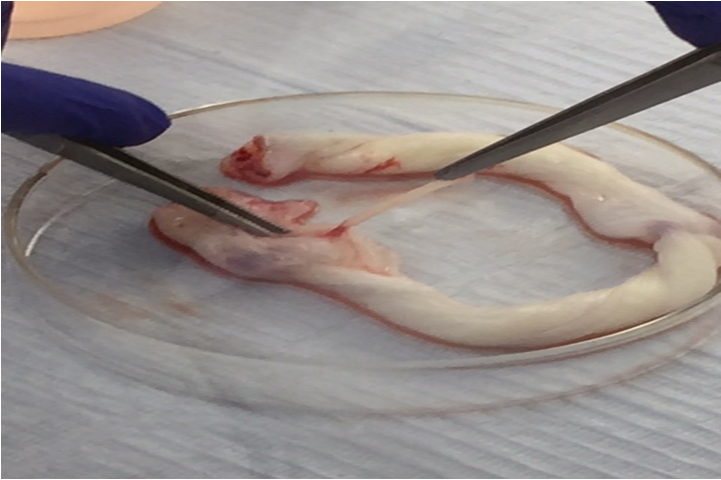
Aseptically collect samples of UC 10 – 15 cm in length, Immediately after C/S, the cords were transferred to sterile containers containing PBS W/O Mg, W/O Ca. & AB. transported to Mansoura Research Center for Cord Stem Cells (MARC-CSC) in Mansoura University (figure 1). The cells were extracted according to the technique of Beeravolu et al. [13] briefly, the UC was cut into 3-4 pieces, each 2-3cm cut longitudinally with a scalpel. Then, the exposed vein and arteries were removed by pulling. WJ was cut into 3 - 5mm pieces (Figure 2), the tissue pieces were incubated in commercial trypsin solution (0.05%) (Stem Cell technologies- 07910) at 37°C for 30 min in a 5% CO2 incubator for partial digestion of the samples. Addition of equivalent volume of complete medium (C.M.) was performed to neutralize the commercial trypsin, then the partially-digested tissue pieces15 - 20 were plated on a 75-cm2 tissue culture flask (Corning- 9075) and 10 mL of warmed C.M. (DMEM-F12, Hyclone +20% FBS, Gibco +1% AB penicillin streptomycin, Gibco) was added (figure 3).
Aseptically collect samples of UC 10 – 15 cm in length, Immediately after C/S, the cords were transferred to sterile containers containing PBS W/O Mg, W/O Ca. & AB. transported to Mansoura Research Center for Cord Stem Cells (MARC-CSC) in Mansoura University (figure 1). The cells were extracted according to the technique of Beeravolu et al. [13] briefly, the UC was cut into 3-4 pieces, each 2-3cm cut longitudinally with a scalpel. Then, the exposed vein and arteries were removed by pulling. WJ was cut into 3 - 5mm pieces (Figure 2), the tissue pieces were incubated in commercial trypsin solution (0.05%) (Stem Cell technologies- 07910) at 37°C for 30 min in a 5% CO2 incubator for partial digestion of the samples. Addition of equivalent volume of complete medium (C.M.) was performed to neutralize the commercial trypsin, then the partially-digested tissue pieces15 - 20 were plated on a 75-cm2 tissue culture flask (Corning- 9075) and 10 mL of warmed C.M. (DMEM-F12, Hyclone +20% FBS, Gibco +1% AB penicillin streptomycin, Gibco) was added (figure 3).
The tissue culture flasks were incubated at 37°C in a 5% CO2 incubator (Binder15-03520, Germany) for 2 - 3 days without disturbance to permit the adherence of tissue pieces. Following three days of incubation, the CM was changed & after 7 - 10 days of incubation, tissues were removed.
