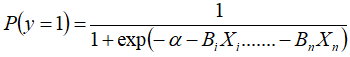Research Article
Volume 2 Issue 1 - 2020
Effect of Thermal Stress on Physiological Parameters in Dogs from the Tropical Region of Camaguey, Cuba.
1,3University of Camagüey Ignacio Agramonte Loynaz, Cuba
2Faculty of Veterinary Medicine, Technical University of Manabí, Ecuador
2Faculty of Veterinary Medicine, Technical University of Manabí, Ecuador
*Corresponding Author: Ivan Pena G, University of Camagüey Ignacio Agramonte Loynaz, Cuba.
Received: April 12, 2020; Published: May 05, 2020
Summary
Climate change is one of the main challenges we face in recent years; As a consequence, heat stress results in the inability of the animal to maintain its body temperature. The study aimed to verify the effect of thermal stress on physiological constants: respiratory, heart rate and rectal temperature in dogs from the tropical region of Camagüey, Cuba. A total of 245 dogs, 113 females and 132 clinically healthy males with different breeds and ages were sampled from February 2017 to February 2018, each with defined owner’s resident in the municipality of Camagüey, who were measured for respiratory rate, heart rate and rectal temperature at different times of the day. The average monthly air temperature and the monthly relative humidity were included. It was determined that the months of greatest temperature-humidity index (ITH) were: July, August and September. The average values of the physiological constants are kept within the normal values for the species under study. The logistic regression model chosen was effective, in terms of correct predictions (91, 6%), which shows the dependence on the respiratory rate of the groups of variables used. Thermal stress causes variation in animal behavior and physiological constants, causing deterioration of well-being and the correlations found between physiological constants with average air temperature, these physiological indicators are suggested as predictors of thermal stress.
Keywords: Dogs; Thermal stress; Heat stress; Physiological constants; Logistic regression; Climate change.
Introduction
Climate change is one of the main challenges we face in recent years; the weather begins to be extreme in places where it was not before. Factors associated with climate change such as the greenhouse effect, ozone, aerosols and land use, as well as other factors where the human being has less control, such as solar variations and volcanoes, exacerbate the increase in temperature and precipitation (Easterling et al., 2016).
In the last 66 years, globally, there is a tendency for hotter days and nights, as well as increased rainfall in some regions or, conversely, less precipitation (Heim, 2015).
All these changes according to, the World Health Organization (2003) cited by Roca (2011), defines stress as the set of physiological reactions that prepares the body for metabolic action.
Stress implies any factor that acts internally or externally to which it is difficult to adapt and that induces an increase in effort on the part of the animal, to maintain a state of balance within itself and with its external environment. Heat stress alters nutritional needs by affecting the gastrointestinal and metabolic system (Hoffmann and Sgrò, 2011; Roca, 2011).
As the ambient temperature rises, the animals begin to use means, to dissipate body heat, and at temperatures above 25°C; You are under heat stress. Changes in rectal temperature and respiratory rate are the two most used physiological parameters as a measure of comfort and adaptability to adverse environments (Hemsworth et al., 1995; Araujo, 2011; Cerqueira, 2013).
The objective of the present study was to evaluate the effect of thermal stress on physiological constants: rectal temperature, respiratory and heart rate in dogs in the tropical region of Camagüey, Cuba.
Materials and Methods
Generalities
The study was carried out in the city of Camagüey, Camagüey province, located in the eastern central region of the island of Cuba; It presents a climate of plains, mainly interior, with seasonal humidification, high evaporation and high air temperature. The average minimum temperature is 22.70°C and the average maximum is 28.90°C. The topography is flat, with values between 100 and 200 meters above sea level altitude (Atlas of the Camagüey province, 1990).
The study was carried out in the city of Camagüey, Camagüey province, located in the eastern central region of the island of Cuba; It presents a climate of plains, mainly interior, with seasonal humidification, high evaporation and high air temperature. The average minimum temperature is 22.70°C and the average maximum is 28.90°C. The topography is flat, with values between 100 and 200 meters above sea level altitude (Atlas of the Camagüey province, 1990).
