Research Article
Volume 3 Issue 1 - 2021
Effects of Some Antiviral Medicines on the Performance and Antibody Titres of Pullets Challenged with Very Virulent Infectious Bursal Disease Virus
1Department of Veterinary Medicine, University of Abuja, Abuja, Nigeria
2Department of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
2Department of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
*Corresponding Author: Ogbe AO, Department of Veterinary Medicine, University of Abuja, Abuja, Nigeria.
Received: July 11, 2021; Published: July 26, 2021
Abstract
Infectious bursal disease (IBD) is an acute, highly contagious immunosuppressive disease of chickens associated with high morbidity and mortality rates. In Nigeria, some antiviral drugs are used for the control of IBD with claims of efficacy. This study evaluated the effects of oral administration of some antiviral medicines on the performance and antibody titres of pullets challenged with very virulent infectious bursal disase virus. A total of 200 day-old pullets were procured from a reputable hatchery and fed chicks’ starter mash containing 20% crude protein, ad libitum. The chicks were vaccinated against Newcastle disease using a live La Sota vaccine at 21 days of age. At day 35 of age, the chicks were randomly assigned into six groups labeled A, B, C, D, E and F, comprising of 32 chicks each. Chicks in groups A, B, C, D and E were inoculated with a very virulent infectious bursal disease virus at 0.05 ml, each via oral route at 35 days of age. Chicks in group F (negative control) were not inoculated. Group A was treated with VHK at 1 g/L, B with SHK at 2 ml/L, C with BVC at 1 g/2 L and D with VRX at 1 g/L. All treatments were administered orally from day 3 post-inoculation (pi) for 5 days, ad-lib. Blood was collected from all the groups after treatments at day 35 and 42 of age to determine IBD antibody titres using enzyme linked immunosorbent assay (ELISA) and haematocrit method was used to determine the packed cell volume (PCV). Clinical signs of depression, prostration, recumbence and inappetance were observed in groups A, B, C, D and E on day 3 to 7 pi. The clinical signs were higher in group E (90.63%), followed by B (87.50%), A (84.38%), C (75.00%) and D (71.88%). The duration of clinical signs in group C was 2 days only and others were 3 to 5 days. Group E had higher morbidity rate (90.63%), followed by A (84.34%), B (75.00%) and D (62.50%). Group C had lower morbidity rate (56.25%). Morbidity score was higher in group A and E with a score of 5 (grave); B and D had a score of 4 (very severe). Group C had the lowest morbidity score of 3. Mortality lasted 4 days in group A and E, and 3 days in B, C and D. Mortality rate was lowest in group A (53.13%). Gross lesions were higher in group E and lowest in group A. Antibody titres were higher in group A (3,076 ±415.78), and C (2,996 ±17.68). Chicks in group A and E had lower PCV (27.5%, each) at 7 dpi. The feed intake and live body weight gains were slightly reduced in group A, B, C, D and E at 7 dpi compared to group F which was slightly increased. The drugs used in this study did not prevent signs or mortalities caused by the very virulent infectious bursal disease virus (vvIBDV). A Further study on the use of BVC, VHK and SHK alone or in combinations was recommended for the control of IBD in Nigeria.
Keywords: Antiviral medicines; Gumboro disease; Clinical signs; Antibody titre
Introduction
Infectious bursal disease (IBD), otherwise called Gumboro disease is an acute, highly contagious virus disease of chickens aged 3-6 weeks, characterized by immunosuppression, high mortality and severe economic loss to the poultry industry (Da Costa et al., 2003; Muller et al., 2003; Musa et al., 2012). IBD is caused by a non-enveloped double stranded ribonucleic acid (dsRNA) virus of the genus, Avibirnavirus and family Birnaviridae (Kibenge et al., 1988; Da Costa et al., 2003). The clinical signs of classical IBD are similar to those caused by the very virulent IBD virus (vvIBDV), except that vvIBDV infection is most acute and pronounced in the individual birds and generalized in flocks with mortality range of 60-100% (Van den-Berg, 2000).
The disease has acquired an endemic status in poultry farms in Nigeria (Durojaiye et al., 1984) and has no cure. Prevention is through biosecurity and vaccination. Despite vigorous vaccinations, IBD outbreaks still occur resulting in high mortality rates in both vaccinated and unvaccinated flocks (Awolaja and Adene, 1995; Musa et al., 2010). Reasons for the inadequate protection despite vaccinations could be poor hygiene practices, inadequate or unsteady storage temperature (Ogbe et al., 2003), reversion to virulence by the classical (attenuated) IBD vaccines (Eteradosi and Saif, 2008) and presence of vvIBD field virus.
In Nigeria, due to the dreaded nature of IBD, farmers and veterinarians have resorted to the use of different alternative therapies in order to control the disease (Balami et al., 2019). This study was aimed at evaluating the effects of some drugs on the performance and antibody titres in pullet chickens challenged with vvIBDV.
Material and Methods
The Study Location
The study was carried out at a private poultry house in Keffi, Nasarawa State, a satellite town near Abuja, where the Faculty of Veterinary Medicine, University of Abuja, Nigeria is situated. Ethical clearance to conduct the research was obtained from University of Abuja Ethics Committee on Animal Use (UAECAU), with approval number: UAECAU/2020/002. The birds were cared for and handled humanely throughout the period of study.
The study was carried out at a private poultry house in Keffi, Nasarawa State, a satellite town near Abuja, where the Faculty of Veterinary Medicine, University of Abuja, Nigeria is situated. Ethical clearance to conduct the research was obtained from University of Abuja Ethics Committee on Animal Use (UAECAU), with approval number: UAECAU/2020/002. The birds were cared for and handled humanely throughout the period of study.
Experimental chicks and care
A total of two hundred (200) day-old brown pullet chicks were procured from a reputable hatchery in Nigeria. The chicks were brooded together for 3 weeks in a deep litter. At 4 weeks of age, the chicks were randomly distributed into six groups (A, B, C, D, E and F) with 32 chicks each, with each compartment measuring 210 cm wide and 240 cm long constructed with wood and wire-meshes. Each compartment was provided with 2 small sizes of round metal feeders, 2 plastic drinkers and 100-watt bulb to supply light and heat. The pens were washed with detergent soaked in water and disinfected with a broad spectrum biocidal disinfectant (Virkon-S®) containing dipotassium peroxodisulphate (1%) at 5 gram per litre of water. Group F (negative control) was isolated from the other groups (A to E) and a different attendant provided care. Pelletized chick feed containing crude protein (17%) (Min.), crude fibre (12%) (Max.), fat (8%) (Max.), calcium (1%) (Min.), phosphorus (0.4%) (Min.) and metabolizable energy (2650 Kcal/Kg) (Min.) was fed ad libitum to the chicks in metallic chicks feeders (2/group) and potable water in plastic drinkers (2/group) from day-old to 9 weeks of age.
