Research Article
Volume 2 Issue 1 - 2020
Formulation and In Vitro Evaluation of Nifedipine Suppositories for Geriatric and Severely Ill Patients with Hypertension
1Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
2Department of Herbal Medicine, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
2Department of Herbal Medicine, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
*Corresponding Author: Dr. Mariam El Boakye-Gyasi, Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Received: August 26, 2020; Published: September 08, 2020
Abstract
Hypertensive patients with swallowing difficulties such as geriatrics and the severely ill require alternative means of continuing oral Nifedipine medications. Current methods used present challenges including increase in undesirable side effects of Nifedipine. This study seeks to provide a safe, convenient and easy to administer alternative to oral nifedipine for these patients. A survey conducted among healthcare givers in three hospitals in Ghana, revealed the use of non-conventional methods such as crushing of Nifedipine tablets and passing through feeding tubes or mixing with food for patients with swallowing difficulty. Moulding from a melt was used to prepare Nifedipine suppositories with polyethylene glycol and glycerogelatin bases respectively. Cracks, pitting, dark regions, holes and exudation were not observed on the suppositories. Acceptable disintegration and uniformity of weight, as well as nifedipine content within the acceptable range of 85-105% were recorded. In vitro dissolution profiles showed the polyethylene glycol based suppositories have a higher cumulative drug release (92.49%) than the glycerogelatin based suppository (89.07%). Stability studies over a period of nine (9) months showed no significant change in physicochemical properties. Polyethylene glycol (6000) or Glycerogelatin based Nifedipine suppository could be a better alternative to oral Nifedipine for patients with swallowing difficulties.
Keywords: Hypertension; Nifedipine Suppository; Geriatric; Polyethylene glycol; Glycerogelatin
Introduction
Hypertension, an age related disease is highly prevalent in the elderly. Hypertension is a global issue that affects about 15-20% of adults. In Ghana nearly one out of every five adults has hypertension [1]. Increase in life expectancy due to improved standards of living as well as access to good quality healthcare is expected to increase the geriatric proportion of the global population and further increase the prevalence of hypertension. It is considered as one of the most vital preventable causes of untimely morbidity and mortality [2].
Change in lifestyle is the first line therapy in the control of hypertension. A combination of lifestyle changes and drug therapy is the second line of therapy when the first line is unable to control the Blood pressure. In the management of hypertension in the elderly, Africans and African Americans, Nifedipine, which is a Calcium channel blocker, is used because of its reported effectiveness [4].
Currently available dosage forms of Nifedipine for hypertensive patients are tablets and capsules all of which are to be swallowed whole. A study in the Kumasi metropolis of Ghana indicated that modified release Nifedipine was the most prescribed antihypertensive for patients with high blood pressure in the study area [5]. However, these dosage forms are certainly not suitable for all categories of patients.
Several reports indicate that crushing of modified release Nifedipine tablets and subsequent administration through feeding tubes has led to the death of elderly hypertensive patients who are unable to swallow the oral tablet [6]. Several dosage forms can be explored to this effect but this study focuses on suppository as the alternative dosage form.
Suppositories offer an advantage of providing an alternative means of administering medications to patients when the oral route cannot be used. In addition, first pass elimination of high clearance drugs by the hepatic system may be partially avoided. These advantages have significantly increased the use of suppositories in both geriatric and paediatric patients [7]. Therefore, the need for a suitable, convenient and alternative dosage form to oral Nifedipine has become imperative and this serves as the basis for the present study.
Materials and Methods
Materials
Polyethylene glycol 6000 (Sigma Aldrich), Nifedipine powder (Anhui Jinao Chemical Co. Ltd), Glycerin (Sigma Aldrich) and gelatin (bloom 180 and 60) from Sigma Aldrich. All reagents used in the study were of analytical grade.
Polyethylene glycol 6000 (Sigma Aldrich), Nifedipine powder (Anhui Jinao Chemical Co. Ltd), Glycerin (Sigma Aldrich) and gelatin (bloom 180 and 60) from Sigma Aldrich. All reagents used in the study were of analytical grade.
Survey
A cross sectional survey on the use of Nifedipine in the treatment of Hypertension in the elderly and severely ill was conducted in three Government hospitals within the Kumasi Metropolis, Ashanti Region, Ghana. Purposive sampling was used to recruit respondents needed for the survey. The study participants who were care givers consisted of seventy (70) Medical Doctors, sixty-nine (69) Pharmacists and one hundred and sixty-one (161) Nurses. These study participants gave their consent for the study and were assured a strict confidentiality of the information they provided. Questionnaires consisting of open and close-ended questions were administered to the respondents. Prior to the administration of the survey, participatory and undeclared pretesting of the questionnaires were done with a Cronbach’s alpha of 0.912.
A cross sectional survey on the use of Nifedipine in the treatment of Hypertension in the elderly and severely ill was conducted in three Government hospitals within the Kumasi Metropolis, Ashanti Region, Ghana. Purposive sampling was used to recruit respondents needed for the survey. The study participants who were care givers consisted of seventy (70) Medical Doctors, sixty-nine (69) Pharmacists and one hundred and sixty-one (161) Nurses. These study participants gave their consent for the study and were assured a strict confidentiality of the information they provided. Questionnaires consisting of open and close-ended questions were administered to the respondents. Prior to the administration of the survey, participatory and undeclared pretesting of the questionnaires were done with a Cronbach’s alpha of 0.912.