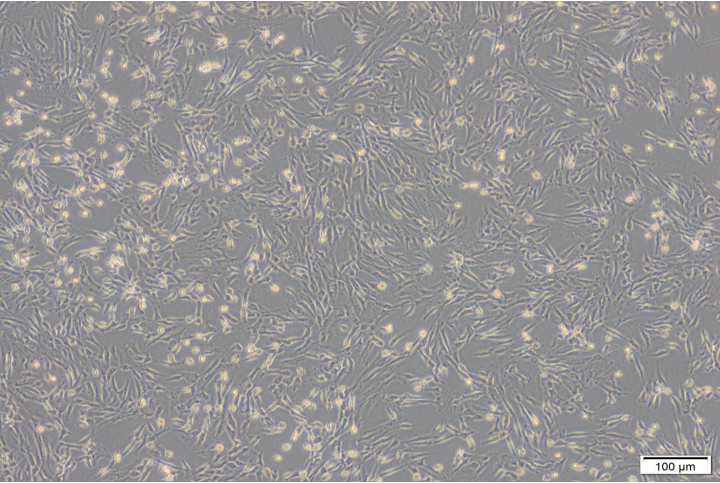
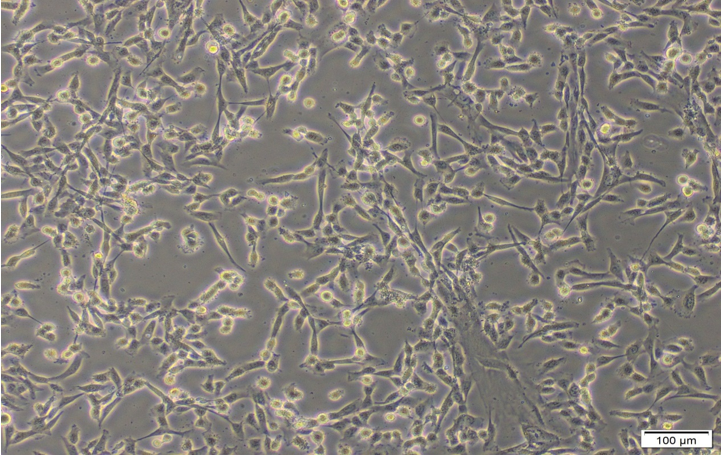
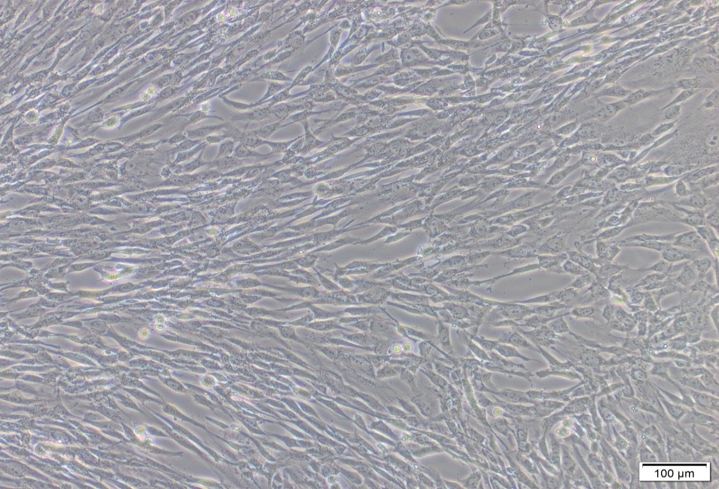
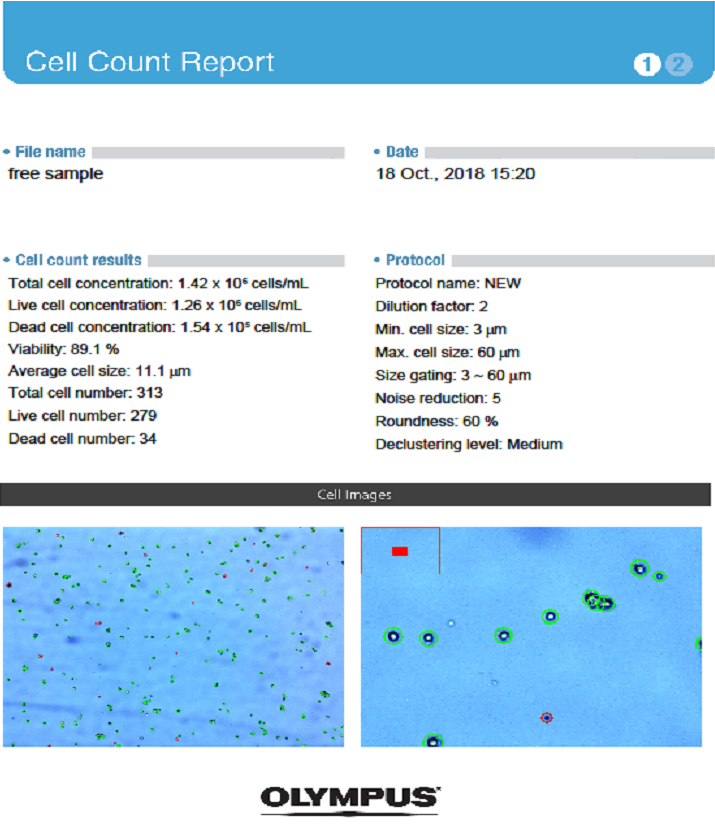
Under inverted microscope, after 17-23 days- the Cells growths were reached 70-80 % of confluence (Olympus SN.7F41627, Japan). The cells were sub cultured to many passages until P4-P8 (figure 4, 6-9). The cells were counted by automated cell counter (Olympus, R1-163-0914 and Japan) for counting & viability (figure 5).

