Research Article
Volume 3 Issue 2 - 2021
Determination of Effective Dose for Ethanol Extract of Dialium guineense Stem Bark
1Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
2Department of Chemistry, College of Arts and Sciences, University of Kentucky, Lexington, USA
2Department of Chemistry, College of Arts and Sciences, University of Kentucky, Lexington, USA
*Corresponding Author: Osahon Abu, Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
Received: October 01, 2021; Published: October 14, 2021
Abstract
Background and Objective: What distinguishes a poison from a remedy is dose.The safety of plant-derived bioactive compounds has become a global concern. The present study was carried out to determine effective dose for ethanol extract of Dialium guineense stem bark.
Methods: Adultmale Wistar rats (n = 30) weighing 160 – 180 g (mean weight = 170 ± 20 g) were randomly assigned to six groups of five rats each. Ethanol extract of the plant stem bark was obtained using cold maceration method. The rats received varied doses of extract (200 - 2000 mg/kg body weight, bwt) orally for 28 days. Concentration of fasting blood glucose (FBG) was used as the therapeutic index.
Results: Ethanol extract of D. guineense stem bark significantly reduced the FBG levels of normal Wistar rats (p < 0.05). The graded and quantal dose response curves showed that1000 mg/kg bwt was effective in reducing the blood glucose of rats.
Conclusion: These results indicate that ethanol extract of D. guineense stem bark possesses hypoglycemic effect at a relatively good dose.
Keywords: Dialium guineense; Dose response; Effective dose; Extract; Therapeutic index
Introduction
Dialium guineense (Velvet Tamarind), a tall, tropical, fruit-bearing tree, belongs to the Leguminosae family. It has small, typically grape-sized edible fruits with brown hard inedible shells. The plant grows in dense forests in Africa along the southern edge of the Sahel and it can be found in West African countries such as Ghana, Sierra Leone, Senegal, Guinea-Bissau and Nigeria. The bark and leaves have medicinal properties and are used against several diseases (Dressler et al., 2014). Despite the widespread use of D. guineense in medical research, the safety of its bioactive components is rarely reported. Extracts of the plant are reported to be rich in important phytochemicals (Abu et al., 2019; Hostettmann and Marston, 1995).
Toxicologists develop extrapolations and hypotheses to explain the adverse effects of chemical agents in situations where there is little or no information (Casarette et al., 1996). Any agent (chemical, drug, food or plant) capable of producing deleterious response in a biological system and seriously injuring functions or causing death is regarded as poison. Similarly, the continuous administration of uncontrolled doses of medicinal plant may possibly lead to any of the following four categories of exposure to poison: acute, sub-acute, sub-chronic and chronic (Abu et al., 2019; Abu and Onoagbe, 2021). The characteristics of exposure and the spectrum of effects come together in a correlative relationship customarily referred to as the dose-response relationship. This is a relationship between exposure and health effect, which can be established by measuring the response relative to an increasing dose. This relationship is important in determining the toxicity of a particular substance. It relies on the concept that a dose, or a time of exposure (to a chemical, drug, or toxic substance), will cause an effect (response) on the exposed organism (Casarette et al., 1996). The present study was carried out to determine effective dose for ethanol extract of Dialium guineense stem bark using Wistar rats.
Materials and Methods
Experimental Rats
Adult male Wistar rats (n = 30) weighing 160 – 180g (mean weight = 170 ± 20g) were obtained from the Department of Anatomy, University of Benin, Benin City, Nigeria. The rats were housed in metal cages under standard laboratory conditions: temperature of 25°C, 55 – 65% humidity and 12-h light/12-h dark cycle. They were allowed free access to rat feed (pelletized growers mash) and clean drinking water. Prior to commencement of the study, the rats were acclimatized to the laboratory environment for one week. The study protocol was approved by the Ethics Committee on Animal Use of the Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
Adult male Wistar rats (n = 30) weighing 160 – 180g (mean weight = 170 ± 20g) were obtained from the Department of Anatomy, University of Benin, Benin City, Nigeria. The rats were housed in metal cages under standard laboratory conditions: temperature of 25°C, 55 – 65% humidity and 12-h light/12-h dark cycle. They were allowed free access to rat feed (pelletized growers mash) and clean drinking water. Prior to commencement of the study, the rats were acclimatized to the laboratory environment for one week. The study protocol was approved by the Ethics Committee on Animal Use of the Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
Collection of Plant Material
The stem barks of D. guineense were obtained from Auchi Area of Edo State, Nigeria and authenticated at the herbarium of the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria.
