Research Article
Volume 4 Issue 1 - 2022
Description for Five Different Forms of Trypanosoma Evansi Infecting Blood of Camelus Dromedarius.
1,2Zoology Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt
*Corresponding Author: Shehata Abd-Elmaleck, Zoology Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt
Received: July 07, 2022; Published: July 22, 2022
Abstract
Only five specimens (2.5%) from 195 Camelus dromedarius were infected with Trypanosoma evansi. Light microscopy was showed different developmental stages.
At the same time five different forms were distinguished beside that, scanning electron microscopy supported these results by revealed different stages such as binary fission. Also appears of different stages of trypomastigote, epimastigote and metacycling stage.
Keywords: Camelus dromedaries; Epimastigote; Scanning; Different forms; Metacycling
Introduction
Hemoparasitic diseases like trypanosomiasis and dipetalonemiasis have adverse impact on health, productivity and working capacity of camel. Local studies on hemoparasites in camel with particular relation to hematochemical and biochemical dimensions are limited. T. evansi is notorious in developing resistance to drugs and infection popularly called “surra” is probably the most serious protozoan disease of camel and is widespread throughout camel rearing areas of the world (Higgins, et al., 1992). This disease adversely affects the growth, reproduction as well as trekking potential of the camel (Ibadullaev & Khalikov, 1972; Boid, et al., 1985; Lohr, et al., 1986). Trypanosomiasis in camel is usually chronic but can be acute with 90% mortality, if not treated (Luckins, 1992). Chronically infected animals may survive for 3-4 years. The disease in this form is characterized by anemia, emaciation, recurrent fever, atrophy of the thigh muscles, edema of the dependent parts, corneal opacity and diarrhea (Singh, et al., 1980).
Other clinical changes recorded in experimentally induced chronic infections include debility alopecia, keratinization and depletion of subcutaneous fat and facial edema (Raisinghani, et al., 1980). Most of the work on the affection until now is done on the incidence (Elamin, et al., 1998; Dia, et al., 1997a), diagnosis (El sawalhy and Seed, 1998; Pathak, et al., 1997; Olaho-Mukani, et al., 1996) and treatment (Nyang’ao, et al., 1995; Musa, et al., 1994) of the affection in different animals. There are only few reports on the pathology of the disease (Yagoub, 1989; Yadvendra, et al., 1997). Very little work have been done on biochemical and hematological alterations induced by natural trypanosomosis in racing camels.
Tsetse flies (Diptera: Glossinidae) are only cyclical vectors of many trypanosome species in Africa. Human sleeping sickness is endemic to 36 countries in Sub-Saharan Africa with 70 million of the 400 million inhabitants at risk. The continent is recovering from an epidemic that there currently 50-70000 people infected per year (Who, 2006). Sleeping sickness is fatal if left untreated. The related disease (nagana) in domesticated animals causes estimated losses to Africa agriculture (Budd, 1999), and had profound effect of the development of the continent (Kabayo, 2002 and Jordan, 1986). The evidence suggests that all 31 species of Tsetse flies can transmit trypanosomes to greater or lesser extent (Aksoy, 2003). Most economically important African trypanosomes are transmitted during the bite of tsetse fly. Humans are only infected T. bruci rhodesiense and T. b. gambiense. The closely related parasite T. bruci is not pathogenic for humans, although it infects all livestock; as a consequence, this species is used most frequently in laboratory studies.
Other major pathogens of domesticated animals that are transmitted by tsetse are T. congolens, T. vivax and T. simiae, while T. evansi, which is most closely related to the brucei group, is transmitted by biting flies. In recent T. evansi in mainland Spain (Tamarit, et al., 2010), several forms of the parasite were commonly observed in blood smear examinations, some of them resembling Trypanosoma vivax. The molecular techniques confirmed that Trypanosoma vivax was the causal agent, discarding other African trypanosomes.
Material and Methods
Out of 195 blood samples of camels (Camelus dromedarius) examined for blood protozoan parasites collected from different localities of Slaughter houses at Assiut city, Egypt (Dairout, Beni ady, Elethamna). These freshly collected blood samples were divided in two groups one in a tube coated with EDTA, and the other in a test tube for Centrifugation to obtain sera. Thick and thin blood smears were made for morphological examination of Trypanosoma evansi. Electron microscopic studies.