Figure 3: The partially- digested tissue pieces about 15 - 20 on a 75-cm2 tissue culture flask in CM in CO2 incubator.
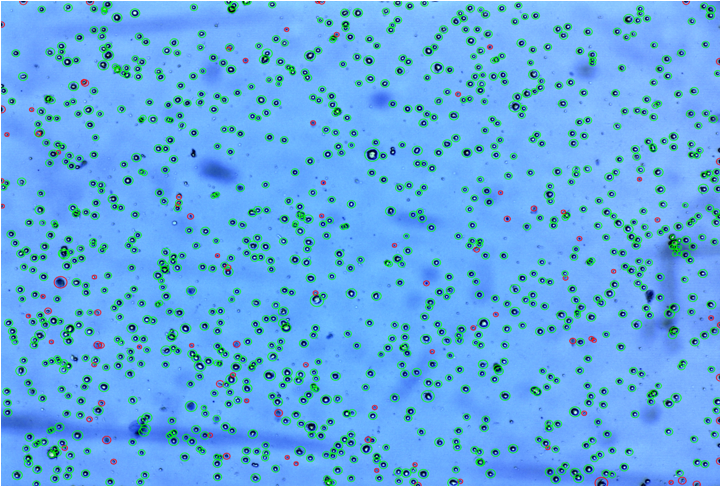
Figure 5: Total cells by automated cells counter (Viability of cells showing live cells with green circle &dead cells with red circle).
Characterization of the isolated cells by flowcytometery (Becton, Dickinson BD Accuri C6)
According to the manufacturer’s instructions (Human Multipotent Mesenchymal Stromal Cell Multi-Color Flow Cytometry Kit Cat. No: FMC002, R&D): incubated 100 μg/106 cells with antibodies conjugated with different fluorescent probes for 30 min in dark at room temperature. The antibodies utilized were: (CD105, CD73, CD90 and CD146 as positive markers and CD45 as negative marker) (Figure10)
According to the manufacturer’s instructions (Human Multipotent Mesenchymal Stromal Cell Multi-Color Flow Cytometry Kit Cat. No: FMC002, R&D): incubated 100 μg/106 cells with antibodies conjugated with different fluorescent probes for 30 min in dark at room temperature. The antibodies utilized were: (CD105, CD73, CD90 and CD146 as positive markers and CD45 as negative marker) (Figure10)

Figure 10: Immunophenotypic analysis of WJ-MSCs representing the flow cytometry performed on the WJ-MSCs shows: CD45 were negative, CD90, CD105, CD73 and CD146 were positive.