A total of two hundred (200) day-old brown pullet chicks were procured from a reputable hatchery in Nigeria. The chicks were brooded together for 3 weeks in a deep litter. At 4 weeks of age, the chicks were randomly distributed into six groups (A, B, C, D, E and F) with 32 chicks each, with each compartment measuring 210 cm wide and 240 cm long constructed with wood and wire-meshes. Each compartment was provided with 2 small sizes of round metal feeders, 2 plastic drinkers and 100-watt bulb to supply light and heat. The pens were washed with detergent soaked in water and disinfected with a broad spectrum biocidal disinfectant (Virkon-S®) containing dipotassium peroxodisulphate (1%) at 5 gram per litre of water. Group F (negative control) was isolated from the other groups (A to E) and a different attendant provided care. Pelletized chick feed containing crude protein (17%) (Min.), crude fibre (12%) (Max.), fat (8%) (Max.), calcium (1%) (Min.), phosphorus (0.4%) (Min.) and metabolizable energy (2650 Kcal/Kg) (Min.) was fed ad libitum to the chicks in metallic chicks feeders (2/group) and potable water in plastic drinkers (2/group) from day-old to 9 weeks of age.
Experimental challenge and treatments
A vvIBDV was obtained from the Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. Chicks in groups A, B, C, D and E were inoculated with 0.05 ml of vvIBDV inoculums per bird equivalent to 1.6 x 104.6 CID50 via oral route at 5 weeks of age. At 3 days post inoculation (pi), chicks in group A were treated with VHK at recommended dose rate of 1 g/L, group B chicks were treated with SHK at a dose rate of 2 ml/L, group C chicks were treated with BVC at a dose rate of 1 g/2 L, group D chicks were treated with VRX at a dose rate of 1 ml/L, while group E (positive control) and F (negative control) were administered water. All treatments were administered ad-libitum for 5 days.
A vvIBDV was obtained from the Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. Chicks in groups A, B, C, D and E were inoculated with 0.05 ml of vvIBDV inoculums per bird equivalent to 1.6 x 104.6 CID50 via oral route at 5 weeks of age. At 3 days post inoculation (pi), chicks in group A were treated with VHK at recommended dose rate of 1 g/L, group B chicks were treated with SHK at a dose rate of 2 ml/L, group C chicks were treated with BVC at a dose rate of 1 g/2 L, group D chicks were treated with VRX at a dose rate of 1 ml/L, while group E (positive control) and F (negative control) were administered water. All treatments were administered ad-libitum for 5 days.
Determination of severity of clinical signs, morbidity and mortality rates and gross lesions
Clinical signs, morbidity and mortality rates were scored as 1 to 5 based on the method of Joseph (2019). Clinical signs were scored as 1 = mild (1 to 20%), 2 = moderate (21 to 40%), 3 = severe (41 to 60%), 4 = very severe (61 to 80%) and 5 = grave (81 to 100%); where the number of birds that showed any clinical sign per day in each group were presented in percentages (%). Post-mortem examination was carried out and gross lesions were presented in percentages (%).
Clinical signs, morbidity and mortality rates were scored as 1 to 5 based on the method of Joseph (2019). Clinical signs were scored as 1 = mild (1 to 20%), 2 = moderate (21 to 40%), 3 = severe (41 to 60%), 4 = very severe (61 to 80%) and 5 = grave (81 to 100%); where the number of birds that showed any clinical sign per day in each group were presented in percentages (%). Post-mortem examination was carried out and gross lesions were presented in percentages (%).
Determination of infectious bursal disease antibody titres
Blood for antibody assay was collected a day prior to inoculation at 5 weeks of age and after 5 days treatment at 6 weeks of age using 25 guage syringes and needles by jugular venipuncture (2 ml/bird) into plain universal serum bottles without ethylene diammine tetra-acetic acid (EDTA) anticoagulant and the sera separated were stored in refrigerator (-20°C) prior to determination of antibody titre by enzyme-linked immunosorbent assay (ELISA). All the sera collected were tested for antibodies against IBD using a standard commercial ELISA Kit (IDEXX Laboratories Inc., USA), and according to the manufacturer’s instruction (Balami et al., 2019; Kassim, 2014). The ELISA kit test was based on solid phase immunoassay on which serum samples from the pullets were added into the wells in rows of the comb coated with purified IBD antigen. Positive and negative control samples of the kit were added into separate wells in the rows of the comb. The wells were washed off two times to remove unbound antibodies. Color changes and its intensity indicate the amount of antibodies in each sample, which are converted to antibody titres using the comb scale (Kassim, 2014).
Blood for antibody assay was collected a day prior to inoculation at 5 weeks of age and after 5 days treatment at 6 weeks of age using 25 guage syringes and needles by jugular venipuncture (2 ml/bird) into plain universal serum bottles without ethylene diammine tetra-acetic acid (EDTA) anticoagulant and the sera separated were stored in refrigerator (-20°C) prior to determination of antibody titre by enzyme-linked immunosorbent assay (ELISA). All the sera collected were tested for antibodies against IBD using a standard commercial ELISA Kit (IDEXX Laboratories Inc., USA), and according to the manufacturer’s instruction (Balami et al., 2019; Kassim, 2014). The ELISA kit test was based on solid phase immunoassay on which serum samples from the pullets were added into the wells in rows of the comb coated with purified IBD antigen. Positive and negative control samples of the kit were added into separate wells in the rows of the comb. The wells were washed off two times to remove unbound antibodies. Color changes and its intensity indicate the amount of antibodies in each sample, which are converted to antibody titres using the comb scale (Kassim, 2014).
Determination of feed intake and live body weight gain
Feed intake of the pullets in each group was determined on daily basis by weighing the feed consumed before inoculation at 5 weeks of age up to 6 weeks of age. Live body weight gain was also determined prior to inoculation at 5 weeks of age up to after treatment.
Feed intake of the pullets in each group was determined on daily basis by weighing the feed consumed before inoculation at 5 weeks of age up to 6 weeks of age. Live body weight gain was also determined prior to inoculation at 5 weeks of age up to after treatment.
Determination of packed cell volume
Blood for determination of PCV was collected into universal bottles containing ethylene diammine tetraacetic acid (EDTA). Packed cell volume (PCV) was determined based on the standard technique described by Rehman et al. (2003). Blood was aspirated into the heparinized capillary tubes and centrifuged using microhaematocrit centrifuge (TG12MX) at 1500 rpm for 2 minute and the packed cell volume (%) read on microhaematocrit reader.
Blood for determination of PCV was collected into universal bottles containing ethylene diammine tetraacetic acid (EDTA). Packed cell volume (PCV) was determined based on the standard technique described by Rehman et al. (2003). Blood was aspirated into the heparinized capillary tubes and centrifuged using microhaematocrit centrifuge (TG12MX) at 1500 rpm for 2 minute and the packed cell volume (%) read on microhaematocrit reader.
Data Analyses
Clinical signs, morbidity and mortality rates were presented in percentages and scores of 1 to 5. Mean ELISA antibody titres and mean optical density (MOD) values were expressed in mean (+ SD) and presented using descriptive statistics (Olawuyi, 1996).