Data processing and analysis of survey
Information gathered from the survey was collated, coded and analyzed using the version 16 of Statistical Package for Social Science (SPSS) software.
Information gathered from the survey was collated, coded and analyzed using the version 16 of Statistical Package for Social Science (SPSS) software.
Formulation and Characterization of nifedipine suppositories
Identity test for nifedipine
Identification tests were carried out on pure Nifedipine powder obtained by methods specified in the British Pharmacopoeia [8].
Identity test for nifedipine
Identification tests were carried out on pure Nifedipine powder obtained by methods specified in the British Pharmacopoeia [8].
Melting pointing determination
The melting point was determined using the Stuart melting point apparatus. Nifedipine powder was packed into a capillary tube. The capillary tube was placed in the melting point apparatus and the temperature switch was turned on. A magnifying lens was used to observe the temperature at which the nifedipine powder melted.
The melting point was determined using the Stuart melting point apparatus. Nifedipine powder was packed into a capillary tube. The capillary tube was placed in the melting point apparatus and the temperature switch was turned on. A magnifying lens was used to observe the temperature at which the nifedipine powder melted.
FTIR of Nifedipine powder
The functional groups present in Nifedipine was confirmed using Fourier Transform Infrared Spectroscopy at the scanning range of 4000- 400 cm-1.
The functional groups present in Nifedipine was confirmed using Fourier Transform Infrared Spectroscopy at the scanning range of 4000- 400 cm-1.
Compatibility studies of drug and excipients
Drug and suppository base interactions were studied. The spectra were recorded for pure drug (Nifedipine), medicated suppository and unmedicated suppository with both PEG (6000) and glycerogelatin at the scanning range of 4000-400 cm-1. The FTIR spectra of drug with bases were superimposed with the FTIR spectrum of the pure drug for comparison.
Drug and suppository base interactions were studied. The spectra were recorded for pure drug (Nifedipine), medicated suppository and unmedicated suppository with both PEG (6000) and glycerogelatin at the scanning range of 4000-400 cm-1. The FTIR spectra of drug with bases were superimposed with the FTIR spectrum of the pure drug for comparison.
Preparation of nifedipine suppositories
Suppositories formulation was done using moulding from a melt method [9,10]. A 2g mould was used in preparing the suppositories (30 mg of nifedipine was incorporated into each suppository). Water-soluble bases; polyethylene glycol (6000) and Glycerogelatin were used in preparing the suppositories taking into account the displacement value of nifedipine in each base. The displacement value of nifedipine in polyethylene glycol was 1.17 and that of glycerogelatin was 1.19. The prepared suppositories were packaged in aluminum foil and stored in airtight containers in a humidity chamber for further investigation.
Suppositories formulation was done using moulding from a melt method [9,10]. A 2g mould was used in preparing the suppositories (30 mg of nifedipine was incorporated into each suppository). Water-soluble bases; polyethylene glycol (6000) and Glycerogelatin were used in preparing the suppositories taking into account the displacement value of nifedipine in each base. The displacement value of nifedipine in polyethylene glycol was 1.17 and that of glycerogelatin was 1.19. The prepared suppositories were packaged in aluminum foil and stored in airtight containers in a humidity chamber for further investigation.
External and Internal Appearance of formulated suppositories
Four suppositories from each batch were physically examined using the following parameters; odour, colour, surface condition (cracks, pitting) and shape. Two suppositories were randomly selected from each batch. Each suppository was cut longitudinally into two halves and the internal surfaces examined with a hand lens.
Four suppositories from each batch were physically examined using the following parameters; odour, colour, surface condition (cracks, pitting) and shape. Two suppositories were randomly selected from each batch. Each suppository was cut longitudinally into two halves and the internal surfaces examined with a hand lens.
Uniformity of weight and disintegration tests
The uniformity of weight and disintegration tests were determined using pharmacopeial methods [8].
The uniformity of weight and disintegration tests were determined using pharmacopeial methods [8].
Drug content determination
One randomly selected suppository from each formulation batch (Polyethylene glycol formulation and glycerogelation formulation) was weighed, crushed and dissolved in 100 mL of phosphate buffer (7.4). The resulting mixture was filtered and made up to 200 mL using phosphate buffer (pH 7.4) in a volumetric flask. The final solution was diluted and the absorbance was measured at 238 nm using phosphate buffer as blank. This procedure was repeated on nine other suppositories from each batch [11,12].
One randomly selected suppository from each formulation batch (Polyethylene glycol formulation and glycerogelation formulation) was weighed, crushed and dissolved in 100 mL of phosphate buffer (7.4). The resulting mixture was filtered and made up to 200 mL using phosphate buffer (pH 7.4) in a volumetric flask. The final solution was diluted and the absorbance was measured at 238 nm using phosphate buffer as blank. This procedure was repeated on nine other suppositories from each batch [11,12].