The sampling was carried out in the period between February 2017 and February 2018, to a total of 245 dogs, 113 females and 132 clinically healthy males with different breeds and ages, each with defined owner’s resident in the municipality of Camagüey, a who respiratory, heart rate and rectal temperature were measured at three times of the day, classified as follows: from 7 am to 12 pm; between 1 p.m. and 5 p.m. and from 6 p.m. at 11 p.m. The average monthly air temperature and the monthly relative humidity were included.
A stethoscope was used to measure the respiratory and heart rate. Rectal temperature was measured following the rectal temperature taking technique according to (Mccoll, 2013) with a mercury thermometer.
Data from the meteorological records were collected through the national agrometeorological bulletins, issued by the Center for Agricultural Meteorology. Ministry of Science, Technology and Environment, Institute of Meteorology. The meteorological records were collected and used to determine heat stress, as well as to estimate the temperature and humidity index (ITH) for months.
Heat stress indices
The ITH is an index that, as the name implies, combines the temperature and humidity to which animals are exposed; it is the index of thermal comfort as an estimator of heat stress.
The ITH is an index that, as the name implies, combines the temperature and humidity to which animals are exposed; it is the index of thermal comfort as an estimator of heat stress.
To evaluate the environmental impact, work has been done on the development of ITH, which combines two or more elements. It is possible to quantify the monthly heat stress, through the calculation of the ITH, according to the modification proposed by (Valtorta and Gallardo, 1996).
ITH = (1, 8 Ta + 32) - (0, 55 - 0.55 HR / 100) (1, 8 Ta - 26)
Ta = average air temperature (ºC)
RH = relative humidity (%)
ITH = (1, 8 Ta + 32) - (0, 55 - 0.55 HR / 100) (1, 8 Ta - 26)
Ta = average air temperature (ºC)
RH = relative humidity (%)
The ITH scale defines critical points or severity levels of heat stress, in the range of 72 to 79 it is considered moderate stress, 80 to 89 is moderate to severe stress and 90 to 98 is severe or even stress lethal (Zimbelman and Collier, 2011).
ITH <= 72 No heat stress
ITH = 72-79 Moderate heat stress
ITH = 80-89 Moderate to severe heat stress
ITH> = 90-98 Severe heat stress
ITH <= 72 No heat stress
ITH = 72-79 Moderate heat stress
ITH = 80-89 Moderate to severe heat stress
ITH> = 90-98 Severe heat stress
Statistical processing
The average values of the different physiological variables contemplated in the investigation were estimated; the existence or not of correlation between the variables contemplated in the study was determined.
Description of the variables used in the analyzes:
Sex. Binomial variable (1 = male, 2 = female).
Rectal temperature Binomial variable (1 = 38 – 38, 5; 2 = 38,6 -…)
Average air temperature Binomial variable (1 = 1-27ºC; 2 = 28-40ºC)
Hours of the day It was coded into three categories (1 = 7 am - 12 pm, 2 = 1 pm - 5 pm; 3 = 6 pm - 11 pm)
The average values of the different physiological variables contemplated in the investigation were estimated; the existence or not of correlation between the variables contemplated in the study was determined.
Description of the variables used in the analyzes:
Sex. Binomial variable (1 = male, 2 = female).
Rectal temperature Binomial variable (1 = 38 – 38, 5; 2 = 38,6 -…)
Average air temperature Binomial variable (1 = 1-27ºC; 2 = 28-40ºC)
Hours of the day It was coded into three categories (1 = 7 am - 12 pm, 2 = 1 pm - 5 pm; 3 = 6 pm - 11 pm)
Also, to quantify the risk factor that causes heat stress in the environmental order by increasing the respiratory rate, the binomial logistic regression with the following general mathematical model was used as an analytical procedure:
Where
P (y = 1): Respiratory rate
a: Constant
Bi: Coefficient
Xi: Explanatory variable (Sex, rectal temperature; average air temperature, daytime hours).
exp = exponential function (neperian antilogarithm).