Differentiations of MSCs into hepatocytes
According to the technique of Campard et al. [14]: UCMSCs (from passage 4 to 8) were seeded at a density of 1.5 x 104 cells/cm2 in 24-well plates coated with 5µ/cm2 of rat tail collagen type I (Sigma -Aldrich, Cat. No. C3867, USA).The cells were supplemented in DMEM- low glucose supplemented with 20% fetal bovine serum and penicillin and streptomycin. Culture medium was switched for one day to IMDM (Iscove’s Modified Dulbecco’s Medium) (Sigma life science, Cat. No. 16529, UK) containing 20 ng/mL epidermal growth factor (R&D, Cat. No.233-FB-025, England) and 10 ng/mL b-FGF (R&D, Cat. No.236-EG-200, England) for two days. Step 1: Induction of differentiation was stimulated through culturing UCMSCs for ten days with IMDM containing 20 ng/mL HGF (R&D, Cat. No.294-HG-005, England), 10 ng/mL b-FGF, 0.61 g/L nicotinamide (Sigma-Aldrich, Cat. No.N0636, USA) and 1% insulin-transferrin-selenium premix (ITS-P) (Sigma -Aldrich, Cat. No. I3146, USA). The terminal maturation step: composed of management with IMDM containing 20 ng/mL oncostatin M (Sigma-Aldrich, Cat. No. H6541, USA), 1µmol/L dexamethasone (Sigma -Aldrich, Cat. No.D4902, USA), and 1% ITS-P for 10 days. For all steps, changing the medium was performed every three days.
According to the technique of Campard et al. [14]: UCMSCs (from passage 4 to 8) were seeded at a density of 1.5 x 104 cells/cm2 in 24-well plates coated with 5µ/cm2 of rat tail collagen type I (Sigma -Aldrich, Cat. No. C3867, USA).The cells were supplemented in DMEM- low glucose supplemented with 20% fetal bovine serum and penicillin and streptomycin. Culture medium was switched for one day to IMDM (Iscove’s Modified Dulbecco’s Medium) (Sigma life science, Cat. No. 16529, UK) containing 20 ng/mL epidermal growth factor (R&D, Cat. No.233-FB-025, England) and 10 ng/mL b-FGF (R&D, Cat. No.236-EG-200, England) for two days. Step 1: Induction of differentiation was stimulated through culturing UCMSCs for ten days with IMDM containing 20 ng/mL HGF (R&D, Cat. No.294-HG-005, England), 10 ng/mL b-FGF, 0.61 g/L nicotinamide (Sigma-Aldrich, Cat. No.N0636, USA) and 1% insulin-transferrin-selenium premix (ITS-P) (Sigma -Aldrich, Cat. No. I3146, USA). The terminal maturation step: composed of management with IMDM containing 20 ng/mL oncostatin M (Sigma-Aldrich, Cat. No. H6541, USA), 1µmol/L dexamethasone (Sigma -Aldrich, Cat. No.D4902, USA), and 1% ITS-P for 10 days. For all steps, changing the medium was performed every three days.
Assay methods for differentiated MSCs into hepatocyte like cells:
1. Periodic Acid–Schiff Staining for Glycogen Accumulation according to manufacture of kit (Sigma,cat.no.395B,USA):
The cells were fixed by 4% formaldehyde. Incubation of the cells for ten minutes in 1% periodic acid were done, and then cleaned with distilled water. The cells were incubated with Schiff’s reagent for 15 min. Following ten minutes, cells were washed in water. Karazi’s hematoxylin counterstain was conducted for ten minutes and after that washed. The cells were examined under inverted microscope for glycogen storage inside cells.
1. Periodic Acid–Schiff Staining for Glycogen Accumulation according to manufacture of kit (Sigma,cat.no.395B,USA):
The cells were fixed by 4% formaldehyde. Incubation of the cells for ten minutes in 1% periodic acid were done, and then cleaned with distilled water. The cells were incubated with Schiff’s reagent for 15 min. Following ten minutes, cells were washed in water. Karazi’s hematoxylin counterstain was conducted for ten minutes and after that washed. The cells were examined under inverted microscope for glycogen storage inside cells.
2. Immunocytochemistry for Cellular serum albumin
The cells were washed two times with PBS (1 mL/well for a 24-well plate). The cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature. The cells were cleaned three times with 1% BSA in PBS for five minutes (0.5 mL/well for a 24-well plate). Then the instructions were followed according to manufacture of kit included Anti-Human Serum Albumin antibody (R&D, Cat. No. SC033, USA) then fluorescein- coupled goat anti mouse immunoglobulin secondary antibody for one hour.