In vitro dissolution study
The dissolution study was carried out using the USP basket dissolution apparatus I. Each of the vessels of the dissolution apparatus was filled with 900 mL of freshly prepared phosphate buffer of pH 7.4 and maintained at 37±1°C. Stirring was at a speed of 100 revolutions per minute. At specified time intervals (10, 20, 30, 45, 60, 90, 120, 180 and 240 minutes) 5 mL sample was withdrawn, filtered, diluted and transferred into appropriately labelled amber coloured bottles. The 5 mL aliquot was replaced with equal volume of fresh dissolution medium to maintain sink conditions. The absorbances of these solutions were determined using ultra violet spectrometry at a wavelength of 238 nm. The absorbances recorded were used to determine the percentage cumulative drug released at each time interval [11,12,13].
The dissolution study was carried out using the USP basket dissolution apparatus I. Each of the vessels of the dissolution apparatus was filled with 900 mL of freshly prepared phosphate buffer of pH 7.4 and maintained at 37±1°C. Stirring was at a speed of 100 revolutions per minute. At specified time intervals (10, 20, 30, 45, 60, 90, 120, 180 and 240 minutes) 5 mL sample was withdrawn, filtered, diluted and transferred into appropriately labelled amber coloured bottles. The 5 mL aliquot was replaced with equal volume of fresh dissolution medium to maintain sink conditions. The absorbances of these solutions were determined using ultra violet spectrometry at a wavelength of 238 nm. The absorbances recorded were used to determine the percentage cumulative drug released at each time interval [11,12,13].
Stability studies
Stability studies were carried out according to the ICH guidelines on the formulated suppositories at 4°C/ 60±5% RH (refrigerated temperature) as well as 27°C/ 65±5% RH (room temperature). Physical appearance tests, disintegration, drug content as well as dissolution tests were subsequently carried out on the formulated suppositories at three monthly intervals for a period of nine months [14,15].
Stability studies were carried out according to the ICH guidelines on the formulated suppositories at 4°C/ 60±5% RH (refrigerated temperature) as well as 27°C/ 65±5% RH (room temperature). Physical appearance tests, disintegration, drug content as well as dissolution tests were subsequently carried out on the formulated suppositories at three monthly intervals for a period of nine months [14,15].
Results and Discussions
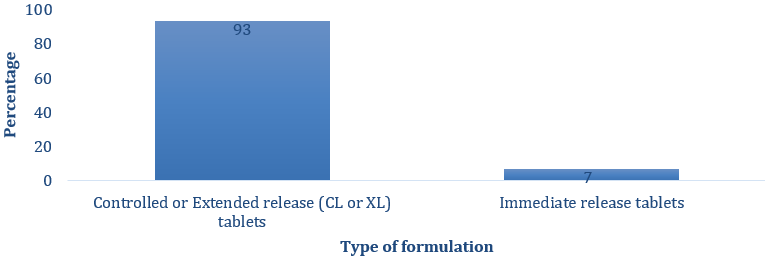
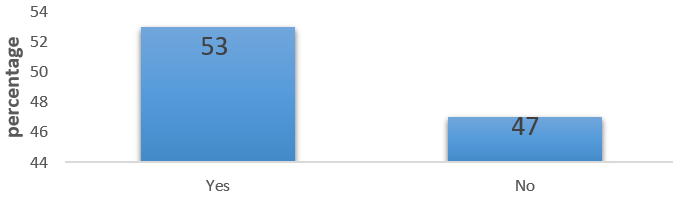
The Nifedipine tablets available in the 3 hospitals and prescribed most often for hypertensive patients were extended release ones (Figure 1). However, more than half the number of respondents (53%) indicated that they had challenges administering Nifedipine tablets to geriatrics (63%) and the severely ill (37%) hypertensive patients due to their inability to swallow solid dosage forms (Figures 2 and 3).
Inability to swallow Nifedipine tablets
Figure 2: Existence of Complaints of inability to swallow Nifedipine tablets.
Figure 2: Existence of Complaints of inability to swallow Nifedipine tablets.
Swallowing is an involuntary physiological process, which may be compromised as one ages or as a result of a disease. Several Disease conditions such as stroke, Parkinson’s disease, muscular dystrophy and tonsillitis may bring about swallowing difficulties (dysphagia). The existence of inability to swallow Nifedipine tablets in these groups of patients necessitated the discontinuation of therapy or the use of alternative means of administration. Whilst 34% of respondents indicated that they discontinued therapy with tablets, 66% of respondents indicated that they adapted alternative means of administration which involved crashing of the tablets and passing it through feeding tubes (37%) or mixing it with food for the patients (29%) (Table 1).
| Characteristic | Frequency(n) | Percentage (%) |
| Alternative means of administering Nifedipine | ||
| Crushing of the Nifedipine tablets and passing it through feeding tubes for patients | 111 | 37 |
| Crushing of the Nifedipine tablets and mixing it with food for patients | 87 | 29 |
| Discontinuing Nifedipine use | 102 | 34 |
| Presence of undesirable effects after unconventional use of Nifedipine tablets | ||
| Yes | 168 | 56 |
| No | 132 | 44 |
| The need to formulate alternative dosage form of Nifedipine | ||
| Yes | 294 | 98 |
| No | 6 | 2 |
Table 1: Summary of Respondents’ information about Nifedipine use.