Clinical signs, morbidity and mortality rates were presented in percentages and scores of 1 to 5. Mean ELISA antibody titres and mean optical density (MOD) values were expressed in mean (+ SD) and presented using descriptive statistics (Olawuyi, 1996).
Results
Clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
Table 1 and figure 1 showed the percentage (%) clinical signs of depression, prostration, recumbence and inappetance observed in all the groups except the negative control (group F). Group B treated with SHK and the positive control (group E) was more depressed with 28.13%, respectively. Group C treated with BVC had lower depression (18.75%) and inappentance (21.88%). Group D treated with VRX had lower recumbence (3.13%), followed by group A treated with VHK and B with 9.38% respectively.
Table 1 and figure 1 showed the percentage (%) clinical signs of depression, prostration, recumbence and inappetance observed in all the groups except the negative control (group F). Group B treated with SHK and the positive control (group E) was more depressed with 28.13%, respectively. Group C treated with BVC had lower depression (18.75%) and inappentance (21.88%). Group D treated with VRX had lower recumbence (3.13%), followed by group A treated with VHK and B with 9.38% respectively.
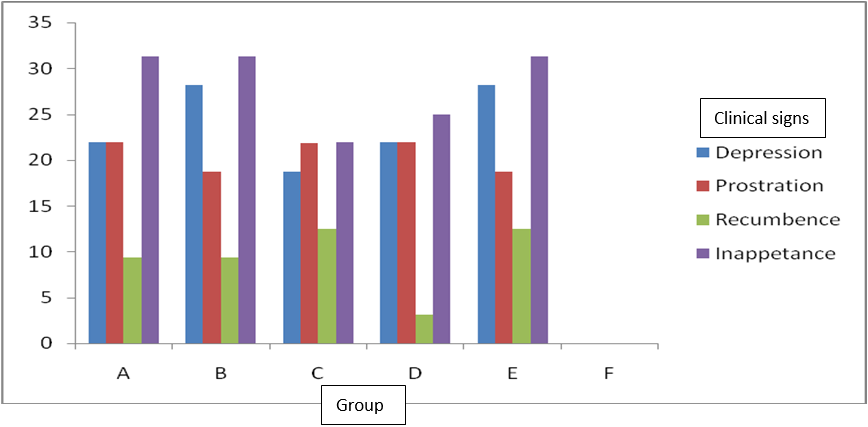
Figure 1: Percentage (%) pullets with clinical signs of virulent infectious bursal disease virus and treated with some antiviral drugs.
| Clinical Signs | Percentage (%) of clinical signs of infectious bursal disease in pullet chicks | |||||
| A | B | C | D | E | F | |
| Depression | 21.88 | 28.13 | 18.75 | 21.88 | 28.13 | 0 |
| Prostration | 28.89 | 18.75 | 21.88 | 21.88 | 18.75 | 0 |
| Recumbency | 9.38 | 9.38 | 12.50 | 3.13 | 12.50 | 0 |
| Inappentance | 31.26 | 31.25 | 21.88 | 25.00 | 31.25 | 0 |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 1: Clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 1: Clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Severity of clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
The severity of clinical signs were higher in chicks of group E (90.63%), followed by group B (87.54%), A (84.42%) and C (75.01%) (Table 2). Chicks treated with VRX (group D) had the least clinical signs (71.92%). Clinical signs observed in groups A, B and E showed higher score of 5, which was grave (serious). Chicks treated with BVC (group C) and VRX (group D) had a score of 4, which was very severe but lower than those treated with VHK (group A), SHK (group B) and untreated but infected birds (group E) (Table 2).
The severity of clinical signs were higher in chicks of group E (90.63%), followed by group B (87.54%), A (84.42%) and C (75.01%) (Table 2). Chicks treated with VRX (group D) had the least clinical signs (71.92%). Clinical signs observed in groups A, B and E showed higher score of 5, which was grave (serious). Chicks treated with BVC (group C) and VRX (group D) had a score of 4, which was very severe but lower than those treated with VHK (group A), SHK (group B) and untreated but infected birds (group E) (Table 2).
| Clinical Signs | Days post-inoculation of pullet chicks (age of chicks in days) | ||||||
| 2 (37) | 3 (38) | 4 (39) | 5 (40) | 6 (41) | 7 (42) | ||
| Group | Severity of clinical signs (%) | Total (%) | |||||
| A | 0 (0.00) | 6 (18.76) | 9 (28.13) | 5 (15.63) | 3 (9.39) | 4 (12.51) | 27 (84.42%) |
| B | 0 (0.00) | 9 (28.14) | 13 (40.63) | 4 (12.51) | 0 (0.00) | 2 (6.26) | 28 (87.54%) |
| C | 0 (0.00) | 10 (31.25) | 14 (43.76) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 24 (75.01%) |
| D | 0 (0.00) | 2 (6.26) | 11 (34.39) | 8 (25.01) | 2 (6.26) | 0 (0.00) | 23 (71.92%) |
| E | 0 (0.00) | 9 (28.14) | 14 (43.75) | 5 (15.64) | 1 (3.13) | 0 (0.00) | 29 (90.63 %) |
| F | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 2: Severity of clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 2: Severity of clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Duration of clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
The duration of clinical signs of depression, prostration, inappetance and morbidity rate in group C was 2 days only (Table 3 and 4). In group A, B and D, the signs lasted for 3 to 5 days. Mortality lasted for 4 days in groups A and E but 3 days in group B, C and D (Table 3 and 5).
The duration of clinical signs of depression, prostration, inappetance and morbidity rate in group C was 2 days only (Table 3 and 4). In group A, B and D, the signs lasted for 3 to 5 days. Mortality lasted for 4 days in groups A and E but 3 days in group B, C and D (Table 3 and 5).
| Clinical Signs | Number of days clinical signs of infectious bursal disease were observed | |||||
| A | B | C | D | E | F | |
| Depression | 5 | 4 | 2 | 4 | 4 | 0 |
| Prostration | 5 | 3 | 2 | 2 | 3 | 0 |
| Recumbency | 1 | 2 | 2 | 1 | 2 | 0 |
| Inappentance | 5 | 4 | 2 | 4 | 3 | 0 |
| Morbidity | 5 | 4 | 2 | 4 | 4 | 0 |
| Mortality | 4 | 3 | 3 | 3 | 4 | 0 |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 3: Duration of clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 3: Duration of clinical signs of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Morbidity rates of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
Morbidity rate observed on day 4 pi was higher in group E (71.88%), A treated with VHK (46.88%) and B treated with SHK (53.13%) (Table 4). Chicks in group C treated with BVC had morbidity rate of 43.75% on day 4 pi, and those in group D treated with VRX had morbidity rates of 40.63% on day 4 pi. By day 7 pi, group E (positive control) had the highest morbidity rate (90.63%), followed by A (84.38%), B (75.0%) and D (62.5%). Group C had lowest morbidity rate (56.25%) (Table 4). Group A and E had higher morbidity score of 5. Group C had the lowest score of 3.
