Research Article
Volume 1 Issue 2 - 2019
Empirical Study of Hydrodynamic Flow of Sour Gas in the Porous Bed in Sweetening Process
1Department of Petroleum Engineering, Khomeinishahr Branch, Islamic Azad University, Khomeinishahr, Iran
2Department of Mechanical Engineering, khomeinishahr Branch, Islamic Azad University, Khomeinishahr/Isfahan, Iran
3Department of Chemical Engineering, Firoozabad Branch, Islamic Azad University, Firoozabad, Iran
2Department of Mechanical Engineering, khomeinishahr Branch, Islamic Azad University, Khomeinishahr/Isfahan, Iran
3Department of Chemical Engineering, Firoozabad Branch, Islamic Azad University, Firoozabad, Iran
*Corresponding Author: Arezoo Khosravi, Department of Mechanical Engineering, khomeinishahr Branch, Islamic Azad University, Khomeinishahr/Isfahan, Iran.
Received: November 09, 2019; Published: December 04, 2019
Abstract
Many different processes are used to treat raw natural gas to pipeline quality. Sulfur is commonly present as an impurity in fossil fuels. The sulfur compounds can threat the pipe lines and all of the devices, severely. In addition, the mechanism of sulfur absorption by nano fluid in a packed bed under the magnetic field is considered, in this study. The experimental and theoretical investigation is done to obtain the outlet amount of sulfur in gas stream. Results show, the amounts of hydrogen sulfide catch from branch line defined the process performance. Inlet sour gas contains 3.4 ppm H2S, temperature of 33 C is fed into the packed bed with 20m3/m3, 30m3/m3 and 40m3/m3 porosity and also, magnetic field occurred with current of one Ampere.
Key words: Hydrodynamic; Gas; Packed bed; Aluminum oxide nano fluid; Sulfur
Introduction
Many natural gases are produced from wells containing hydrogen sulphide, sulphur compounds and carbon dioxide. Perry and Chilton, et al, 1999 these gases are treated prior to sale or entry to a process plant. A gas which has high concentration of sulphur compounds is called 'Sour' gas. (Also called 'Acid' gas). The H2S and CO2 are known as acid gases, and are corrosive, if water or oxygen is present.The hydrogen sulphide H2S must be removed from the Natural Gas before it can be used, due to the fact that it is highly corrosive and deadly toxic. The MDEA solution selectively removes the H2S, however, some of the CO2 and sulphur compounds during the sweetening process also absorbed, CO2 concentration in the first gas plant is below the allowable concentration for the pipe line specification (less than 5%).
Amine Sweetening Process
There is a number of absorption gas treating processes, but the most common is the amine process in which acid gases react chemically. The 'rich' amine solution is heated under low pressure to regenerate the liquid by driving off the acid gases. Several different amine solutions can be used. These amine mixtures have been called a variety of names including formulated amines and MDEA based amines. Historically, MDEA has been recognized primarily for its ability to selectively absorb H2S from a gas while leaving large amounts of CO2 in the gas. The selective absorption characteristics of MDEA have been widely reported in the above literature. Until the last few years, MDEA has not been associated with cases where the removal of large amounts of CO2is desired. The type of solution used in the process will depend upon the type and quantity of acid gas contained in the sour gas stream and the volume of sour gas to be treated. From Sahl Operating Manual, 1983, Sirte Oil Company, the process flow diagram for Amine sweetening system show the process details carried upon the Sour gases in the plant. The GPSA (Gas Processors Suppliers Association), et al, 2004, the general operating problems in the amine system are centered around the following five major areas:-
There is a number of absorption gas treating processes, but the most common is the amine process in which acid gases react chemically. The 'rich' amine solution is heated under low pressure to regenerate the liquid by driving off the acid gases. Several different amine solutions can be used. These amine mixtures have been called a variety of names including formulated amines and MDEA based amines. Historically, MDEA has been recognized primarily for its ability to selectively absorb H2S from a gas while leaving large amounts of CO2 in the gas. The selective absorption characteristics of MDEA have been widely reported in the above literature. Until the last few years, MDEA has not been associated with cases where the removal of large amounts of CO2is desired. The type of solution used in the process will depend upon the type and quantity of acid gas contained in the sour gas stream and the volume of sour gas to be treated. From Sahl Operating Manual, 1983, Sirte Oil Company, the process flow diagram for Amine sweetening system show the process details carried upon the Sour gases in the plant. The GPSA (Gas Processors Suppliers Association), et al, 2004, the general operating problems in the amine system are centered around the following five major areas:-
- Amine loss from the system
- Amine foaming problem
- Corrosion problem
- Concentration of residual gas in lean amine solution
- Winterization.