The stem barks of D. guineense were obtained from Auchi Area of Edo State, Nigeria and authenticated at the herbarium of the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria.
Plant Preparation and Extraction
The stem bark was brushed and shade- dried at 30°C for a period of two weeks and crushed into small pieces using clean mortar and pestle. Ethanol extract of the stem bark was obtained using cold maceration method as described previously (Abu et al., 2015).
The stem bark was brushed and shade- dried at 30°C for a period of two weeks and crushed into small pieces using clean mortar and pestle. Ethanol extract of the stem bark was obtained using cold maceration method as described previously (Abu et al., 2015).
Dose Response Study
The rats were randomly assigned to six groups of five rats each. They received varied doses of extract (200 - 2000 mg/kg bwt) orally with the aid of gavage for 28 days. Concentration of FBG was used as the therapeutic index.
The rats were randomly assigned to six groups of five rats each. They received varied doses of extract (200 - 2000 mg/kg bwt) orally with the aid of gavage for 28 days. Concentration of FBG was used as the therapeutic index.
Statistical Analysis
Data are expressed as mean ± SEM (n = 5), and statistical analysis was performed using Graph Pad Prism Demo (6.07). Groups were compared with Duncan multiple range test. Statistical significance was assumed at p < 0.05.
Data are expressed as mean ± SEM (n = 5), and statistical analysis was performed using Graph Pad Prism Demo (6.07). Groups were compared with Duncan multiple range test. Statistical significance was assumed at p < 0.05.
Results
Concentrations of Fasting Blood Glucose
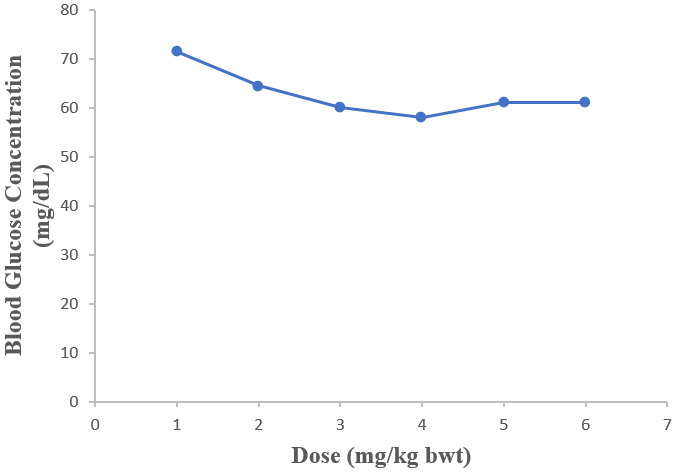
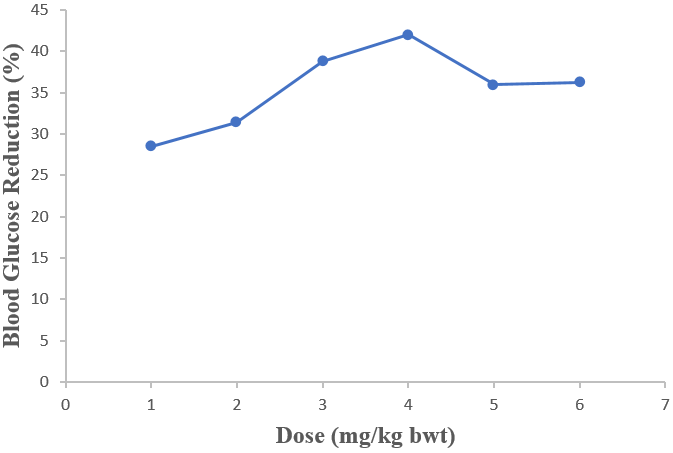
Ethanol extract of D. guineense stem bark significantly reduced the FBG levels of normal Wistar rats (p < 0.05). The graded and quantal dose response curves showed that 1000 mg/kg bwt was effective in reducing the blood glucose of rats (Tables 1 and 2).