Morbidity rate observed on day 4 pi was higher in group E (71.88%), A treated with VHK (46.88%) and B treated with SHK (53.13%) (Table 4). Chicks in group C treated with BVC had morbidity rate of 43.75% on day 4 pi, and those in group D treated with VRX had morbidity rates of 40.63% on day 4 pi. By day 7 pi, group E (positive control) had the highest morbidity rate (90.63%), followed by A (84.38%), B (75.0%) and D (62.5%). Group C had lowest morbidity rate (56.25%) (Table 4). Group A and E had higher morbidity score of 5. Group C had the lowest score of 3.
| Number of days post-inoculation (age in days) | |||||||
| Group | 2 (37) | 3 (38) | 4 (39) | 5 (40) | 6 (41) | 7 (42) | |
| Morbidity rate (%) | Total (%) | ||||||
| A | 0 (0.00) | 3 (9.38) | 15 (46.88) | 5 (15.63) | 2 (6.25) | 2 (6.25) | 27 (84.38) |
| B | 0 (0.00) | 4 (12.50) | 17 (53.13) | 2 (6.25) | 0 (0.00) | 1 (3.13) | 24 (75.00) |
| C | 0 (0.00) | 4 (12.50) | 14 (43.75) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 18 (56.25) |
| D | 0 (0.00) | 1 (3.13) | 13 (40.63) | 5 (15.63) | 1 (3.13) | 0 (0.00) | 20 (62.50) |
| E | 0 (0.00) | 3 (9.38) | 23 (71.88) | 2 (6.25) | 1 (3.13) | 0 (0.00) | 29 (90.63) |
| F | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 4: Morbidity rates of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 4: Morbidity rates of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Mortality rates of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
Mortality rates on day 4 pi were highest in group B and E with 53.13%, each, followed by group C with 37.50% (Table 5). Group A and D had the lowest mortality rate with 31, 25%, respectively. By the end of treatments, group B had highest mortality rate (81.25%) with a score of 5 (serious). Group C and D had mortality rates of 71.88% and 62.50% with a score of 4 (very severe), each. Group E had mortality rate of 78.13% with a score of 4. Mortality rate (53.13%) was still lowest in group A with a score of 3 (severe).
Mortality rates on day 4 pi were highest in group B and E with 53.13%, each, followed by group C with 37.50% (Table 5). Group A and D had the lowest mortality rate with 31, 25%, respectively. By the end of treatments, group B had highest mortality rate (81.25%) with a score of 5 (serious). Group C and D had mortality rates of 71.88% and 62.50% with a score of 4 (very severe), each. Group E had mortality rate of 78.13% with a score of 4. Mortality rate (53.13%) was still lowest in group A with a score of 3 (severe).
| Number of days post-inoculation (age in days) | |||||||||
| Group | 2 (37) | 3 (38) | 4 (39) | 5 (40) | 6 (41) | 7 (42) | Total (%) | ||
| Mortality rate (%) (n = 32) | |||||||||
| A | 0 (0.00) | 0 (0.00) | 10 (31.25) | 4 (12.50) | 2 (6.25) | 1 (3.13) | 17 (53.13) | ||
| B | 0 (0.00) | 3 (9.38) | 17 (53.13) | 6 (18.76) | 0 (0.00) | 0 (0.00) | 26 (81.25) | ||
| C | 0 (0.00) | 2 (6.25) | 12 (37.50) | 9 (28.13) | 0 (0.00) | 0 (0.00) | 23 (71.88) | ||
| D | 0 (0.00) | 0 (0.00) | 10 (31.25) | 6 (18.75) | 4 (12.50) | 0 (0.00) | 20 (62.50) | ||
| E | 0 (0.00) | 1 (3.13) | 17 (53.13) | 6 (18.75) | 1 (3.13) | 0 (0.00) | 25 (78.13) | ||
| F | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 5: Mortality rates of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 5: Mortality rates of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Gross lesions of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
The gross lesions observed in groups A to E were haemorrhages of the skeletal muscles (thigh and legs), haemorrhages of the pectoral (breast) muscles, enlarged bursa of Fabricius, haemorrhages of the bursae, haemorrhages of the mucosal junction between the proventriculus and ventriculus, enlarged spleen and congested kidneys (Table 6). Group E (positive control) had higher gross lesions with 24 out of 25 birds (96%), followed by group D with 18 out of 20 (90%). Group C had 19 out of 23 (82.61%) and B with 21 out of 26 (80.77%). Group A chicks treated with VHK had the least gross lesions (12 out of 17) amounting to 70.58% (Table 6). Plates 1 - IX showed some of the gross lesions observed in the pullet chicks.
The gross lesions observed in groups A to E were haemorrhages of the skeletal muscles (thigh and legs), haemorrhages of the pectoral (breast) muscles, enlarged bursa of Fabricius, haemorrhages of the bursae, haemorrhages of the mucosal junction between the proventriculus and ventriculus, enlarged spleen and congested kidneys (Table 6). Group E (positive control) had higher gross lesions with 24 out of 25 birds (96%), followed by group D with 18 out of 20 (90%). Group C had 19 out of 23 (82.61%) and B with 21 out of 26 (80.77%). Group A chicks treated with VHK had the least gross lesions (12 out of 17) amounting to 70.58% (Table 6). Plates 1 - IX showed some of the gross lesions observed in the pullet chicks.
| Group gross lesions | Number of occurrence of gross lesions in pullets (n = number examined) | |||||
| A (n = 17) | B (n = 26) | C (n = 23) | D (n = 20) | E (n = 25) | F | |
| Haemorrhagic thigh/leg muscles | 2 | 4 | 0 | 3 | 3 | 0 |
| Haemorrhagic breast muscles | 0 | 7 | 7 | 3 | 6 | 0 |
| Enlarged bursa of Fabricius | 8 | 9 | 11 | 10 | 10 | 0 |
| Haemorrhagic bursa | 0 | 0 | 0 | 0 | 1 | 0 |
| Haemorrhagic mucosal junction between the proventriculus and ventriculus | 1 | 0 | 0 | 0 | 0 | 0 |
| Enlarged and congested kidneys | 1 | 0 | 0 | 1 | 1 | 0 |
| Enlarged spleen | 0 | 1 | 1 | 1 | 3 | 0 |
| Group total gross lesions (average/bird) = | 12 (0.71) | 21 (0.81) | 19 (0.83) | 18 (0.90) | 24 (0.96) | 0 |
| Percentage (%) gross lesion/bird = | 70.58 | 80.77 | 82.61 | 90.00 | 96.00 | 0 |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 6: Gross lesions of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 6: Gross lesions of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
ELISA antibody titres of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
None of the chicks tested for IBDV antibodies at day 35 of age prior to inoculation had high antibody titres as indicated by the ELISA mean antibody titres and by mean optical density (MOD) values (Table 7). High antibody titre was detected in group A (3,076), B (1,483), C (2,996), D (1,898) and E (2,083) when the chicks were tested on day 7 pi (42 days of age). Group F (negative control) had low antibody titre. The antibody titre of pullets in group A treated with VHK was significantly higher (P<0.05), possibly due to its immune modulatory effect.