TEM
Few drops from blood which is highly infected with Trypanosoma evansi immediately fixed in 3 ml. of 3% glutaraldehyde solution in phosphate buffer (PH 7.2), for 24 hours and kept at 4°C in refrigerator. The samples were post fixed in 1% Osmium tetra-oxide in phosphate buffer (PH 7.2, 300 mom), for 30 minutes. They were washed several times with phosphate buffer solution. The samples were then embedded in Epon which can preserve in structure from distortion during processing then ultra-thin sections were cut by an Ultra microtome and examined by JEOL, 100 CXII operating at 80 KV (TEM).
Few drops from blood which is highly infected with Trypanosoma evansi immediately fixed in 3 ml. of 3% glutaraldehyde solution in phosphate buffer (PH 7.2), for 24 hours and kept at 4°C in refrigerator. The samples were post fixed in 1% Osmium tetra-oxide in phosphate buffer (PH 7.2, 300 mom), for 30 minutes. They were washed several times with phosphate buffer solution. The samples were then embedded in Epon which can preserve in structure from distortion during processing then ultra-thin sections were cut by an Ultra microtome and examined by JEOL, 100 CXII operating at 80 KV (TEM).
SEM
For scanning electron microscope of blood; few drops were fixed in 3% glutaraldehyde in buffer for 24 hours. Specimens were washed three times in Phosphate buffer and post fixed in 1% Osmium tetra-oxide for 2 hours and then washed in the same buffer. They were Dehydrated in different grades of ethyl alcohol and then mounted on special holders and coated with gold. Then they were examined in a JSM-T 200 L.V. 5400 Scanning Electron Microscopy (SEM)
For scanning electron microscope of blood; few drops were fixed in 3% glutaraldehyde in buffer for 24 hours. Specimens were washed three times in Phosphate buffer and post fixed in 1% Osmium tetra-oxide for 2 hours and then washed in the same buffer. They were Dehydrated in different grades of ethyl alcohol and then mounted on special holders and coated with gold. Then they were examined in a JSM-T 200 L.V. 5400 Scanning Electron Microscopy (SEM)
Results
Microscopy of the examined trypanosomes from dromedary camel in Assiut were morphologically consistent with T. evansi where they were slender with either tapered or blunt ends,only five specimens (2.5%) from 195 Camelus dromedarius were infected with Trypanosoma evansi.
Light microscopy was showed different developmental stages figures. (1-6). At the same time five different forms were distinguished as in table (!) and figures. (7-11). they were slender with either tapered or blunt ends, with long free flagellum and well-developed undulating membrane as in figure (7), with two small folded and sharp posterior end as on figure (8), or no folds and truncated posterior end as in figure (9), or with pulp body shape and Vacuolated like structure with granules as in figure (10) and with with well developed undulating membrane with two to four folds and Normal posterior end as in figure (11).
This indicated that the high prevalence infection in the blood in the same infected specimen. Scanning electron microscopy supported these results by revealed different stages such as binary fission figure. (12), elongated long trypomastigote figure. (13), short epimastigote figure. (14) and metacyclic form figure. (15).
| Type or form no. | 1 | 2 | 3 | 4 | 5 |
| Total length | 23-30 | 20-27 | 28-30 | 20-30 | 36-44 |
| Total width | 2.5-5 | 3-4 | 4-6 | 13.5-20 | 5-7 |
| Nucleus index | 6 x 2.5 | 2 x 5 | 2.5 x 4 | 4 x 4 | 3 x 6 |
| Kinetoplast to the posterior end | 2 | 4 | Contact with it | 10-13 | 3-5 |
| Free flagellum and undulating membrane | Free flagellum length 7 μm with two small folds. | Free flagellum 8 μm with one to two small folds | 8 with no folds | 17-20 with pulp body shape | 10-12 with well developed undulating membrane with two to four folds. |
| Shape of the posterior end | Pointed posterior end | Sharp posterior end | Truncate-d posterior end | Vacuolated like structure with granules | Normal posterior end |
| Nucleus to posterior end | 15 | 15 | 8 | 3 | 15 |
| Nucleus to anterior end | 7 | 13 | 8 | 12 | 10 |
| Figs. no. | Fig. (7). | Fig. (8). | Fig. (9). | Fig. (10). | Fig. (11 ). |
Table 1: Showing differences between different forms of Trypanosoma evansi (measurements with µm).