P (y = 1): Respiratory rate
a: Constant
Bi: Coefficient
Xi: Explanatory variable (Sex, rectal temperature; average air temperature, daytime hours).
exp = exponential function (neperian antilogarithm).
The model included respiratory rate as a dependent variable.
To estimate the reason for the advantages, the (odds ratio) is used, for each variable independently of the model, with a 95% confidence interval, through SPSS version 23 for Windows.
In all statistical analyzes a level of significance of 5% was used.
Results
The influence of monthly heat stress in dogs is shown in Table 1.
| Months | ITH | Category | Interpretation |
| February 2017 | 74 | Alert | Do not leave the pet exposed to the sun |
| March 2017 | 72 | Normal | Suitableconditions, the animal doesnot |
| April 2017 | 76 | Alert | Do not leave the pet exposed to the sun |
| May 2017 | 77 | Alert | |
| June 2017 | 79 | Alert | |
| July 2017 | 80 | Danger | Do not subject animals to too many movements. |
| August 2017 | 80 | Danger | |
| September 2017 | 80 | Danger | |
| October 2017 | 78 | Alert | Do not leave the pet exposed to the sun |
| November 2017 | 76 | Alert | |
| December 2017 | 73 | Alert | |
| January 2018 | 73 | Alert | |
| February 2018 | 73 | Alert |
The ITH was greater than 72 in all months of the year except in March (table 1), being higher in the summer months compared to the winter months. Likewise, it was found that the months with the highest ITH were: July, August and September. Likewise, it is observed that heat stress not only occurs in the summer months, as it is considered, but it is a problem that is present in most months of the year, only that its effect is accentuated more severely during the summer. Normally, in August the average temperature increases with respect to June and July, and is often a very hot month, the hottest month of the year.
| Mean | Standard deviation | ||
| Statistical | Standard error | ||
| Breathing Frequency | 23 | 0,559 | 11,935 |
| Heartrate | 73 | 0,565 | 12,056 |
| Rectal temperature | 38,2 | 0,0086 | 0,1836 |
Table 2: Descriptive analysis of physiological variables.
The average values of the physiological constants; Heart rate, respiratory rate and rectal temperature remain within normal values for the species under study.
| TMA | FR | FC | TR | |
| Average air temperature (TMA) | NS | ** | * | ** |
| RespiratoryRate (FR) | ** | NS | ** | ** |
| Heartrate (HR) | * | ** | NS | ** |
| Rectal Temperature (TR) | ** | ** | ** | NS |
| **. The correlation is significant at the 0.01 level (bilateral). *. The correlation is significant at the 0.05 level (bilateral). |
||||
Not significant (NS)
Table 3: Correlation between climatic and physiological variables.
Table 3: Correlation between climatic and physiological variables.
The correlations between the physiological constants studied and the average air temperature showed positive correspondence.
| Step 1a | B | Standard error | Wald | gl | Sig. | Exp(B) | 95% C.I. for EXP(B) | |
| Lower | Superior | |||||||
| Sex | -2.596 | .596 | 18.989 | 1 | .000 | .075 | .023 | .240 |
| TR | 3.216 | .977 | 10.833 | 1 | .001 | 24.938 | 3.673 | 169.307 |
| TMA | 3.456 | .757 | 20.812 | 1 | .000 | 31.679 | 7.178 | 139.816 |
| Schedule | .763 | .337 | 5.114 | 1 | .024 | 2.144 | 1.107 | 4.152 |
| Constant | -129.239 | 37.649 | 11.784 | 1 | .001 | .000 | ||
| a. Variables in the equation specified in step 1: Sex, TR (rectal temperature), TMA (average air temperature), Schedule. | ||||||||
Table 4: Potential risk factors of temperature rise.
The OR indicates how many times dogs exposed to variations in the average air temperature are likely to suffer from heat stress, compared to those not exposed. We can observe the presence of statistical significance, which reflects differences in the physiology of these animals, as the average air temperature varies. All independent variables used had significant effects; the effect of TR and TMA with odds ratio reached (24,938) and (31,679) is highlighted. We can notice the action exerted by the rectal temperature, an aspect that is motivated by the presence of correlation with the average air temperature.