Ethanol extract of D. guineense stem bark significantly reduced the FBG levels of normal Wistar rats (p < 0.05). The graded and quantal dose response curves showed that 1000 mg/kg bwt was effective in reducing the blood glucose of rats (Tables 1 and 2).
| Days | 200 mg/kg bwt (mg/dL) | 600 mg/kg bwt (mg/dL) | 800 mg/kg bwt (mg/dL) | 1000 mg/kg bwt (mg/dL) | 1500 mg/kg bwt (mg/dL) | 2000 mg/kg bwt mg/dL) |
| 0 | 100.00 ± 2.50 | 94.00 ± 2.80 | 98.00 ± 3.00 | 100.00 ± 4.00 | 96.94 ± 3.10 | 97.19 ± 4.00 |
| 7 | 88.50 ± 5.02 | 87.50 ± 4.10 | 86.50 ± 4.10 | 80.00 ± 3.00 | 85.00 ± 3.50 | 85.00 ± 1.90 |
| 14 | 80.00 ± 3.00 | 70.00 ± 4.50 | 77.00 ± 2.00 | 72.50 ± 3.70 | 73.00 ± 3.08 | 74.00 ± 3.50 |
| 21 | 75.50 ± 3.50 | 68.00 ± 3.00 | 67.50 ± 3.06 | 64.00 ± 4.02 | 65.00 ± 3.00 | 69.00 ± 2.01 |
| 28 | 71.50 ± 3.10 | 64.50 ± 4.01 | 60.00 ± 2.50 | 58.00 ± 2.50 | 61.00 ± 3.00 | 61.00 ± 2.50 |
Table 1: Concentrations of Fasting Blood Glucose in Rats Treated with Ethanol Extract of D. guineense Stem Bark Data are concentrations of FBG and are expressed as mean ± SEM (n = 5).
| Days | 200 mg/ kg bwt (mg/dL) | 600 mg/kg bwt (mg/dL) | 800 mg/kg bwt (mg/dL) | 1000 mg/kg bwt (mg/dL) | 1500 mg/kg bwt (mg/dL) | 2000 mg/kg bwt (mg/dL) |
| 7 | 11.50 ± 1.05 | 6.91 ± 0.52 | 11.73 ± 2.81 | 20.00 ± 2.00 | 11.46 ± 1.33 | 19.05 ± 2.01* |
| 14 | 20.00 ± 2.00 | 25.53 ± 2.07 | 21.43 ± 2.03 | 27.50 ± 1.90 | 23.96 ± 2.72 | 29.52 ± 2.83 |
| 21 | 24.50 ± 1.50 | 27.66 ± 2.83 | 31.12 ± 2.11 | 36.00 ± 2.30 | 32.29 ± 4.19 | 34.29 ± 2.84 |
| 28 | 28.50 ± 1.95 | 31.38 ± 2.89 | 38.78 ± 2.44 | 42.00 ± 2.00 | 35.94 ± 2.92 | 36.19 ± 3.04 |
Table 2: Glycemic Change in Rats Treated with Ethanol Extract of D. guineense Stem Bark.
Data are concentrations of FBG and are expressed as mean ± SEM (n = 5). *p < 0.05, when compared with group 1 (200 mg/kg bwt group).
Discussion
According to Paracelsus “the right dose differentiates a poison from a remedy”. The science of toxicology is based on the principle that a relationship exists between a toxic reaction (the response) and the amount of toxic substance received (the dose). An important assumption in this relationship is that there is almost always a dose below which no response occurs or can be measured (Eaton and Klaassen, 1996). Another assumption is that when the maximum dose is reached any further increase in dose will produce no increase in effect. Exposure refers to any condition which provides an opportunity for an external environmental agent to enter the body, while dose refers to the amount of agent actually deposited within the body. Response refers to the biological effect elicited by the agent. Dose and response are related and can be represented by a dose-response curve. Data from toxicological testing can be represented by a dose-response curve. A dose-response curve is a curve which shows the relationship between the dose administered and the observed response (Eaton and Klaassen, 1996).