The cells were washed two times with PBS (1 mL/well for a 24-well plate). The cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature. The cells were cleaned three times with 1% BSA in PBS for five minutes (0.5 mL/well for a 24-well plate). Then the instructions were followed according to manufacture of kit included Anti-Human Serum Albumin antibody (R&D, Cat. No. SC033, USA) then fluorescein- coupled goat anti mouse immunoglobulin secondary antibody for one hour.
Results
Count & viability
Cells count & viability from 1ry culture to passage 8 was determined in range from 70.0% to 90.0% by automated cell counter (figure 11).
Cells count & viability from 1ry culture to passage 8 was determined in range from 70.0% to 90.0% by automated cell counter (figure 11).
Immuno phenotypically Characteristics of WJ-MSCs Shows mean expressions of WJ-MSCs by flow cytometry for CD105 which show positive with percentage of (95.4%), CD73 which show positive with percentage of (99.0%), CD90 which show positive with percentage of (88.2%), CD146 which show positive with percentage (54.4%) and CD45 which show negative with percentage of (98.1%) (Figure 10).
Detection of Glycogen Storage by Periodic acid-Schiff Staining Assay
An important role of hepatic cells is glycogen storage. Glycogen in tissues can be recognized by staining with PAS reagent, (with glycogen oxidized to aldehyde), creating a purple color. Outcomes of the present experiment with PAS staining showed that WJ-MSCs 20 days following differentiation had purple stain (Figure12). The results showed that the differentiated WJ-MSCs into hepatocyte like cells had elevated glycogen storage.
An important role of hepatic cells is glycogen storage. Glycogen in tissues can be recognized by staining with PAS reagent, (with glycogen oxidized to aldehyde), creating a purple color. Outcomes of the present experiment with PAS staining showed that WJ-MSCs 20 days following differentiation had purple stain (Figure12). The results showed that the differentiated WJ-MSCs into hepatocyte like cells had elevated glycogen storage.
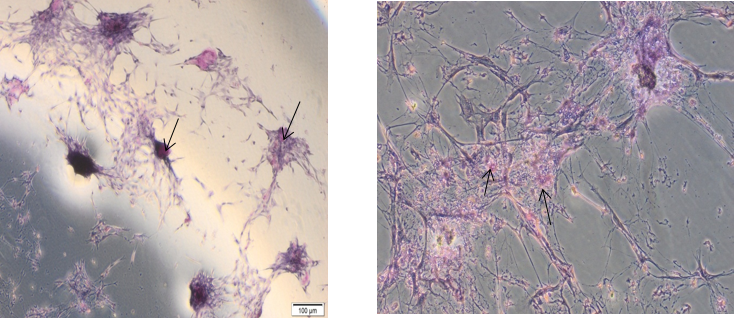
Figure 12: Periodic acid-Schiff staining for differentiated WJ-MSCs into hepatocyte like cells showed Glycogen storage presented by red color in cells.
Immunohistochemical evidence of albumin expression in differentiated WJ-MSCs
The outcomes of immunofluorescence analysis of albumin expression in undifferentiated as well as differentiated MSCs. Following 20 days culture in the hepatogenic differentiation medium, expression of albumin was detected in almost all of the differentiated –MSCs into hepatocyte like cells presented by green fluorescence dots (Figure 13).
The outcomes of immunofluorescence analysis of albumin expression in undifferentiated as well as differentiated MSCs. Following 20 days culture in the hepatogenic differentiation medium, expression of albumin was detected in almost all of the differentiated –MSCs into hepatocyte like cells presented by green fluorescence dots (Figure 13).