The logistic regression model chosen was effective, in terms of correct predictions (91, 6%), which shows the dependence on the respiratory rate of the groups of variables used.
Discussion
There are few studies that have been able to quantify the negative effect of heat stress in affective animals specifically in dogs. The ITH has been used to assess the environmental impact; because it can more accurately describe the effects of the environment on the ability of animals to dissipate heat (West, 1999). Changes in temperature and respiratory rate may be indicative of stress or infection (Sharma et al., 2013; Sanmiguel et al., 2018).
The ITH, as shown in table 1, allows us to estimate the adverse effects of heat stress on animal welfare, in our study we confirm the existence of weather conditions conducive to causing heat stress, proving in our case that the month of greatest danger Animal welfare turns out to be the month of August, coinciding with other studies conducted in other species where the greatest impact of heat stress occurs during the summer months (Rodríguez et al., 2005; Domínguez, 2008; Contreras, 2009; Sandoval et al., 2017). Heat stress has a significant impact on all livestock species causing economic losses and much concern regarding animal welfare (Brown-Brandl et al., 2016).
There are tolerance ranges against environmental temperature, called thermal well-being for animals. The best temperature and relative humidity conditions for animals, in general, are around 13 to 18 ° C and 60 to 70%, respectively (Pires and Campos, 2003).
Animal welfare is evaluated through two types of indicators: those based on the animal, such as: physiological (physiological constants), such as: Heart Rate, Respiratory Rate, Temperature, and Pulse and, paraclinical tests, such as: Cortisol and Hemoleukogram. In addition to the aforementioned, there are the Behavioral Indicators, which are the presence of clinical signs compatible with behavioral alterations and, related to the environment such as: accommodation, animal/man, and management (Cano, 2003).
Changes in rectal temperature and respiratory rate are the two most commonly used physiological parameters to measure animal comfort and the ability to adapt to adverse environments (Hemsworth et al., 1995).
Changes in habitual behavior can be seen as the environmental temperature increases, the gasping showing as the respiratory rate increases; the animals remain lying in places with better ventilation, increase water consumption, decrease food consumption, results that coincide with research conducted in livestock (Arias et al., 2008).
Feeding as a fundamental part of maintaining the internal balance, through obtaining nutritional and functional components, is strongly linked to the pleasure pathways that allow the animal to perform behavioral repertoires that allow it to obtain these resources in the environment (Kringelbach et al., 2012).
The decrease in the consumption of a food is not only affected by the characteristics of the product consumed, but also by the physiological state (hunger, satiety, disease, internal temperature, among others) and the psychological state (stress, anxiety etc.) In the case of psychological status, certain stressors could generate changes in the feeding behavior of various animals (Willner, 1991; Grønli et al., 2005; Álvarez and Figueroa, 2015), observing a decrease in the preference or acceptability of palatable substances offered in low concentrations (Willner el al., 1987; Matthews et al., 1995; Álvarez and Figueroa, 2015; Figueroa et al., 2012; Ho and Sommers, 2013). This change does not lie in the ingested substance, but in the animal's ability to recognize its reward (Der-Avakian and Markou, 2012).
The system that regulates stress responses (Hypothalamic-Pituitary-Adrenal Axis: HHA) plays an important role in food-related responses because the neural circuits that regulate energy intake converge in the paraventricular nucleus which contains the cell bodies where the hormone corticotrophin is secreted. So in this area there is a cross between responses to stress and food. (Álvarez and Figueroa, 2015). Additionally, glucocorticoids stimulate the secretion of insulin, orexigenic neuropeptide "Y" in the hypothalamus (Álvarez and Figueroa, 2015) and the expression of other neuropeptides related to food. Glucocorticoids also interact with the hormone leptin producing changes in food responses (Cavagnini et al., 2000; Odeón and Romera, 2017).