None of the chicks tested for IBDV antibodies at day 35 of age prior to inoculation had high antibody titres as indicated by the ELISA mean antibody titres and by mean optical density (MOD) values (Table 7). High antibody titre was detected in group A (3,076), B (1,483), C (2,996), D (1,898) and E (2,083) when the chicks were tested on day 7 pi (42 days of age). Group F (negative control) had low antibody titre. The antibody titre of pullets in group A treated with VHK was significantly higher (P<0.05), possibly due to its immune modulatory effect.
| Day pre-inoculation | Day post-inoculation | |||
| 0 | 7 | |||
| Group | MOD values | Mean ELISA ( ± SD) antibody titre | MOD values | Mean ELISA ( ± SD) antibody titre |
| A | 0.112 ± 0.01 | 206 ± 38.18 | 0.709 ± 0.01 | 3,076 ± 415.78 |
| B | 0.124 ± 0.01 | 252 ± 43.84 | 0.382 ± 0.40 | 1,483 ± 170.01 |
| C | 0.124 ± 0.01 | 252 ± 55.86 | 0.694 ± 0.01 | 2,996 ± 17.68 |
| D | 0.172 ± 0.05 | 463 ± 242.54 | 0.538 ± 0.30 | 1,898 ± 155.64 |
| E | 0.116 ± 0.01 | 219 ± 49.50 | 0.691 ± 0.09 | 2,983 ± 442.65 |
| F | 0.101 ± 0.01 | 159 ± 18.39 | 0.201 ± 0.04 | 589 ± 159.10 |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 7: Mean ELISA antibody titres of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 7: Mean ELISA antibody titres of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Average feed intake and live body weight of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs
Average feed intake at day 7 pi was reduced in group A (45.31 to 27.94 g/bird), B (48.78 to 29.15 g/bird), C (46.21 to 29.72 g/bird), D (47.65 to 30.74 g/bird) and group E (48.89 to 27.92 g/bird). There was increased average feed intake in group F from 48.44 to 58.57 g/bird (Table 8). Group F also had an increased in live body weight gain (0.12 Kg/bird) (Table 9).
Average feed intake at day 7 pi was reduced in group A (45.31 to 27.94 g/bird), B (48.78 to 29.15 g/bird), C (46.21 to 29.72 g/bird), D (47.65 to 30.74 g/bird) and group E (48.89 to 27.92 g/bird). There was increased average feed intake in group F from 48.44 to 58.57 g/bird (Table 8). Group F also had an increased in live body weight gain (0.12 Kg/bird) (Table 9).
| Group | ||||||||
| Number of days post-infection | Age (week) | A | B | C | D | E | F | |
| Average feed intake (g/bird) | ||||||||
| Pre-infection (day 0) | 5 | 45.31 | 48.78 | 46.21 | 47.65 | 48.89 | 48.44 | |
| Post-infection (day 7) | 6 | 27.94 | 29.15 | 29.72 | 30.74 | 27.92 | 58.57 | |
| Decline in feed intake (g/bird) | -17.37 | -19.63 | -16.49 | -16.91 | -20.97 | + 10.13 | ||
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 8: Average feed intake of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 8: Average feed intake of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
| Group | |||||||
| Number of days post-infection | Age (week) | A | B | C | D | E | F |
| Average live body weight (kg/bird) | |||||||
| Pre-infection (day 0) | 5 | 0.37 | 0.37 | 0.37 | 0.36 | 0.37 | 0.37 |
| Post-infection (day 7) | 6 | 0.42 | 0.44 | 0.44 | 0.40 | 0.40 | 0.48 |
| After treatment (day 9) | 7 | 0.46 | 0.47 | 0.47 | 0.45 | 0.44 | 0.49 |
| Difference in live body weight gain (day 0 – 9) | 0.09 | 0.10 | 0.10 | 0.09 | 0.07 | 0.12 | |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with VRX; E = infected but not treated; F = not infected and not treated.
Table 9: Average live body weight gain of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 9: Average live body weight gain of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Packed cell volume (%) of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Group A had a drop in packed cell volume (PCV) from 32.0% to 27.5% (Table 10). Group E (positive control) also had a drop in PCV from 29.0% to 27.5% with a further reduction of PCV to 27.0%. This drop in PCV may be caused by the loss of blood from haemorrhages of the thigh/legs and pectoral (breast) muscles (Plate IV). The loss of blood from these haemorrhages could lead to low PCV.
Group A had a drop in packed cell volume (PCV) from 32.0% to 27.5% (Table 10). Group E (positive control) also had a drop in PCV from 29.0% to 27.5% with a further reduction of PCV to 27.0%. This drop in PCV may be caused by the loss of blood from haemorrhages of the thigh/legs and pectoral (breast) muscles (Plate IV). The loss of blood from these haemorrhages could lead to low PCV.
| Group | |||||||
| Number of days post-infection | Age (days) | A | B | C | D | E | F |
| Average packed cell volume (%) | |||||||
| Pre-infection (day 0) | 35 | 32.00 | 31.00 | 28.50 | 27.00 | 29.00 | 29.00 |
| Post-infection (day 7) | 42 | 27.50 | 31.50 | 29.00 | 30.00 | 27.50 | 29.50 |
| After treatment (day 9) | 44 | 30.00 | 30.00 | 29.50 | 30.50 | 27.00 | 28.50 |
| Reduction in PCV (%) | -2 | -1 | + 1 | + 3 | -2 | - 0.5 | |
A = infected and treated with VHK; B = infected and treated with SHK; C = infected and treated with BVC; D = infected and treated with.
Table 10: Average packed cell volume (%) of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Table 10: Average packed cell volume (%) of pullets challenged with virulent infectious bursal disease virus and treated with some antiviral drugs.
Discussion
The onset of clinical signs of IBD in this study was sudden after an incubation period of 3 days and the affected birds showed depression, reduced feed intake and drop in weight gain. These observations were similar to those reported by other authors (Abdu, 2007; Ogbe et al., 2003; Abdu and Saidu, 2002). Group E (positive control) recorded higher clinical signs with a score of 5. Clinical signs of depression and inappentance were low in group C and the duration of the clinical signs was short (2 days) because the birds drank the medicated water (BVC). The medicated water (BVC) contained vitamin B1, B2 and B6, which are essential for prevention and treatment of viral diseases and recovery of damaged organs thereby reducing the duration of clinical signs due to IBDV infection. Groups A, B, D and E had high morbidity rates possibly because the birds did not drink the medicated waters resulting in longer duration of clinical signs of depression and inappetance. VHK, SHK and VRX could not reduce duration of clinical signs of vvIBDV. In this study, the duration of clinical signs and morbidity lasted for 4 days compared to that reported by Kassim (2014), which was 6 dpi in chicks inoculated with vvIBDV but were not treated.