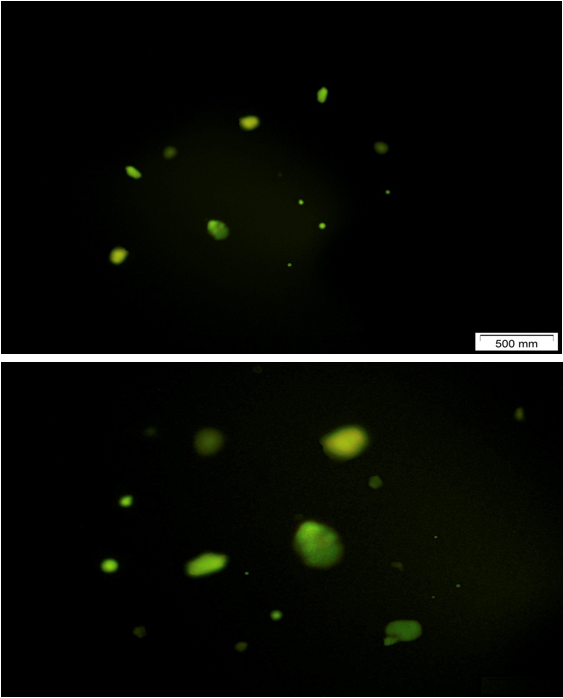
Figure 13: Immunofluorescence staining for differentiated WJ-MSCs into hepatocyte like cells showed albumin stained fluorescence green dots inside cells.
Discussion
The ability of MSCs from several sources to undergo trans-differentiation and maturation into hepatocyte-like cells has laid down the solid foundation for application of MSCs as a substitution for donor liver to meet the major requirement in transplanted hepatocytes. Several clinical and pre-clinical researches were carried out in the usage of MSCs for management of end stages of hepatic disorders with different degrees of successfulness [7].
The aim of the current study was to produce functioning hepatocyte like cells from human umbilical Wharton’s jelly derived MSC. The main findings displayed that WJ-MSCs may be triggered to differentiate into functioning hepatocyte like cells (PAS staining for glycogen storageand immunofluorescence analysisfor intra cellular albumin granules).
The outcomes of the procedure evaluated that incomplete digestion of UC tissue pieces 3-5mm provided outgrowth of cells from pieces without induction of cellular injury, preserved viability, and provided major quantities of MSCs but the use of mixed enzymes for inappropriate time & amount might lead to cell lysis, affecting cell proliferation & adherence to tissue culture flask.
These results agreed with Beeravolu et al. [13]who confirmed that explant- trypsin method is a simple approach for the dissection of the UC for the isolation of MSCs and revealed that the incomplete digestion of tissue pieces 1-2 mm in size by utilizing a commercial trypsin solution provided outgrowth of cells without causing extensive cell damage, maintained viability cells from the explant tissues and yielded greater quantities of MSCs.
However, Trivanovic et al. [15] recorded those greater tissue explants of UC about 10 mm in size were optimum for cell isolation.
In our study, we evaluated the efficacy of MSCs isolation protocol from human UC-WJ and determine the number of available cells & their viability from passage 0 to passage 8 with increasing in count.
In our study, we obtained sufficient number of live MSCs to transplant into an adult ranging from 1.7 x 106 to 3.6 x 106 with cord segment of 8-10cm. Thus a normal term UC of 30-50 cm can generate high numbers of cells for clinical application.
These results agreed with De Bruyn et al. [16]who obtained 2.7 x 106 cells at passage 1, at passage 2 were 4.6 x 106 and passage 3were 7.7 x 106.
In our study, the cultured cells from explant-trypsin method were analyzed by flow cytometry and the results showed that the cells were +ve for MSCs surface markers CD90, CD105, CD73 and CD146 with 88.2%, 95.4%, 99.0% and 54.4% respectively and negative for hematopoietic cell surface markers CD45 with 98.1%.
These results was explained as regards to MSCs criteria described by the ISCT as positive for specific cell surface markers (CD90,CD105,CD73) and absence CD45 [17].
The current study revealed that the typical pericyte markers CD146 were expressed in WJMSC belonging to MSCs family surround the blood vessels.
These results agreed with Campard et al. [14] who showed that the cells were positive for CD90 & CD73 with high expression level 99.5% and 81.6% respectively. However, CD105 was expressed positive marker only with 28.8% may be depending on the different location in the cord where the cells are removed.
Magar et al. [18]agreed with our results in which WJ-MSCs were positive for CD90 with percentage 88.21% and negative for the hematopoietic markers CD45 with percentage 99.39%.