It is important to note that the lake of the hair or the density of hair in some races caused an accentuated predisposition to thermal stress and with this a lower well-being results that coincide in studies conducted in other species (Berman, 2003; Araúz, 2017).
As can be seen in Table 3, the existence of bilateral correlations between physiological and environmental variables can be observed, except for heart rate with average air temperature, similar results are shown (Arias et al., 2008; Alzinaet al., 2001). Nienaber et al., (2003) indicated that respiratory rate as body temperature are the main variables affected in relation to thermoregulatory processes, also indicating that environmental temperature and ITH have a marked effect on rectal temperature, respiratory rate and frequency cardiac of the animal. Cerqueira et al., (2016) found in their study that all the correlations analyzed were significant and of high value, suggesting the use of physiological indicators respiratory rate and rectal temperature as predictors of heat stress.
Rectal temperature (TR) is recognized as an important measure of physiological status, as well as an ideal indicator for the evaluation of thermal stress in animals (Srikandakumar and Johnson, 2004; Marko et al., 2011).
Logistic regression is a widely disseminated procedure in health sciences (Silva, 1995; González, 2014) due to the possibilities it offers for prognosis, in studies related to animal health, Table 4 shows how temperature Average air is a potential risk factor in the face of physiological constants and animal welfare, confirming that the main metabolic and physiological changes in situations of heat stress are represented by an increase in body temperature, respiratory and heart rate. These changes characterize the response to stress situations, however, they can have deleterious effects on the physiological status of the animal (West, 2003; Arias et al., 2008; Nardone et al., 2010; Barragán et al., 2015).
Modifications of the photoperiod, temperature, rainfall, among others are environmental factors that affect different moments of the biological development of an individual. These events seem to synchronize physiological and metabolic aspects, according to the conditions in which the animals are found (Souza et al., 2006; Vélez and Uribe, 2010).
Under the loss of efficiency to lose heat in sensitive responses, insensitive response mechanisms are activated. It has been shown that increased respiratory rate is an efficient mechanism to lose heat in situations of heat stress (Ferreira et al., 2006; Arias et al., 2008). However, this increase in respiratory rate alters the acid-basic balance of the blood by loss of CO2, reducing the concentration of carbonic acid (H2 CO3), with the consequent increase in the concentration of bicarbonate (HCO3 -), resulting in a respiratory alkalosis, and subsequently a metabolic acidosis is triggered by over excretion of HCO3 - (West, 2003; Nardone et al., 2010).
Conclusions
- It is shown that thermal stress causes variation in animal behavior and physiological constants, causing deterioration of well-being, justified through the logistic regression model that explains 91.8% of cases.
- The high correlations found between respiratory rate, heart rate and rectal temperature with the average air temperature allow these physiological indicators to be used as predictors of heat stress.
Cited Literature
- Álvarez D, Figueroa j. Anhedonia. (2015). Effect of Stress on Food Behavior and its Possible Implication in Domestic Dogs. Advances in Veterinary Sciences V 30, Nº 1 and 2.
- Alzina A, Farfán J, Valencia E, Yokoyama J. (2001). Environmental condition and its effect on rectal temperature and respiratory rate in crossed cattle (Bostaurus x Bosindicus) in the state of Yucatan, Mexico. Rev Biomed 12: 112-121.
- Araujo, R. (2011). Heat stress in dairy cows, El Salvador.
- Araúz E. (2017). Influence of fur color on body thermal behavior, kinetics of caloric overload and circadian cardiorespiratory alteration in crossed dairy cows (6/8 Bostaurus x 2/8 Bosindicus) under heat stress in the humid tropics. Electronic Veterinary Magazine - ISSN 1695-7504. Volume 18 No. 7.
- Arias R, Mader T, Escobar P. (2008). Climate factors that affect the productive performance of beef and milk cattle. Arch Med Vet 40, 7-22
- Barragán W, Mahecha L, Boxes Y. (2015). Physiological-metabolic variables of heat stress in cows under silvopastoral and meadow without trees. Mesoamerican Agronomy, vol. 26, no. 2, pp. 211-223.