Morbidity rates were highest on the fourth day of commencement of clinical signs in all the groups. The observed short duration of morbidity rate in group C may be due to the slightly low depression and inappentance of the pullets in this group and so the birds were able to drink the medicated water. Aliyu et al. (2016) reported a high morbidity (80-100%) in pullets infected with virulent IBDV. The high morbidity in this study could be due to the virulent nature of the IBDV inoculated into the chicks.Mortality rates were higher in group B treated with SHK and group E (positive control). Pullets in group D treated with VRX had lower mortality rate. Mortalities due to vvIBDV in pullets in Nigeria are usually high (50-100%) and up to 70% of the total mortalities occur by the third or fourth day of appearance of clinical signs (Abdu et al., 2001; Okoye, 2005).
In this study, the mortalities lasted 4 days in group A and E. While those in group B, C and D lasted 3 days. Possibly, treatments with SHK, BVC and VRX may be responsible for the reduction in the duration of mortalities in these groups. There was a day delay in the onset of mortality in pullets treated with either VHK or VRX and the mortalities on the fourth day were also low. This study showed that treatment with either VHK or VRX could reduce onset and rate of mortality in pullets. While treatments with SHK, BVC and VRX reduced the duration of mortalities, VHK and VRX reduced the onset of mortalities in pullets.
Gross lesions of enlarged bursa of Fabricius, enlarged spleen, enlarged and congested kidneys, haemorrhages of the thigh, leg and breast muscles, haemorrhages of the mucosal junction between the proventriculus and ventriculus observed in this study were similar to those reported by Abdu and Saidu (2002) and Abdu (2007). Haemorrhagic bursa of Fabricius was reported by Tsukamoto et al. (1999). Enlargement of the spleen and kidneys were reported by Igwe et al. (2017). The gross lesions were high in all the groups (70-96%) in which each bird showed at least one gross lesion due to IBDV infection (Plates I - IX). The presence of gross lesions in the chicks in all the treated groups implies that the drugs used could not prevent the development of gross lesions caused by vvIBDV infection.
None of the groups tested for IBDV antibodies prior to inoculation (day 35 of age) had high antibody titres as indicated by the low ELISA mean antibody titres and mean optical density (MOD). The high antibody titres detected in groups A, B, C, D and E (2,083), when the pullets were tested on day 7 pi (day 42 of age) could be as a result of responses to vvIBDV infection. Group F (negative control) had lowest mean antibody titre and optical density because the birds were not infected. Low mean optical density (MOD) was reported in pullets pre-vaccination with a rise in MOD at 4 weeks after vaccination against IBD (Ogbe et al., 2008). The higher ELISA antibody titres observed in chicks treated with VHK may be attributed to its immunostimulation activity.
The reduction in the average feed intake was lowest in chicks in group C than those in group A, B, D and E (positive control). Group F (negative control) had an increase in average feed intake. Increase in average live body weight gain was higher in groups B and C, followed by groups A, D and E. Group F had the highest average live body weight gain. The decrease in packed cell volume (PCV) in groups A, B and E at 9 dpi may be due to the haemorrhages (blood loss) in the skeletal muscles, proventriculus and ventriculus. Kassim (2014) reported low PCV in chicks infected with vvIBDV and thus suggested that the virus has effect on PCV, thereby decreasing it. In chickens, PCV count of less than 30% indicates anaemia (Kassim, 2014).
There were increases in PCV after treatments in group D and C and decreases in groups A and E. The medicines used in group D contained vitamin K (an essential factor) that plays a role in the synthesis of prothrombin (a precursor of thrombin), which converts fibrinogen in blood plasma into fibrin that holds blood clots together to prevent blood loss or haemorrhage in chicks (McDonald et al., 1995). The deficiency of vitamin K can be one of the aetiological factors of haemorrhagic syndrome in poultry resulting in blood tinged faeces, haemorrhages in the thigh, breast muscles and other organs (Chauhan and Roy, 2007). The drugs used in treatment of chicks in group C contained vitamins B1, B2 and B6, which are required to improve appetite. Chicks deficient in vitamins B1, B2 and B6 have poor appetite and may develop anaemia (McDonald et al., 1995).
Conclusions and Recommendations
In conclusion, this study showed that clinical signs in pullet chickens challenged with virulent IBDV and treated with VRX and BVC were reduced. The duration of clinical signs of pullets treated with BVC lasted 2 days only. Morbidity rate (56.25%) was ameliorated in pullets treated with BVC and VRX. Pullets challenged with virulent IBDV and treated with VHK had lower mortality rate (53.13%). Gross lesions were lower in pullets treated with VHK. Pullets treated with VHK had significantly higher antibody titres (p<0.05) against the virulent IBDV. The decline in feed intake due to IBDV was ameliorated by treatment with either VRX or BVC. Chicks treated with either SHK or BVC had slightly improved live body weight gain. Treatment with either VRX or BVC ameliorated the reduction of packed cell volume caused by the virulent IBDV in pullet chickens.
It was therefore recommended that BVC can be used in pullets to reduce the duration of clinical signs caused by virulent IBDV; BVC or VRX can be used to ameliorate the morbidity rate of IBDV in pullets, and VHK can be used to ameliorate the mortality rate and gross lesions caused by IBDV. SHK or BVC can be used to improve the live body weight gain of pullets infected with virulent IBDV. VRX or BVC can be used to ameliorate the decline in feed intake and reduction of packed cell volume of pullets infected with virulent IBDV. VHK can be used as immune modulator in pullets to stimulate high antibody titre against virulent IBDV.
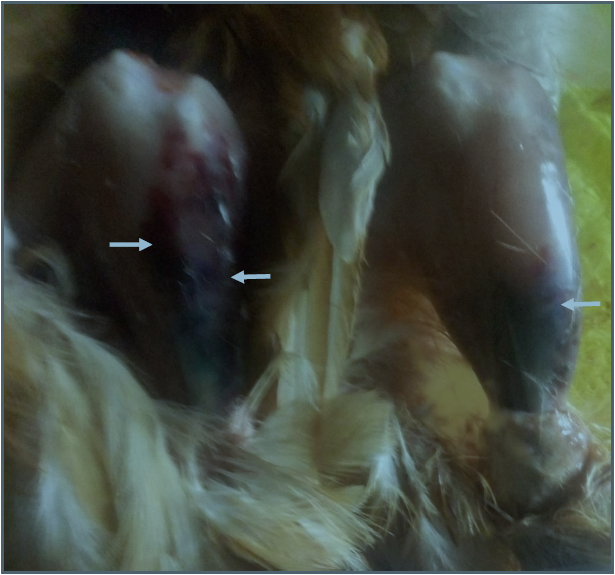
Plate I: Ecchymotic haemorrhages of the legs muscles in pullets (group B); (white arrows showed ecchymotic haemorhage).