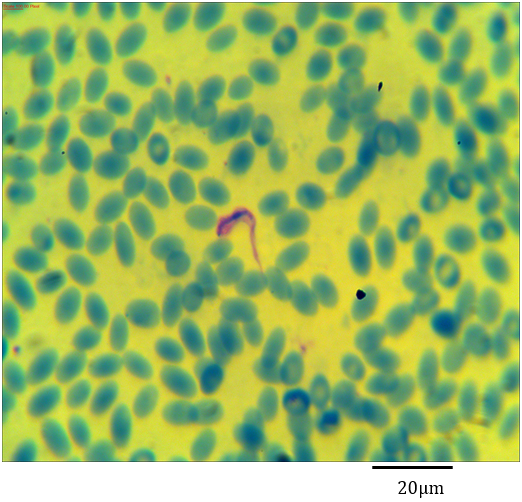
Figure 1: Photomicrograph showing Trypanosoma evansi with an axostyle shaped flagellum in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
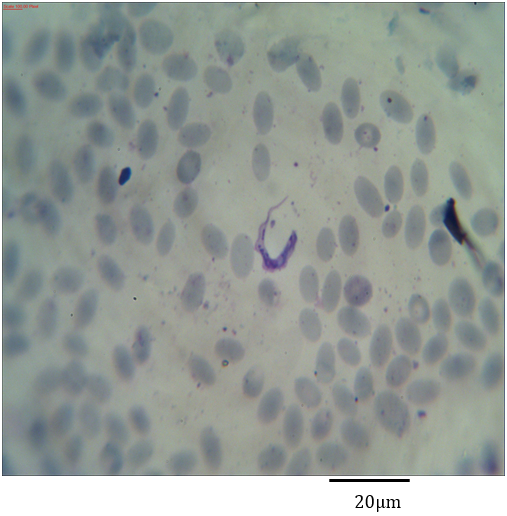
Figure 2: Photomicrograph showing Trypanosoma evansi with granulated cytoplasm in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
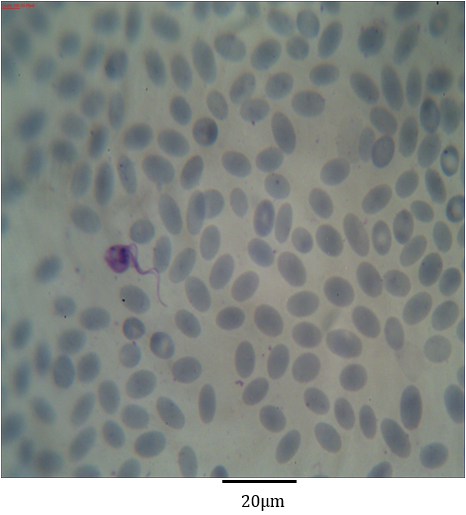
Figure 3: Photomicrograph showing Trypanosoma evansi with kinetoplast similar to the nucleus in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
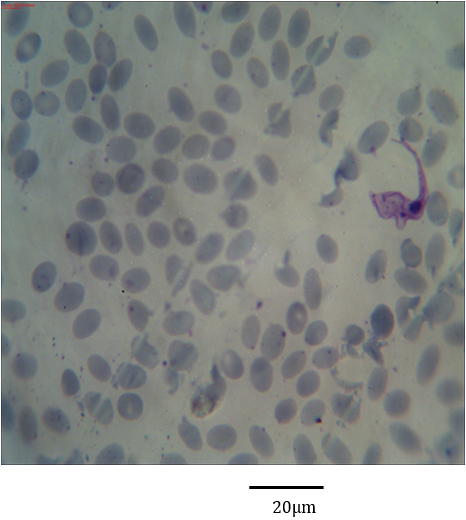
Figure 4: Photomicrograph showing Trypanosoma evansi with clear undulating membrane in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