Amine loss from the system
A certain amount of MDEA will be continuously lost from the amine sweetening system due to the vapour pressure of the amine.
A certain amount of MDEA will be continuously lost from the amine sweetening system due to the vapour pressure of the amine.
The largest amine losses are usually through the amine absorber as carry over with the treated gas. Some amine is also lost through the amine stripper, amine flash tank, pumps packing etc. The losses vary for different plants, but usually are between 0.05 to 0.5 gallons per Mmscf of treated gas. These losses can be much higher depending upon several factors such as sour gas flow rate, reboiler temperature, high differential temperature between amine solution and the sour gas in the amine absorber, bad mist eliminator on the absorber top, bad filtration system and foaming. Here are some guidance to help in minimizing the amine solution losses from the system:
- Maintain the top temperature of the amine absorber as low as possible.
- Maintain proper amine solution concentration.
- Ensure good clean amine solution - good filtration, proper reboiler control to avoid chemical breakdown of amine and avoid all amine contamination. Regularly check all operating parameters & process variables to maintain steady running of the amine unit.
Amine foaming
Foaming is a common problem that results in a decrease of treating capacity of the plant and amine losses. It is usually detected by a sharp rise in the pressure drop across the amine absorber. The amine foaming can occur from the reaction of the amine solution with organic acids or because of the presence of hydrocarbon contaminants, ferric sulphide, or other sludge. Several anti-foaming agents have been developed to combat this and some products which are primarily corrosion inhibitors have shown good anti-foaming properties. Where foaming is caused by hydrocarbon contamination, it may be eliminated by maintaining the temperature of the absorber above the hydrocarbon condensation temperature. Foaming can be prevented in the following ways:
Foaming is a common problem that results in a decrease of treating capacity of the plant and amine losses. It is usually detected by a sharp rise in the pressure drop across the amine absorber. The amine foaming can occur from the reaction of the amine solution with organic acids or because of the presence of hydrocarbon contaminants, ferric sulphide, or other sludge. Several anti-foaming agents have been developed to combat this and some products which are primarily corrosion inhibitors have shown good anti-foaming properties. Where foaming is caused by hydrocarbon contamination, it may be eliminated by maintaining the temperature of the absorber above the hydrocarbon condensation temperature. Foaming can be prevented in the following ways:
- Do not overload the inlet separator in your plant.
- Avoid condensation of liquid hydrocarbons in the amine absorber by keeping the temperature in the absorber above the hydrocarbon dew point.
- Keep field corrosion inhibitors, soap based lubricants and lube oil out of the amine system.
- Degradation of amine can cause foaming, so avoid it by preventing oxidation and by proper reboiler control.
- Maintain the charcoal filter in good working condition, because this is the filter which absorbs the liquid contaminants.
- If these remedies fail, try a de-foaming agent and evaluate the results.
- Always keep in mind that the de-foamers are only a temporarily answer for the foaming problem, and the best cure for the problem is to avoid the main causes.
Corrosionproblem
Corrosion problem is commonly encountered in the amine system and generally occurs in the amine regenerator, heat exchanger, amine stripper and amine pumps etc. Most corrosion occurs in areas where the acid gases are actually released from the solution i.e. in the reboiler, stripper tower and its overhead systems. The cause of corrosion is traced to gaseous H2S & CO2 which comes out of the amine solution while the rich amine solution is receiving heat in the heat exchanger prior to the regenerator. These acid gases combine with water to form acids, which will attack the metal surfaces in contact with the amine solution.
Corrosion problem is commonly encountered in the amine system and generally occurs in the amine regenerator, heat exchanger, amine stripper and amine pumps etc. Most corrosion occurs in areas where the acid gases are actually released from the solution i.e. in the reboiler, stripper tower and its overhead systems. The cause of corrosion is traced to gaseous H2S & CO2 which comes out of the amine solution while the rich amine solution is receiving heat in the heat exchanger prior to the regenerator. These acid gases combine with water to form acids, which will attack the metal surfaces in contact with the amine solution.
Corrosion problems can be minimized by the following practices:
- Keep the amine solution clean. Do not over load the inlet separator, which prevents solids entering in the system. Other solids that contribute to corrosion are removed by amine filters. So, it’s very important to maintain a good amine filtration system.