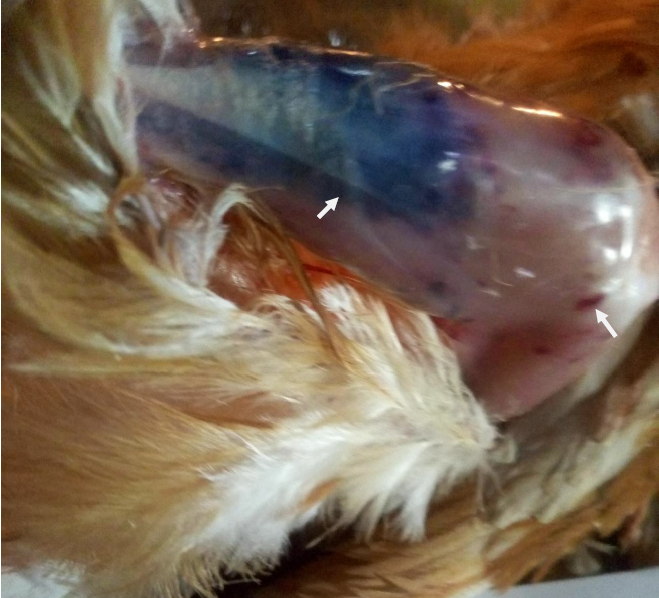
Plate II: Ecchymotic haemorrhages of thigh and leg muscles in pullets (group C); (white arrow showed ecchymotic haemorrhage).
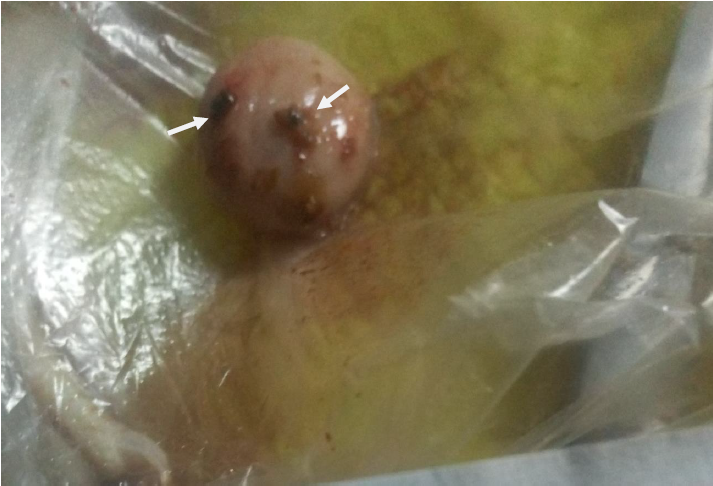
Plate III: Enlarged and haemorrhagic bursa of Fabricius in pullets (group B); (white arrows showed ecchymotic haemorrhage)
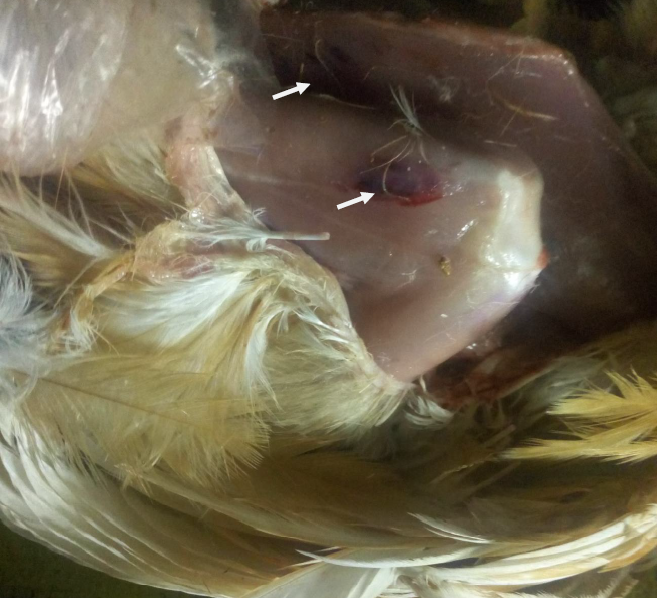
Plate IV: Ecchymotic haemorrhages of the thigh and breast muscles in pullets (group E); (white arrows showed ecchymotic haemorrhage)
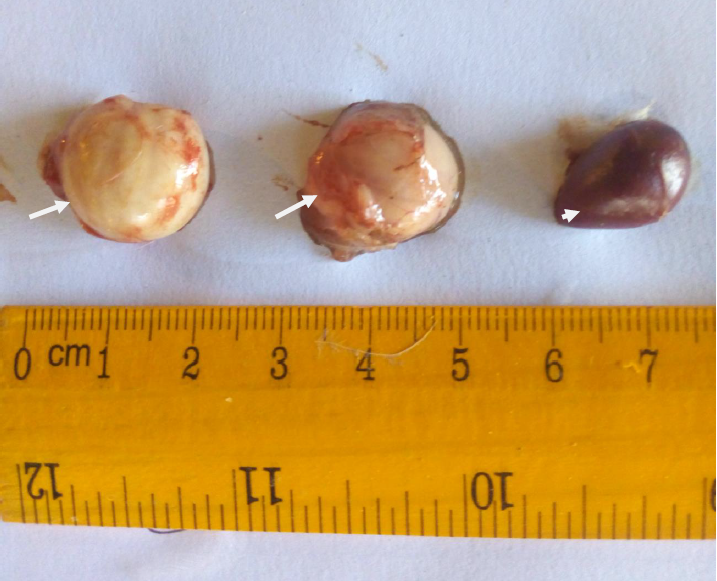
Plate V: Enlarged bursa of Fabricius and spleen in pullets (group B); (white arrows showed enlarged bursae; white arrow head showed enlarged spleen).

Plate VI: Enlarged bursa of Fabricius and spleen in pullets (group C); (white arrows showed enlarged bursae; white arrow head showed enlarged spleen).

Plate VII: Enlarged bursa of Fabricius and spleen in pullets (group D); (white arrows showed enlarged bursae; white arrow head showed enlarged spleen).

Plate VIII: Enlarged bursa of Fabricius and spleen in pullets (group E); (white arrows showed enlarged bursae; white arrow head showed enlarged spleen)
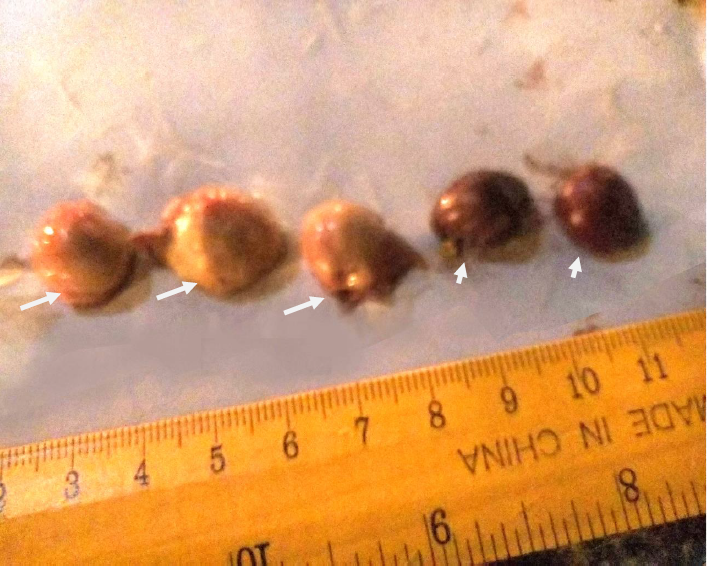
Plate IX: Bursae and spleen in pullets (group F); (white arrows showed normal bursae; white arrow heads showed normal spleen)
References
- Abdu PA (2007). Gumboro disease. Manual of Important Poultry Diseases in Nigeria. Second Edition. Printed by MacChin Multimedia Designers, Samaru, Zaria, Nigeria. pp. 15-24.