In the present study, the hepatic differentiation potential of our WJ-MSCs was assessed among the 4th passage to the 8th passage by combination of HGF, basic-FGF, Oncostatin M, Dexamethasone and Nicotinamide which markedly improves the invitro maturation of MSCs into hepatocyte like cells.
To be sure that this is a functioning hepatocyte like cells, several criteria must be fulfilled:
- Functional assays of Hepatocyte like cells by periodic acid Schiff staining (PAS) after 20 days of growth in hepatogenic culture media(figure 12).
Our study showed that differentiated WJ-MSCs into Hepatocyte like cells became positive by PAS staining for glycogen storage producing a purple color which represents the main functions of mature hepatocytes on day 20 after differentiation, while undifferentiated MSCs did not have these hepatogenic features.
This result agreed with Doan et al. [19]in whichcells were analyzed for hepatocyte differentiation by PAS staining for glycogen accumulation showing positively stained cells in compared to MSCs as negative control.
In agreement with our dataKao et al. [20]who detected the glycogen inside cells by staining with PAS reagent producing a purple color.
- Immunofluorescence analysis for intracellular granules of albumin (the abundant protein created by mature hepatocytes) revealed that undifferentiated MSCs stained -ve for cellular albumin, but +ve staining was visualized for hepatocyte like cells after 20 days of differentiations presented by fluorescence green dots inside cells (figure 13).
This result agreed withYu et al. [21]who detected hepatocyte - specific marker expression by immune-fluorescence. UC?MSCs became positive for ALB after they were incubated in hepatic differentiation medium for 4 weeks.
In conclusion
Our findings indicate that MSCs derived from UC-WJ can differentiate into functioning hepatocyte-like cells in vitro. The outcomes of this study continue to challenge the perspective future for liver diseases regeneration in vivo. Essentially, MSCs might act as a cell source for tissue engineering or cell therapy of hepatic diseases. Finally, UCMSCs appear to be a technically and ethically appropriated extra hepatic source for generation of hepatocyte-like cells.
Recommendations
- We recommend the use of WJMSCs in the development of functional hepatocytes in vitro as a cell line for research use.
- WJMSCs exhibit low immunogenic features, thus we recommend performing a clinical trial as a pilot study on liver disease patient in Mansoura university hospital.
- We recommend the use of UC tissues as a valid source for MSCs in transplantation for therapy.
Conflict of interest statement
This work funded by the science and Technology Development fund (STDF) project no. 5202 under super vision of prof. Dr. Farha El-chennawi.
This work funded by the science and Technology Development fund (STDF) project no. 5202 under super vision of prof. Dr. Farha El-chennawi.
Acknowledgments
Thanks to all team workers at Mansoura Research Center for Cord Stem Cells (MARC-CSC) in faculty of medicine Mansoura university-Egypt.
Thanks to all team workers at Mansoura Research Center for Cord Stem Cells (MARC-CSC) in faculty of medicine Mansoura university-Egypt.
References
- Prasajak P and Leeanansaksiri W. (2014). Mesenchymal Stem Cells: Current Clinical Applications and Therapeutic Potential in Liver Diseases. J Bone Marrow Res., 2(1): 137.
- Upadhyay R.K. (2017): Stem Cell Therapeutics of Acute Liver Diseases, Transplantation and Regeneration. J. Cell Sci. Ther., 8(4): 275.
- Herrero A., Prigent J., Lombard C., Rosseels V., Daujat-Chavanieu M.Breckpot K., Najimi M., Deblandre G and Sokal E.M. (2016): Adult-derived human liver stem/progenitor cells infused 3 days post-surgery improve liver regeneration in a mouse model of extended hepatectomy. Cell Transplant, 26(2): 351-364.
- Robert C., Huebert M.D., and Jorge Rakela M.D. (2014): Cellular Therapy for Liver Disease. Mayo. Clin. Proc., 89(3): 414-424.
- Wang Y., Yu X., Chen E. and Li L. (2016): Liver-derived human mesenchymal stem cells: a novel therapeutic source for liver diseases. Stem Cell Research & Therapy, 7(1):71.
- Zhang Y., Li Y., Zhang L., Li J. and Zhu C. (2018): Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis. Stem Cell Research & Therapy, 9(1):59.