- The presence of air will cause the amine to degrade into heat stable salts, so there should be a gas blanket on all the amine storage tanks to exclude air.
- Maintain acid-gas loading within the proper ranges.
- Corrosion problems become severe at high temperatures with the rich amine solutions, so keep the amine solution concentration up to the recommended value.
- Amine reboiler temperature should be kept at the recommended range to avoid any amine decomposition or any extra water losses which will affect the amine solution concentration.
- Maintain a regular corrosion testing programmed for an early detection of any corrosion problems in the system.
Concentration of residual gas in lean amine solution
- The Concentration of residual gas in the lean amine solution should be controlled at a specified level for the plant. This is the gas which remains in the MDEA solution at all times.
- The amount of residual acid gas in the solution depends upon the heat used in the stripper reboiler. Increasing the heat reduces the residual acid gas and vice versa.
- If the concentration of residual gas is low then more acid gas can be absorbed by the lean amine solution in the absorber. This will also, allow a reduction in the circulation rate of the amine solution.
- If the inlet sour gas rate changes, the amine solution flow rate and the reboiler heat should be changed in same proportion to match the sour gas changes.
Note: The rich amine solution fed to the amine stripper contains acid gas from two sources:
- Acid gas absorbed in the absorber.
- Residual acid gas from the amine stripper.
Therefore, the sum of the two is the total acid gas content of the rich amine solution.
Winterization
Cold weather operation requires special attention for plants using MEA, DEA or Sulfinol. The freezing point of these solutions is about the same as that of the water. Consequently, lines or vessels in which there is no continuous flow should be given a special consideration during cold weather. If the plant and / or the amine unit are shut down for an extended period during freezing weather, the amine solution should be completely drained from the system.
Cold weather operation requires special attention for plants using MEA, DEA or Sulfinol. The freezing point of these solutions is about the same as that of the water. Consequently, lines or vessels in which there is no continuous flow should be given a special consideration during cold weather. If the plant and / or the amine unit are shut down for an extended period during freezing weather, the amine solution should be completely drained from the system.
The freezing point of MDEA and DGA is about (-40°F) at the recommended amine concentration which is about 50% by weight for MDEA.
Therefore, the amine system at the hot areas gas plant does need special attention during the cold weather other than not allowing excessive cooling. As a conclusion, this problem is an occasional one, but it is rather important and must be considered while working in the amine system.
Burning fuels, the sulfur is released as sulfur dioxide—an air pollutant responsible for respiratory problems and acid rain [1-6]. Environmental regulations have increasingly restricted sulfur dioxide emissions, forcing fuel processors to remove the sulfur from both fuels and exhaust gases. The cost of removing sulfur from natural gas and petroleum in the United States was about $1.25 billion in 2008. In natural gas, sulfur is present mainly as hydrogen sulfide gas (H2S), while in crude oil it is present in sulfur-containing organic compounds which are converted into hydro aluminum oxides and H2S during the hydro desulphurization [7, 8 and 9]. In both cases, corrosive, highly-toxic H2S gas must be converted into elemental sulfur and removed for sale or safe disposal [10, 11 and 12]. Formation fluids that contain Hydrogen Sulfide-By-product from anaerobic bacterial action on sulfur compounds present in the mud (i.e. Sodium Sulfite). Thermal degradation of mud additives containing sulfur with tool joint lubricants containing sulfur [13, 14 and 15].
H2S is a weak acid that can go through the following 2 stages when dissolved in water or water based mud: 1. H2S ↔ H+ + HS- - both steps (1 and 2) can go back and forth depending on the pH. 2. HS- + OH- ↔ S= + H2O.
Combination of absorption mechanism and magnetic field are used to remove hydrogen sulfide from sour natural gas. Mass transfer rate and mass transfer coefficient is measured and calculated experimentally and theoretically.
Materials and Method
Sour gasses which contain different amounts of hydrogen sulfide are reactor bed feed. Two gray 20 lit of volume pressurized vessel contain sour gas can be joint to the experimental line. In 40 cm of efficient volume of glass balls with 2.5 cm in diameter are used as the packing. Two meshes are applied for separating the reactor vessel to the three sections. One polymeric weir with mesh size of 0.02 cm is on the top of vessel as a holder to let the only treated gas stream.