- Berman A. (2003). Effects of body surface area estimates on predicted energy requirements and heat stress. Journal for Dairy Science. 86: 3605-3610.
- Brown-Brandl T, Chitko-McKown G, Eigenberg R, Mayer J, Welsh T, Davis J, Purswell J. (2016). Physiological responses of feedlot heifers provided access to different levels of shade. Animal.
- Cano F. (2003). Animal welfare: experimentation, production, company and zoos: Book of abstracts. Copisterías Don Folio SL. pp. 38-42.
- Cavagnini F, Croci M, Putignano P, Petroni M, Invitti C. (2000). Glucocorticoids andneuroendocrine function. Int. J. Obes. RelatMetabDisord; 24: S77 – S79.
- Cerqueira J, Araújo J, Blanco-Penedo I, Cantalapiedra J, Silvestre M, Silva S. (2013). Study of physiological indicators as predictors of thermal stress of dairy cows in northern Portugal. AIDA, XV Conference on Animal Production,; Volume I, 40-42.
- Cerqueira J, Araújo J, Blanco-Penedo I, Cantalapiedra J, Silvestre A, and Silva S. (2016). Prediction of thermal stress in dairy cows using environmental and physiological indicators. Arch. Zootec. 65 (251): 357-364.
- Contreras MA. (2009). Effect of heat stress on production in a dairy stable in the Trujillo area. Thesis of Zootechnical Engineer. Lima: National Agrarian University La Molina. 26 p.
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Cell 2012; 35: 68–77.
- Dominguez CE. (2008). Effects of heat stress in dairy cows in Lima. Thesis of Zootechnical Engineer. Lima: National Agrarian University La Molina. 37 p.
- Easterling D, Kunkel K, Wehner M, and Sun L. (2016). Detection and attribution of climate extremes in the observed record. Weather Clim. Extrem. 11: 17-27.
- Ferreira F, Pires M, Martínez S, Coelho A, Carvalho P, Ferreira E, Facury F, Fields W. (2006). Physiological parameters of crossed cattle submerged to or heat stress. Architect Bras. Med. Vet. Zootec 58: 732-738.
- Figueroa J, Solà-Oriol D, Pérez J, Manteca X. (2012). Acute stress in post-weaned pigs increases the detection threshold for sucrose. 46 th International Congress of the International Society for Applied Ethology, Vienna, Austria.
- González L, GÓMEZ C, Chemello C, Cubiles M, Santos J, Ortega M. (2014). Triangulation of a qualitative study through logistic regression. Index Enferm (Great). 23 (1-2): 80-84
- Grønli J, Murison R, Fiskea E, Bjorvatnb B, Sørensen E, Portas C, Ursina R. (2005). Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. PhysiolBehav 84: 571-577.
- Heim Jr R. (2015). An overview of weather and climate extremes - Products and trends. Weather Clim. Extrem. 10, Part B: 1–9.
- Hemsworth P, Barnett J, and Beveridge L. (1995). The welfare of extensively managed dairy cattle: a review. ApplAnimBehavSci. 42: 161-182.
- Ho N, Sommers M. (2013). Anhedonia: A Concept Analysis. Arch. Psychiat. Nurs 27: 121-129.
- Hoffmann, A., Sgrò, C. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485.
- Institute of Meteorology of the Ministry of Science Technology and Environment. National Agrometeorological Bulletin. 2017; VOL. 36, No. 04-36. VOL. 37, No. 2-6.
- Kringelbach M, Stein A, van Hartevelt, T. The functional human neuroanatomy of food pleasure cycles. PhysiolBehav 2012; 106: 307-316.
- Marko R, Beli? B, Toholj B, Stevan?evi? M, Potkonjak A, and Lako P. (2011). Influence of respiration rate and rectal temperature in Holstein cows to milk production during heat stress. Serb J. Agri. Sci., 60: 183-189.
- Matthews K, Forbes N, Reid I. (1995). Sucrose Consumption as an Hedonic Measure Following Chronic Unpredictable Mild Stress. PhysiolBehav 57: 241–248.