- Li Z., He C., Xiao J. and Chen Z.Y. (2013): Treating end-stage liver diseases with mesenchymal stem cells: an oak is not felled at one stroke. OA Tissue Engineering, 1(1):3.
- Deuse T., Stubbendorff M., Tang-Quan K., Phillips N., Kay M.A., Eiermann T., Phan T.T., Volk H.D., Reichenspurner H.& Robbins R.C. (2011): Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant, 20: 655–667.
- Kim D.W., Staples M., Shinozuka K., Pantcheva P., Kang S. D. and Borlongan C.V. (2013): Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci., 14(6):11692-11712.
- Prasanna S.Jand Jahnavi V.S. (2011): Wharton’s jelly mesenchymal stem cells as off-the -shelf cellular therapeutics: A closer look into their regenerative and immunomodulatory properties. Open Tissue Eng. Regen. Med. J., 4: 28–38.
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D.j. and Horwitz E.(2006): Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4): 315-317.
- Seo K.W., Lee S.R., Bhandari D.R., Roh K.H., Park S.B., So A.Y., Jung J.W., Seo M.S., Kang S.K., Lee Y.S. & Kang K.S. (2009): OCT4A contributes to the stemness and multi-potency of human umbilical cord blood-derived multipotent stem cells (hUCB-MSCs). Biochem. Biophys Res. Commun., 384(1): 120-125.
- Beeravolu N., McKee C., Alamri A., Mikhael S., Brown C., Cruet M. and Chaudhry R. (2017): Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J.Vis. Exp., 122: 1 -13.
- Campard D., Lysy P.A., Najimi, M. and Sokal E.M.( 2008): Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology, 134: 833–848.
- Trivanovic D., Kocic J., Mojsilovic S., Krstic A.,Iliac V. , Djordjevic I.O., Santibanez J.F., Jovcic G., Terzic M and Bugarski D. (2013): Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton's jelly. Srp Arh Celok Lek, 141 (3-4): 178-186.
- De Bruyn C., Najar M., Raicevic G., Meuleman N., Pieters K., Stamatopoulos B., Delforge A., Bron D. and Lagneaux L. (2011): A Rapid, Simple, and Reproducible Method for the Isolation of Mesenchymal Stromal Cells from Wharton’s Jelly Without Enzymatic Treatment. Stem cells and development, 20(3): 547-557.
- Bai L., Li D., Li J., Luo Z., Yu S., Cao S., Shen L., Zuo Z. and Ma X. (2016): Bioactive molecules derived from umbilical cord mesenchymal stem cells. Acta Histochemica, 118: 761-769.
- Magar R. W., Ismail A. M., Barakat N. M., Elghzaly A. A. , Khater S. M. and El-chennawi F. A. (2017): Hyaluronic Acid Potentiates the Renoprotective Effects of Wharton Jelly –Derived Mesenchymal Stem Cells In a rat Model of Renal Ischemia Reperfusion Injury. International Journal of Scientific & Engineering Research, 8(10)165-174.
- Doan C.C., Le T.L. , Hoang N.S., Ky D.N., Nguyen H.C., Le V.D. and Do M.S. (2013): Differentiation of Human Umbilical Cord Lining Membrane-Derived Mesenchymal Stem Cells into Hepatocyte-Like Cells. ISRN Stem Cells, Volume 2013:10 pages.
- Kao S.Y., Shyu J.F. and Wang H.W. (2015): Transplantation of Hepatocyte-like Cells Derived from Umbilical Cord Stromal Mesenchymal Stem Cells to Treat Acute Liver Failure Rat, J Biomedical Sci., 7(1:2).
- Yu Y.B., SONG Y., CHEN Y., ZHANG F. and QI F. Z. (2018): Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Molecular Medicine Reports, 18(2): 2009-2016.
Citation: Mervat Abd EL-Haleem Ahmed Dawood. (2020). In Vitro Differentiation of Human Mesenchymal Umbilical Cord Stem Cells into Hepatocytes. Journal of Medical Research and Case Reports 2(2).
Copyright: © 2020 Mervat Abd EL-Haleem Ahmed Dawood. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.