Generally, tablets which are not scored and tablets which are in the modified release form, should not be split, or crushed [16]. Crushing of the Nifedipine tablets which are not scored will result in uneven distribution of Nifedipine within the two halves of the tablets and can result in under dosing or overdosing. Overdosing leads to exacerbation of the side effects of Nifedipine in the Patient as well as blood pressure reduction, which may be more than therapeutically desired. Conversely, under dosing leads to sub therapeutic activity of Nifedipine in the patient and this can worsen the prognosis of the disease. Furthermore, most of the Nifedipine tablets that are crushed are available as modified release tablets. Thus crushing of these tablets will alter the release of the Nifedipine and can cause dose dumping with serious adverse effects to the patient [17]. This was evident where 56 % of the respondents indicated that the patients complained of several undesirable effects when these alternative modes were used to administer Nifedipine tablets (Table 1).
The presentation of undesirable effects of administering Nifedipine and the various challenges associated with attempting to administer Nifedipine originally formulated into tablets in a non-convenient dosage form and non-conventional modes or the fact that therapy had to be stopped due to swallowing difficulties according to the respondents necessitates the need to formulate a suitable, convenient, and effective dosage form for Nifedipine. A need that was supported by 95% of the respondents. This finding was consistent with previous reports [6,18] that suggest the need to investigate other dosage forms as alternative means of administering drugs to adult patients with swallowing difficulties such as geriatrics and the severely ill.
Formulation and characterization of Nifedipine suppositories
Identification of Nifedipine
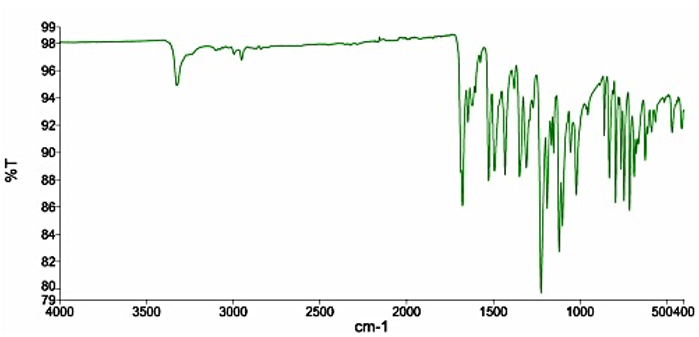
The Nifedipine powder used had a melting point range of 172°C-175°C which was within the acceptable range stipulated (171°C-175°C) [8] FTIR spectrum of pure Nifedipine powder is characterized by; υN-H stretching at 3326 cm-1, absorption bands at 3236 cm-1 and 2997 cm-1 assigned to the stretching vibrations of aromatic υC -H. There is also a characteristic band stretching at 2841 cm-1 specific to the υC-H aliphatic group (-CH3). At 1677 cm–1 there is a characteristic band indicative of υC=O stretching of ester group. Absorption bands at 1648 cm-1 and 1617 cm-1 are attributable to pyridine group, high intensity peak present at 1526 cm-1 is characteristic δN–H bending vibrations and a peak at 1493 cm-1 is indicative of δC-H. High intensity bands characteristic of υC-O stretching vibrations occur at 1224 cm-1, and between 829-712 cm-1 characteristic stretching vibration band appears υC-N (specific aryl-nitro) and δC-H bending vibrations of aromatic substances [19,20]. The IR spectrum obtained for the pure Nifedipine powder (Figure 4) exhibited absorption bands at aforementioned wavenumbers. The pure Nifedipine powder was therefore considered suitable for use in the formulation of the suppository.
Identification of Nifedipine
The Nifedipine powder used had a melting point range of 172°C-175°C which was within the acceptable range stipulated (171°C-175°C) [8] FTIR spectrum of pure Nifedipine powder is characterized by; υN-H stretching at 3326 cm-1, absorption bands at 3236 cm-1 and 2997 cm-1 assigned to the stretching vibrations of aromatic υC -H. There is also a characteristic band stretching at 2841 cm-1 specific to the υC-H aliphatic group (-CH3). At 1677 cm–1 there is a characteristic band indicative of υC=O stretching of ester group. Absorption bands at 1648 cm-1 and 1617 cm-1 are attributable to pyridine group, high intensity peak present at 1526 cm-1 is characteristic δN–H bending vibrations and a peak at 1493 cm-1 is indicative of δC-H. High intensity bands characteristic of υC-O stretching vibrations occur at 1224 cm-1, and between 829-712 cm-1 characteristic stretching vibration band appears υC-N (specific aryl-nitro) and δC-H bending vibrations of aromatic substances [19,20]. The IR spectrum obtained for the pure Nifedipine powder (Figure 4) exhibited absorption bands at aforementioned wavenumbers. The pure Nifedipine powder was therefore considered suitable for use in the formulation of the suppository.