A dose-response curve can be developed for most chemicals. From these curves the threshold level and relative toxicity of chemicals can be obtained to establish safe levels of chemical exposure. There are two types of dose-response curves: one that describes the graded responses of an individualto varying doses of the chemical and one that describes the distribution of responses to different doses in a populationof individuals. The dose is represented on the x-axis, while the response is represented on the y-axis (Eaton and Klaassen, 2001). An important aspect of dose-response relationships is the concept of threshold. This is the dose below which no adverse effect is observed. The identification of the threshold beyond which the human body cannot remain healthy depends on the type of response that is measured and can vary depending on the individual being tested (Eaton and Klaassen, 2001; Marczewski and Kamrin, 1987). In this study, ethanol extract of D. guineense stem bark significantly reduced the blood glucose levels of normal Wistar rats. The graded and quantal dose response curves showed that1000 mg/kg bwt was effective in reducing the blood glucose of rats.
Conclusion
The results obtained in this study indicate that ethanol extract of D. guineense stem bark possesses hypoglycemic effect at a relatively good dose.
References
- Abu, O. D., Imafidon, K. E. and Iribhogbe M. E. (2015). Biochemical effect of aqueous leaf extract of Icacina trichanta Oliv. on urea, creatinine and kidney oxidative status in CCl4-induced Wistar rats. Nigerian Journal of Life Sciences. 5 (1): 85 - 89.
- Abu, O.D and Onoagbe, I.O. (2021). Acute toxicity of aqueous and ethanol extracts of Dialium guineense stem bark. Journal of Bioinnovation. 10 (2): 427 – 432.
- Abu, O.D., Adeogun, E.F. and Ebhohon S.O. (2019). Oral LD50 of total saponins and tannins isolated from Dialium guineense stem bark. European Journal of Experimental Biology. 9 (2): 11- 13.
- Abu, O.D.,Aleogho, B.M. and Omoregie F.O. (2019). Aqueous leaf extract of Icacina trichanta Oliv. Improves lipid profile and CCl4 - induced histological changes in the liver and kidney of Wistar rats. Asian Journal of Research in Biochemistry. 4 (1): 1 – 11.
- Cassette, I., Kaasen, C.D., Amur, M.O. and Dolls, J. (1996). Principles of Toxicology In: Cassarett and Doul’s Pharmacology, The Basic Science of Poison Edited by Curtis, D. Klaassen, 5th edition copyright McGraw – Hill (USA) Health Professional Division New York. Pp 13 – 33; 403-414.
- Dressler, S., Schmidt, M. and Zizka, G. (2014). Dialium guineense: African Plants – A photo guide. Frankfurt/Main: Forschungsinstitut Senckenberg
- Eaton, D.L. and Klaassen, C.D. (1996). Principles of toxicology in Casarett and Doull's Toxicology: The Basis Science of Poisons. 6th edition. McGraw-Hill. Pp. 13 - 33.
- Eaton, D.L., Klaassen, C.D. (2001). Principle of Toxicology, in Klassen CD (ed) Casaret and Doll’s Toxicology. The Basic Science of Poisons. 6th edition. McGraw-Hill. Pp. 11 – 34.
- Hostettmann, K. and Marston A. (1995). Saponins. Cambridge: Cambridge University press. Pp. 3.
- Marczewski, A.E. and Kamrin, M.A. (1987). Toxicology for the Citizen. Michigan State University, Center for Environmental Toxicology. Pp. 1- 16.
Citation: Osahon Abu, IO Onoagbe and I Ojo. (2021). Determination of Effective Dose for Ethanol Extract of Dialium guineense Stem Bark. Journal of Medical Research and Case Reports 3(2).
Copyright: © 2021 Osahon Abu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.