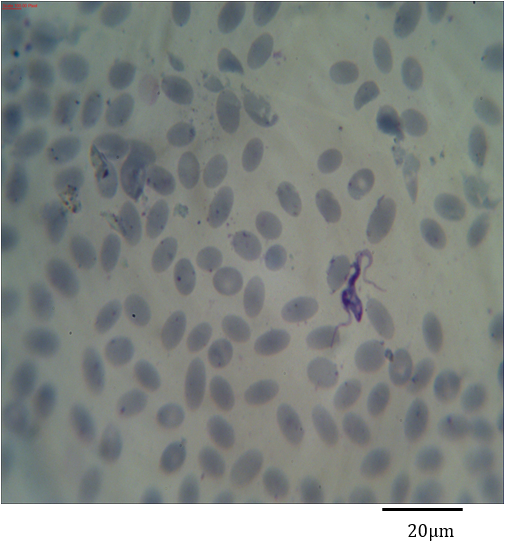
Figure 5: Photomicrograph showing symmetric binary fission of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
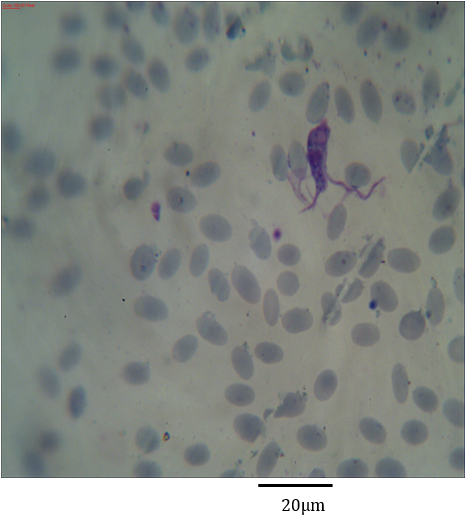
Figure 6: Photomicrograph showing asymmetric binary fission of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
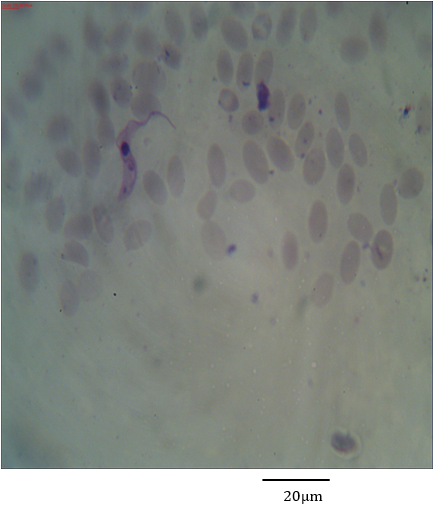
Figure 7: Photomicrograph showing form (1) of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
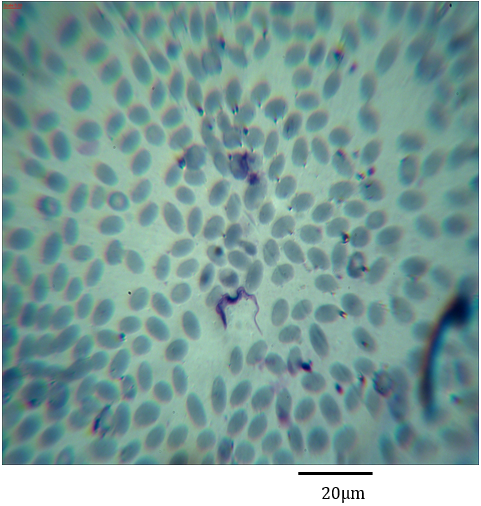
Figure 8: Photomicrograph showing form (2) of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
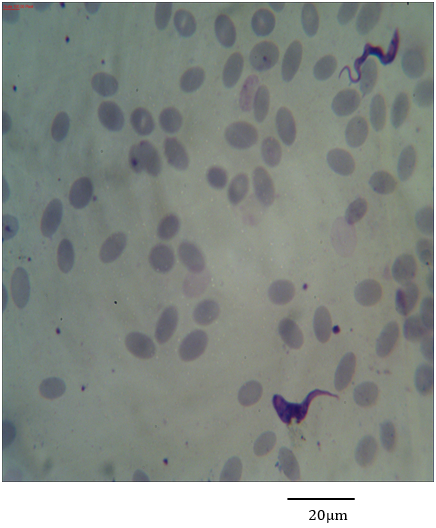
Figure 9: Photomicrograph showing form (3) of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa x bar = 20 µm.