H2S+1/2O2à1/xSx+H2O (1)
H2S+1/2O2à1/xSx+H2O (1)
Control and measuring instruments for pressure, temperature and flow rate evaluation are set in stream lines. All the used valves, pipes and equipment’s are corrosion resisted. The bed porosity, e, changes when the number of ball changes, Equation 2 shows the porosity evaluation. Vnb is nanoball volume and V is efficient volume of bed.
e= 1-Vnb./V. (2)
e= 1-Vnb./V. (2)
Water is applied to enhance the amount of absorbed H2S in to the nano fluid. A Nano aluminum oxide particle with 3% wt is mixed with pure water for 180 min and 4000 Watt. This time is considered for stability of nano aluminum oxide into the pure water. This step is an exothermic process. Water helps oxidation of H2S and also increases the nano aluminum oxide capacity for H2S absorption. Equation 3, 4, 5, 6 and 7 show the related mechanisms in H2S adsorption.
H2S (g) → H2S (aqua.) (3)
H2S (aqu.) → H2S (aqua-absorp.) (4)
H2S (aqua-absorp.) → HS-(ads.)+H+ (5)
HS-(ads.)+O (ads.) → S (ads.)+ OH- (6)
HS-(ads.)+3O (ads.) → SO2 (ads.)+OH- (7)
After absorption of H2Sinto the aluminum oxide nano fluid, the increase in the outlet gas temperature is observed. Also, the increase in temperature is obtained using magnetic fields. Since of some safety limitations, lower appropriate ranges of feed temperature, below 40 C, are chosen beside the application of magnetic field. The basic parameters in operation of a packed bed are evaluated experimentally and theoretically in this paper.
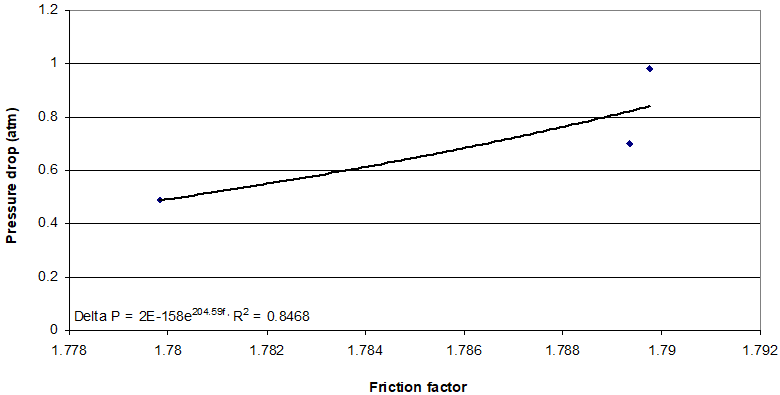
Totally, the oxygen can be react with hydrogen sulfide, and then water molecules and sulfur elements can be produced in the absorption process of sulfur compound removal from sour gas. Changes in the amount of bed porosity are evaluated by measuring pressure drop and minimum velocity. The experiments are done more than minimum velocity values. Pressure drop between top and bottom of fluidized bed is considered. The friction is appeared between the gas stream and the surface of packing’s. The Figure 1 shows the effect of friction factor on pressure drop. This also, affects the friction factor and pressure drop. The higher value of friction factor causes higher values of pressure drops. Although the small changes in values of higher friction factor causes harp change in value of pressure drop.
Results and Discussion
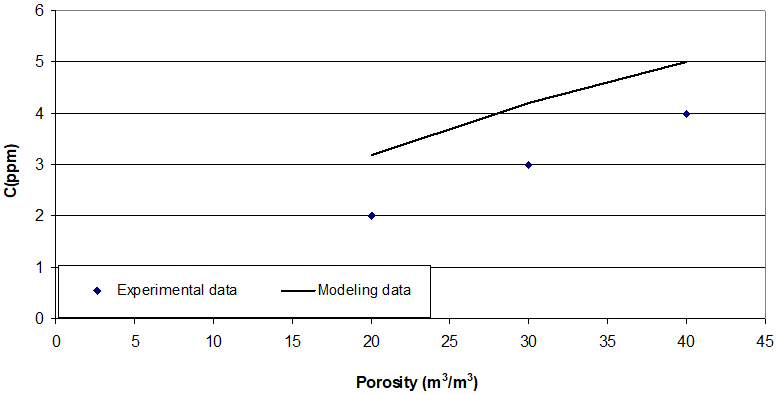
Bed porosity, inlet gas temperature, inlet concentration of H2S, magnetic field which influences the mass transfer area, mass transfer coefficient and rate of reaction are considered here. Three different amounts of porosity are achieved by three groups of packing’s. In this case higher porosity presents lower amount of packing’s and also lower amount of mass transfer surface area. In the packed bed the channeling malfunction is occurred. So, the increase trend of C is predicted by increase in the amount of porosity. Outlet concentration below 4 ppm is acceptable result due to commercial rules. Experimental data are in higher values of hydrogen sulfide comparing with ones from modeling data. Figure 2 shows the trend of outlet hydrogen sulfide concentration with bed porosity, Vvoid/V. In this experiment, the amounts of hydrogen sulfide catch from branch line defined the process performance. Inlet sour gas contains 3.4 ppm H2S, temperature of 33 C is fed into the packed bed with 20m3/m3, 30m3/m3 and 40m3/m3 porosity and also, magnetic field occurred with current of one Ampere.