Drug and excipients’ compatibility study
The FTIR spectra of drug and the two bases with their corresponding bland formulations (Figure 5 and Figure 6) showed that there was no disappearance of the characteristic functional peaks of Nifedipine and the various bases. Thus, there was no interaction between the drug and the various bases used in the formulation.
The FTIR spectra of drug and the two bases with their corresponding bland formulations (Figure 5 and Figure 6) showed that there was no disappearance of the characteristic functional peaks of Nifedipine and the various bases. Thus, there was no interaction between the drug and the various bases used in the formulation.
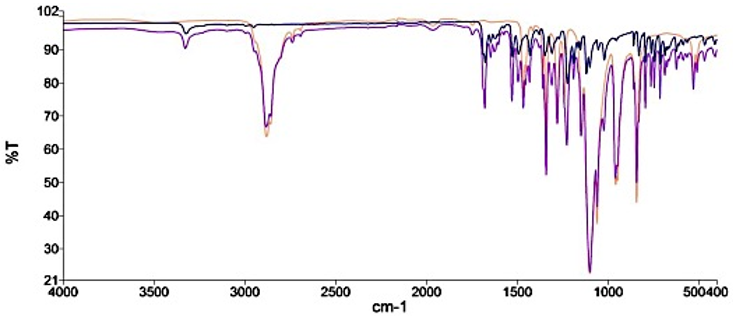
Figure 5: FTIR of Nifedipine powder, bland and medicated polyethylene glycol suppositories. Key - Nifedipine only, - Polyethylene glycol only, - Polyethylene glycol plus Nifedipine.
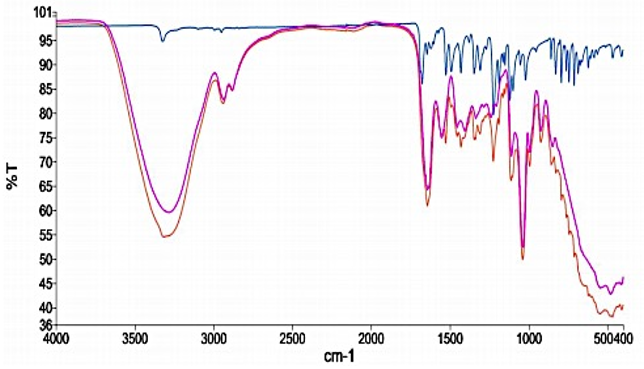
Figure 6: FTIR of Nifedipine powder, bland and medicated polyethylene glycol suppositories. Key - Nifedipine only, - Glycerogelatin only,- Glycerogelatin plus Nifedipine.
Quality assessment of formulated Suppositories
The prepared suppositories were assessed for visual parameters such as, colour, shape, feel and surface appearance as well as surface conditions such as cracks, holes, exudation, pitting and migration of active ingredient. Samples of each formulation were subjected to organoleptic examination and the results were found to be satisfactory. All formulated Suppositories were found without any cracks, pitting, dark regions, holes and exudation.
The prepared suppositories were assessed for visual parameters such as, colour, shape, feel and surface appearance as well as surface conditions such as cracks, holes, exudation, pitting and migration of active ingredient. Samples of each formulation were subjected to organoleptic examination and the results were found to be satisfactory. All formulated Suppositories were found without any cracks, pitting, dark regions, holes and exudation.
Uniformity of weight of Suppositories
The polyethylene glycol medicated suppositories, as well as the Glycerogelatin medicated suppositories had their weight variation within the limit stipulated by the International Pharmacopoeia [8] (Table 2). This indicates that the method used in incorporating the Nifedipine powder into the respective bases was consistent and therefore other quality assessment issues such as probability of overdosing and underdosing are avoided.
The polyethylene glycol medicated suppositories, as well as the Glycerogelatin medicated suppositories had their weight variation within the limit stipulated by the International Pharmacopoeia [8] (Table 2). This indicates that the method used in incorporating the Nifedipine powder into the respective bases was consistent and therefore other quality assessment issues such as probability of overdosing and underdosing are avoided.
Suppository Disintegration time
The formulated polyethylene glycol and glycerogelatin medicated suppositories disintegrated between ten (10) minutes to twenty (20) minutes (Table 2), hence both formulations passed the disintegration test according to the International Pharmacopoeia [8]. However, the disintegration time of the medicated glycerogelatin suppository (10.81 ± 1.128 minutes) was faster than that of the polyethylene glycol (19.29 ± 1.091 minutes). This may be attributed to the fact that the glycerogelatin medicated suppository was relatively softer and hence disintegrated faster than the polyethylene glycol medicated suppository. The nature of the glycerogelatin base also enhances its ability to be easily wetted and this facilitates the disintegration process. This implies that nifedipine suppositories formulated with the glycerogelatin base may produce a faster drug release and potentially increase the bioavailability of the administered nifedipine.