- Mccoll P, Cohen K, Soto-Aguilar F, Caro A. (2013). Comparison of temperature values obtained with digital otic thermometer and with axillary and rectal mercury thermometers in children under 5 years. Rev ChilPediatr. 84 (3): 293-299.
- Nardone A, Ronchi B, Lacetera N, Ranieri M, Bernabucci U. (2010). Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 130: 57-69.
- Nienaber J, Hahn G, Brown-Brandl T, Eigenberg R. (2003). Heat stress climatic conditions and the physiological responses of cattle. 5th International Dairy Housing Proceedings of the 29-31 January Conference, Fort Worth Texas, USA. ASAE publication No. 701P0203, Pp 255-262.
- Odeón M, &Romera S. (2017). Stress in cattle. Rev. vet. 28: 1, 69-77
- Pires M, Campos A. (2003). Relação two climatic dice with animal performance. in: Resende H, Campos AT, Pires MF. (Orgs) Climatic data and use of the activity at leiteira, 1 ed, Juiz de Fora: EMBRAPA Gado de Leite; (1): 250.
- Roca, A. (2011). Effect of heat stress on animal welfare, a review in time of climate change. SPAMCIENCE, Volume. 2, Number 1.
- Rodríguez L, Ara M, Huamán H, Echevarría L. (2005). Adjustment models for lactation curves of cows in intensive rearing in the Lima basin. Rev Inv Vet Peru 16: 1-12.
- Sandoval R, Ruiz L, Carcelén F. (2017). Determination of the service rate and the factors that affect it in intensive dairy stables in Lima, Peru. RevInv Vet Peru 28: 314-326.
- Sanmiguel R, Plazas F, Trujillo D, Pérez M, Peñuela L, DiGiacinto A. (2018). Requirements for measuring invasive and non-invasive stress indicators in animal production. Rev Inv Vet Peru 29 (1): 15-30.
- Sharma S, Ramesh K, Hyder I, Uniyal S, Yadav VP, Panda, RP, Maurya VP, et al. (2013). Effect of melatonin administration on thyroid hormones, cortisol and expression profile of heat shock proteins in goats (Capra hircus) exposed to heat stress. Small Rumin Res 112: 216-223.
- Silva L. (1995). Excursion to the logistic regression in health sciences. chap. 15. Prediction. Ediciones Díaz de Santos S.A. Madrid Spain. Pp. 121-140.
- Souza M, Uribe L, Ramos A, Oba E. (2006). Plasma levels of total cholesterol, high density lipoproteins (HDL) and cortisol, and its biorritmicidade, in ideal Carneiros-Polwarth. One Hundred Anim Bras; 7 (4): 433-8.
- Srikandakumar A, and Johnson E. (2004). Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian milking zebu cows. Trop Anim. Health Prod., 36 (7): 685
- Valtorta S, Gallardo M. (1996). Heat stress in milk production. In. National Institute of Agricultural Technology. Argentina. Miscellaneous No. 81. 173 - 185 pp.
- Vélez M, Uribe L. (2010). How does heat stress affect reproduction? Biosalud, volume 9 No.2, p. 83-95. ISSN 1657-9550.
- West J. (1999). Nutritional strategies for managing the heat - stressed dairy cow. J AnimSci, 77 (Suppl. 2): 21-35.
- West J. (2003). Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86: 2131-44.
- Willner P, Towell A, Sampson D, Sophokleous, S, Muscat R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93: 358–364.
- Willner P. (1991). Animal models as simulations of depression. Trends Pharmacol Sci. 1991; 12: 131-136.
- Zimbelman R, and Collier R. (2011). Feeding Strategies for High-Producing Dairy Cows During Periods of Elevated Heat and Humidity.
Citation: Ivan Pena G., et al. (2020). Effect of Thermal Stress on Physiological Parameters in Dogs from the Tropical Region of Camagüey, Cuba. Archives of Veterinary and Animal Sciences 2(1).
Copyright: © 2020 Ivan Pena G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.