Conclusion
Combination of absorption mechanism and magnetic field are used to remove hydrogen sulfide from sour natural gas. Mass transfer rate and mass transfer coefficient is measured and calculated experimentally and theoretically. The rate of mass transfer is introduced as function of gas temperature, initial concentration of hydrogen sulfide, gas flow rate, magnetic field, nano fluid flow rate and weight concentration of aluminum oxide nano particle in the pure water. The results show, higher value of friction factor causes higher values of pressure drops. Although the small changes in values of higher friction factor causes harp change in value of pressure drop.
References
- L. Carlos, G. Isabel, B. Irene, D. Luis I. R. Luis M. (2013). Experimental study of SO2 and NOx emissions in fluidized bed oxy-fuel combustion. Fuel Process Techno; 106: 587–594.
- M. de las Obras-Loscertales, A. Rufas, L.F. de Diego, F. García-Labiano, P. Gayán, A. Abad, J. Adánez, (2013). Effects of Temperature and Flue Gas Recycle on the SO2 and NOx Emissions in an Oxy-fuel Fluidized Bed Combustor, Energy Procedia.; 37: 1275-1282.
- W. Kaewboonsong, V.I. Kuprianov, N. Chovichien, (2006). Minimizing fuel and environmental costs for a variable-load power plant (co-)firing fuel oil and natural gas: Part 1. Modeling of gaseous emissions from boiler units, Fuel Processing Technology; 87: 1085-1094.
- A.Irabien, (2012). Environmental and economic evaluation of SO2 recovery in a ceramic hollow fibre membrane contactor. Chem Eng Process: Process Inten., 52: 151-154.
- H. Wang, Sh. Li, F. Lai, B. Wang, (2012). Computational Model of Greenhouse Gas Emissions of Power Station boiler considering Desulphurization, Physics Procedia; 24: 44-49.
- D.L. Stern, K.E. Nariman, J.S Buchanan, N.A. Bhore, D.L. Johnson, R.K. Grasselli, (2000). The Mobil Oil SOx Treatment Process (MOST). Catalytic removal of SOx and H2S from refinery tail gas, Catalysis Today; 55: 311-316.
- W. Zhou, C.S. Zhao, L.B. Duan, XP. Chen, C. Liang. (2011). Two-dimensional computational fluid dynamics simulation of nitrogen and sulfur oxides emissions in a circulating fluidized bed combustor. Chem. Eng. J.; 173: 564-573.
- D. Eow, S. John. (2002). Recovery of sulfur from sour acid gas: A review of the technology Environmental Progress. Americ. Institut. Chem. Eng.; 21: 143 - 162.
- D. Kunii, O. Levenspiel. (1991). Fluidization engineering. First edition. New York: Wiley.
- JF. Davidson. (1991). Fluidization. First edition. USA: Academic Press.
- D. Green, R. Perry. (2007). Perry's Chemical Engineers' Handbook. 8th edition, USA: Mc Graw Hill.
- W.Ch. Yang. (2003). Handbook of Fluidization and Fluid-Particle Systems. First edition. USA: Taylor & Francis.
- L.Davidson, Jr Amick, H.Erwin. (2004). Formation of gas bubbles at horizontal orifices. AIChE J 2: 337–342.
- M. Uzi. (2009). Principles of chemical reactor analysis and design. Second edition. New York: Wiley.
- O. Levenspiel. (1999). Chemical Reaction Engineering. 3th Ed. New York: Wiley.
Citation: Ahamd Reza Gharibi, Arezoo Khosravi and Farshad Farahbod. (2019). Empirical Study of Hydrodynamic Flow of Sour Gas in the Porous Bed in Sweetening Process. Archives of Chemistry and Chemical Engineering 1(2).
Copyright: © 2019 Arezoo Khosravi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.