The formulated polyethylene glycol and glycerogelatin medicated suppositories disintegrated between ten (10) minutes to twenty (20) minutes (Table 2), hence both formulations passed the disintegration test according to the International Pharmacopoeia [8]. However, the disintegration time of the medicated glycerogelatin suppository (10.81 ± 1.128 minutes) was faster than that of the polyethylene glycol (19.29 ± 1.091 minutes). This may be attributed to the fact that the glycerogelatin medicated suppository was relatively softer and hence disintegrated faster than the polyethylene glycol medicated suppository. The nature of the glycerogelatin base also enhances its ability to be easily wetted and this facilitates the disintegration process. This implies that nifedipine suppositories formulated with the glycerogelatin base may produce a faster drug release and potentially increase the bioavailability of the administered nifedipine.
Drug content of formulated suppositories
The percentage drug content in all the formulations was estimated and found to be within the stipulated range (85-105%) [8], indicating the uniform distribution of Nifedipine in all the formulated suppositories (Table 2). The accurate and consistent drug content implies that the patient will receive the needed dose of the drug for therapeutic response to be achieved. This will ultimately reduce the undesirable effects of overdosing and underdosing which occurs due to crushing of the nifedipine tablets for patients who are unable to swallow.
The percentage drug content in all the formulations was estimated and found to be within the stipulated range (85-105%) [8], indicating the uniform distribution of Nifedipine in all the formulated suppositories (Table 2). The accurate and consistent drug content implies that the patient will receive the needed dose of the drug for therapeutic response to be achieved. This will ultimately reduce the undesirable effects of overdosing and underdosing which occurs due to crushing of the nifedipine tablets for patients who are unable to swallow.
In vitro drug release profile of formulated suppositories
All the formulated batches of suppositories produced a cumulative drug release above the stipulated range (≥ 80%). Polyethylene glycol and glycerogelatin are water soluble suppository bases which do not melt at body temperature but rather dissolve slowly in body fluids. This property permits the slow release of drug from the suppository. Initial drug release from the glycerogelatin medicated suppository was higher than that of the polyethylene glycol because the glycerogelatin base dissolved more readily in the dissolution medium in comparison to the polyethylene glycol and made the Nifedipine available for dissolution to occur. However, Polyethylene glycol base has good hydrophilic and solubilizing properties [21,22]. Thus, after the two bases have dissolved, the solubilizing properties of the polyethylene glycol helps with dissolution of nifedipine (a hydrophobic drug) in the dissolution medium (Figure 7 and 8). This explains why polyethylene glycol produced a higher drug release than glycerogelatin at the end of the dissolution study. Polyethylene glycol base will subsequently produce a better drug release and bioavailability of nifedipine even though glycerogelatin base produces a faster disintegration time.
All the formulated batches of suppositories produced a cumulative drug release above the stipulated range (≥ 80%). Polyethylene glycol and glycerogelatin are water soluble suppository bases which do not melt at body temperature but rather dissolve slowly in body fluids. This property permits the slow release of drug from the suppository. Initial drug release from the glycerogelatin medicated suppository was higher than that of the polyethylene glycol because the glycerogelatin base dissolved more readily in the dissolution medium in comparison to the polyethylene glycol and made the Nifedipine available for dissolution to occur. However, Polyethylene glycol base has good hydrophilic and solubilizing properties [21,22]. Thus, after the two bases have dissolved, the solubilizing properties of the polyethylene glycol helps with dissolution of nifedipine (a hydrophobic drug) in the dissolution medium (Figure 7 and 8). This explains why polyethylene glycol produced a higher drug release than glycerogelatin at the end of the dissolution study. Polyethylene glycol base will subsequently produce a better drug release and bioavailability of nifedipine even though glycerogelatin base produces a faster disintegration time.
Stability studies
| Uniformity of weight | |||||||||||
| Type of Suppository | Number of suppositories used | Average weight (g) | Deviation | % Deviation | |||||||
| Min | Max | Min | Max | ||||||||
| Nifedipine in Polyethylene glycol | 20 | 2.63 | 0.000 | 0.050 | 0.000 | 1.901 | |||||
| Nifedipine in Glycerogelatin | 20 | 2.55 | 0.000 | 0.040 | 0.000 | 1.567 | |||||
| Disintegration time | |||||||||||
| Type of suppository | Number of determinations | Average disintegration time (minutes) | |||||||||
| 0 month | 3months | 6 months | 9 months | ||||||||
| 4°C | 27°C | 4°C | 27°C | 4°C | 27°C | ||||||
| Nifedipine in polyethylene glycol | 3 | 19.25 ± 1.21 | 19.21 ± 1.75 | 19.24 ± 0.89 | 19.17 ± 1.80 | 19.14 ± 1.96 | 19.19 ± 0.09 | 19.11 ± 1.07 | |||
| Nifedipine in glycerogelatin | 3 | 10.79 ± 1.06 | 10.17 ± 0.86 | 10.21 ± 1.66 | 10.11 ± 1.47 | 10.20 ± 1.29 | 10.09 ± 0.93 | 10.22 ± 1.45 | |||
| Drug content | |||||||||||
| Type of suppository | Number of determinations | Average drug content (%) | |||||||||
| 0 month | 3 months | 6 months | 9 months | ||||||||
| 4°C | 27°C | 4°C | 27°C | 4°C | 27°C | ||||||
| Nifedipine in polyethylene glycol | 3 | 98.29 ± 0.48 | 96.55 ± 0.54 | 96.45 ± 0.34 | 96.50 ± 0.31 | 96.35 ± 0.45 | 96.46 ± 0.33 | 96.11 ± 0.44 | |||
| Nifedipine in glycerogelatin | 3 | 97.98 ± 0.46 | 94.10 ± 0.56 | 94.01 ± 0.54 | 94.02 ± 0.44 | 93.95 ± 0.34 | 94.04 ± 0.35 | 93.54 ± 0.36 | |||
Table 2: Properties and Stability of Formulated Suppositories.