- Abdu PA and Saidu L (2002). Common poultry diseases in Nigeria and their control. In: A Training Manual on National Training Workshop on Poultry Production in Nigeria held 1-6 September, 2002. Poultry Production in Nigeria Edited by Gefu JO, Adeyinka IA and Sekoni AA. Published by NAPRI, A.B.U, Shika, Zaria, Nigeria. pp. 129-145.
- Abdu PA, Umoh JU, Abdullahi SU and Saidu L (2001). Infectious bursal disease (Gumboro) of chickens in Nigeria. Tropical Veterinarian, 19(4): 216-236.
- Aliyu HB, Sa’idu L, Jamilu A, Andamin AD and Akpavie SO (2016). Outbreaks of virulent infectious bursal disease in flocks of battery cage brooding system of commercial chickens. Journal of Veterinary Medicine, 16: 1-7.
- Awolaja OA and Adene DF (1995). Infectious bursal disease outbreak in a vaccinated poultry flock. Tropical Veterinarian, 13(1/2): 37-43.
- Balami AG, Pilarshimwi JY, Shallmizhili J, Etim AB and Abdu PA (2019): Antibody response to Newcastle disease vaccine of cockerels challenged with virulent infectious bursal disease virus and administered some complementary and alternative therapies. Journal of Applied Sciences, 466-472.
- Chauhan HVS and Roy S (2007). Hypovitaminosis In: Nutritional diseases. Poultry diseases, diagnosis and treatment. Third edition. New Age International Publishers, Delhi, India. p 164.
- Da Costa B, Soignier S, Chevalier C, Henry C, Thory C, Huet JC and Delmas B (2003): Blotched snakehead virus is a new aquatic birnavirus that is more related to avibirnavirus than to aquabirnavirus. Journal of Virology, 77: 715-725.
- Durojaiye OA, Ajibade HA and Olafimiham GO (1984). An outbreak of infectious bursal disease in 20-week old birds. Tropical Veterinarian, 2: 175-176.
- Eterradossi N and Saif YM (2008). Infectious bursal disease. In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK and Swayne DE. (Eds). Diseases of Poultry, 12th Edition, Wiley-Blackwell., Iowa State University Press, Ames., U.S.A. pp. 185-208.
- Joseph G (2019). Evaluation of the prophylactic ability of Chick-on®, King herbs®, Vitalyte extra®, antimicrobial cocktail and Khaya senegalensis leaves on infectious bursal disease in broiler chickens. M.Sc Thesis. Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria, pp. 50-85.
- Igwe AO, Nwachukwu OJ, Chinyere CN and Shittu I (2017): Evaluation of pathological changes of natural infectious bursal disease virus infection in the lymphoid organs of Black Harco pullets. Sokoto Journal of Veterinary Sciences, 15(2): 18-28.
- Kassim I (2014). Pathological, haematological and biochemical changes in cockerels experimentally infected and vaccinated against infectious bursal disease virus. MSc Thesis. Department of Veterinary Pathology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. pp. 25-101.
- Kibenge FSB, Dhillon AS and Russel RG (1988): Biochemistry and immunology of infectious bursal disease virus. Journal of General Virology, 69: 1757-1775.
- McDonald P, Edwards RA, Greenhalgh JFP and Morgan CA (1995). Vitamins. Animal nutrition. Fifth Edition. Pearson Education, Edinburh, United Kingdom. pp. 78-80.
- Muller H, Islam MR and Rane R (2003). Research on infectious bursal disease of poultry- the past, the present and the future. Veterinary Microbiology, 97(1-2): 153-165.
- Musa IW, Saidu L, Adamu J, Mbuko IJ, Kaltungo BY and Abdu PA (2010 ). Outbreak of Gumboro disease in growers in Zaria, Nigeria. Nigerian Veterinary Journal, 31: 306-310.
- Musa IW, Sai’du L and Abalaka ES (2012). Economic impact of recurrent outbreaks of Gumboro disease in a commercial poultry farm in Kano, Nigeria, Asian Journal of Poultry Science, 6(4): 152-159.
- Ogbe AO, Mgbojikwe LO, Owoade AA, Atawodi SE and Abdu PA (2008). The effect of a wild mushroom (Ganoderma lucidum) supplementation of feed on the immune response of pullet chickens to infectious bursal disease vaccine. Electronic Journal of Environmental, Agricultural and Food Chemistry (EJEAFChe), 7(4): 2844-2855.
- Ogbe AO, Adeyefa CAO, Joshua RA and Owoade AA (2003). Effects of different handling temperatures on the immunogenicity of infectious bursal disease vaccines. Nigerian Veterinary Journal, 24(3): 13-18.
- Okoye JOA (2005). The changing faces of infectious bursal disease and the problems in its surveillance and control. Proceedings of the Workshop on Improved Disease Diagnosis, Health, Nutrition and Risk Management Practices in Poultry Production Efficiency; organized by the Department of Veterinary Surgery & Medicine and Veterinary Teaching Hospital, Ahmadu Bello University, Zaria, Nigeria held 29 – 1st December, 2005, pp. 22-34.
- Olawuyi J.F (1996). Biostatistics: A Foundation Course in Health Sciences. First Edition. University College Hospital, Published by Tunji Alabi Printing Co. Total Garden, Ibadan, Nigeria, pp. 1-221.
- Rehman H, Abbas, S and Lohahet N (2003). Laboratory Manual of Physiology. Society of Veterinary Physiology, Lahore, Pakistan., Vol. 1.
- Sharma JM (1984). Effect of infectious bursal disease virus on protection against Marek’s disease by herpesvirus vaccine. Avian Diseases, 28: 629-640.
- Sun Y, Song M, Niu L, Bai X, Sun N, Zhao X, jiang J, He J and Li H (2013): Antiviral effects of the constituents derived from Chinese herb medicines on infectious bursal disease virus. Pharmaceutical Biology, 51(9): 1137-1143.
- Tsukamoto K, Kojima C, Komori Y. Tanimura N, Mase M and Yamaguchi S (1999): Protection of chickens against very virulent infectious bursal disease virus (IBDV) and Mareks disease virus (MDV) with recombinant MDV expressing IBDV VP2. Virology, 257(2): 352-362.
- Van den Berg TP (2000). Acute infectious bursal disease in poultry: A review; Avian Pathology, 29: 175-194.
Citation: Ogbe AO, Shallmizhili J and Abdu PA. (2021). “Effects of Some Antiviral Medicines on the Performance and Antibody Titres of Pullets Challenged with Very Virulent Infectious Bursal Disease Virus”. Archives of Veterinary and Animal Sciences 3(1). DOI: 10.5281/zenodo.5219782
Copyright: © 2021 Ogbe AO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