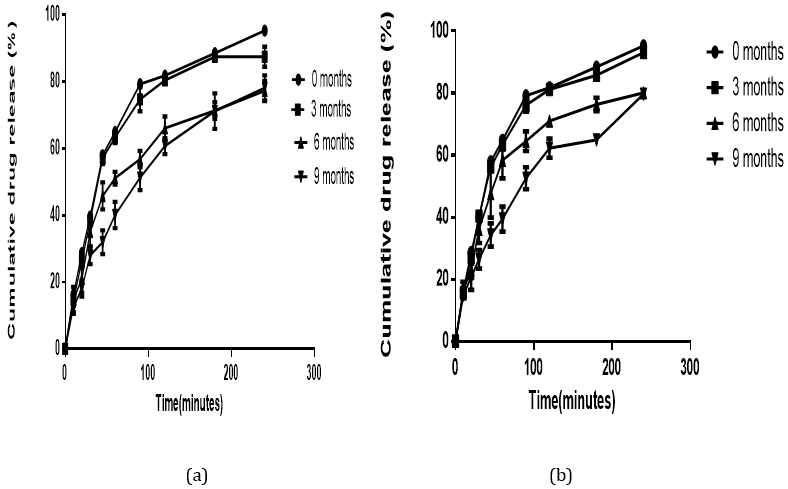
Figure 7: In vitro drug release profile of Nifedipine in Polyethylene glycol base stored at: (a) room (27°C) and (b) refrigerated (4°C) temperature for a period of nine months.
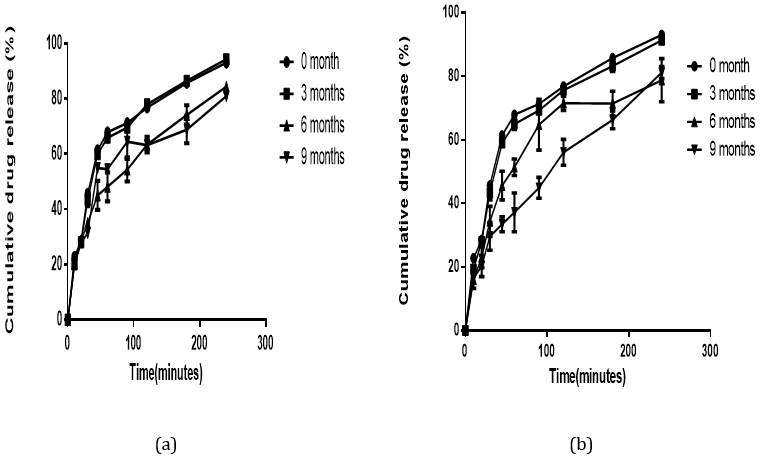
Figure 8: In vitro drug release profile of Nifedipine in Glycerogelatin base stored at; (a) room (27°C) and (b) refrigerated (4°C) temperature for a period of nine months.
The stability test assesses how the quality of a drug substance or a drug product changes with time under the influence of various factors such as temperature, humidity and light. A reduction in drug content to a level of 85% and below may lead to failure of the therapy [14]. None of the formulated suppositories showed any change in physical characteristics indicating that the physical integrity of the formulated suppositories remained intact throughout the period of study. The disintegration time, of the two formulated suppositories remained within the stipulated sixty (60) minutes. The drug content remained above 85% and less than 105% (Table 2) as stated in the ICH guidelines for stability studies. Alterations in temperature can markedly affect the physical and chemical stability of suppositories which can ultimately affect the release profiles. Irrespective of the storage temperature, similar cumulative drug release profiles were observed for glycerogelatin and polyethylene glycol formulated suppositories (Figure 7 and 8b) over the nine months period. This confirms the ability of the formulated suppositories to maintain their release profile irrespective of the storage conditions. Ultimately the formulated suppositories are stable at both room and refrigerated temperatures.
Conclusion
Nifedipine suppositories have been successfully formulated using Glycerogelatin and PEG 6000 as bases with PEG 6000 giving a higher drug release over a four-hour period. All formulated suppositories produced drug contents and release profile within pharmacopeial specifications. Nifedipine suppositories formulated with PEG 6000 and glycerogelatin have potential as alternative dosage forms for hypertensive patients on Nifedipine who have swallowing difficulties.
Acknowledgement
The authors are grateful to the staff of Komfo Anokye Teaching Hospital, KNUST Hospital and Suntreso Hospital. The efforts of Mr. Clement Attor and Mr. Prosper Adzormahe, both of the Department of Pharmaceutics, KNUST, Ghana are greatly appreciated.
The authors are grateful to the staff of Komfo Anokye Teaching Hospital, KNUST Hospital and Suntreso Hospital. The efforts of Mr. Clement Attor and Mr. Prosper Adzormahe, both of the Department of Pharmaceutics, KNUST, Ghana are greatly appreciated.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Ghana Statistical Service (GSS), Ghana Health Service (GHS), and ICF International, Ghana Demographic and Health Survey (2014), GSS, GHS.
- World Health Organization, (2016). The international pharmacopoeia. Sixth edition. World Health Organization
- McManus, R.J., Caulfield, M. and Williams, B., (2012). NICE hypertension guideline 2011: evidence-based evolution., Br. Med. J., 344:181.
- Walker, R. and Edwards, C., (2012). Clinical pharmacy and therapeutics, churchill Livingstone. London: 321-322.
- Osei-Asare, C., Kipo, S.L., Ofori-Kwakye, K. and Boakye-Gyasi, M.E., (2015). Comparative in vitro dissolution of commercially available sustained release nifedipine tablet brands in the Kumasi Metropolis, Ghana., J. Appl. Pharm. Sci,5 (8): 54-60.
- Schier, J.G., Howland, M.A., Hoffman, R.S. and Nelson, L.S., (2003). Fatality from administration of labetalol and crushed extended-release Nifedipine. Ann pharmacother., 37(10):1420-1423.
- Baviskar, P., Bedse, A., Sadique, S., Kunde, V. and Jaiswal, S., (2013). Drug Delivery on Rectal Absorption: Suppositories. J. pharmacol. Sci., 21(1):70-74.
- British Pharmacopoeia (2013) Volume II, Her Majesty’s Stationery Office, London.
- Allen, L.V., Worthen, D.B. and Mink, B., (2008). The art, science, and technology of pharmaceutical compounding (Vol. 2). Washington DC: American Pharmaceutical Association: 150-160.
- Ansel HC, Popovich NG, Allen LV, (2008). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. Lippincott Williams & Wilkins: Philadelphia, PA, USA: 236-250.
- Basappa, Basavaraj, Devi, S., Bharath, S., Deveswaran, R. and Madhavan, V., (2013). Design and Evaluation of Sustained Release Propranolol Hydrochloride Suppositories. J. Appl. Pharm,05 (04): pp100-120.
- Saleem, M.A., Taher, M., Sanaullah, S., Najmuddin, M., Ali, J., Humaira, S. and Roshan, S., (2008). Formulation and evaluation of tramadol hydrochloride rectal suppositories. Indian J. Pharm. Sci., 70(5):640-648.
- Anthony Palmieri. (2008). Suppository Dissolution Testing: Apparatus Design and Release of Aspirin. Drug Dev. Ind. Pharm., 7(2):247-259.
- Bajaj Sanjay, Dinesh Singla and Neha Sakhuja. (2012). Stability testing of pharmaceutical products. J. Appl. Pharm.,2(03):129-138.
- Sah, M.L. and Saini, T.R., (2008). Formulation development and release studies of indomethacin suppositories. Indian J. Pharm. Sci.,70(4):498-502.
- Cornish, P. (2005). “Avoid the crush”: hazards of medication administration in patients with dysphagia or a feeding tube. Can. Med. Assoc. J., 172: 871-872.
- Bowman, C., (2007). Administration of drugs to patients with swallowing difficulties. J. Malta Coll. Pharm. Pract., 12:42-45.
- Logrippo, S., Ricci, G., Sestili, M., Cespi, M., Ferrara, L., Palmieri, G.F., Ganzetti, R., Bonacucina, G. and Blasi, P., (2017). Oral drug therapy in elderly with dysphagia: between a rock and a hard place! Clin. Interv. Aging., 12: pp.241-247.
- Kanagathara, N., Shenbagarajan, P., Jeyanthi, C.E. and Thirunavukkarasu, M., (2011). Fourier transform infrared spectroscopic investigation on nifedipine. Int. J. Pharm. Biol. Sci., 1: 52-56.
- Roxana, Blajovan, and Modra, D., (2013). The study synthesis of nifedipine-calcium antagonist. Front. Chem., 22(2): 47-55.
- Verheyen, S., Blaton, N., Kinget, R. and Van den Mooter, G., (2002). Mechanism of increased dissolution of diazepam and temazepam from polyethylene glycol 6000 solid dispersions. Int. J. Pharm., 249(1): 45-58.
- Riegelman, and W.L. Chiou (1978). "Increasing the Absorption Rate of Insoluble Drugs," US Patent 4151273.
Citation: Frederick William Akuffo Owusu, Philomena Entsie, Mariam El Boakye-Gyasi, Marcel Tunkumgnen Bayor. (2020). Formulation and In Vitro Evaluation of Nifedipine Suppositories for Geriatric and Severely Ill Patients with Hypertension. Journal of Pharmacy and Drug Development 2(1).
Copyright: © 2020 Mariam El Boakye-Gyasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.