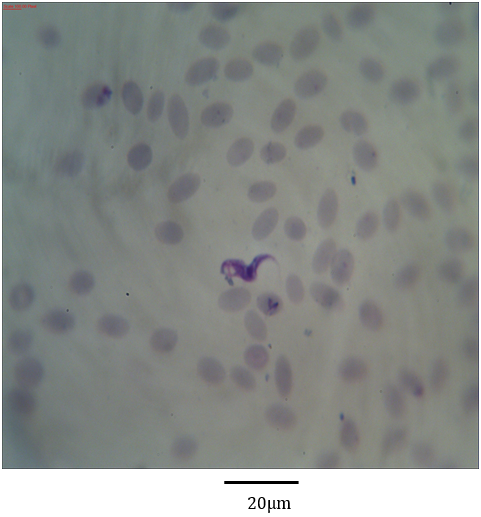
Figure 10: Photomicrograph showing form (4) of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa stain x bar = 20 µm.
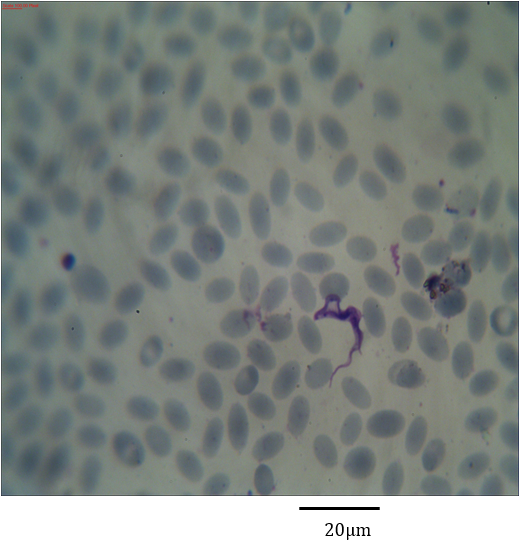
Figure 11: Photomicrograph showing form (5) of Trypanosoma evansi in the blood of Camelus dromedarius stained with Geimsa stain x bar = 20 µm.
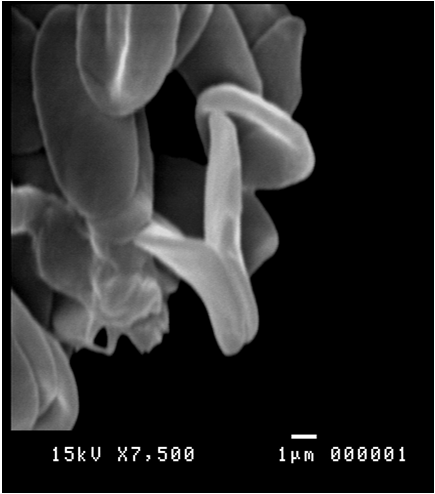
Figure 12: Scanning electron micrograph showing binary fission of Trypanosoma evansi in the blood of Camelus dromedarius x bar = 1 µm.
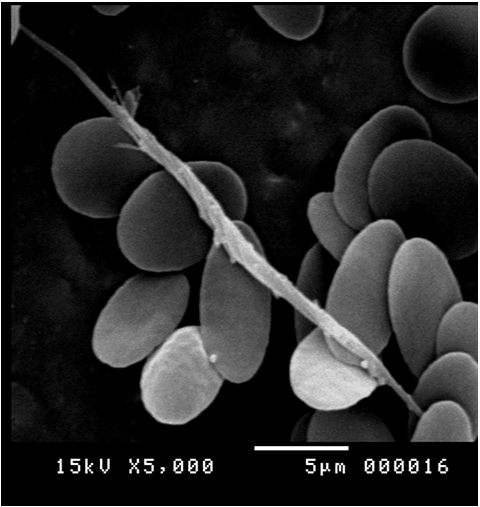
Figure 13: Scanning electron micrograph showing elongated long trypomastigote of Trypanosoma evansi in the blood of Camelus dromedarius x bar = 5 µm.
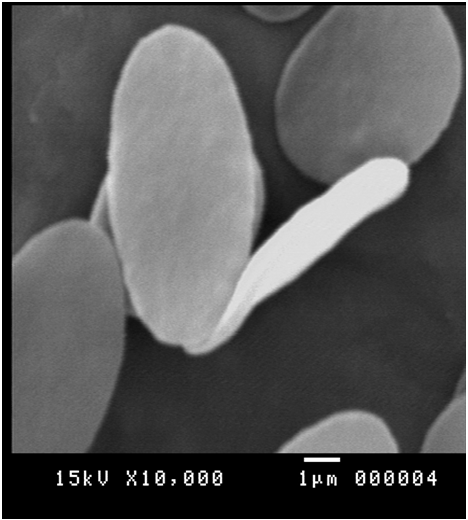
Figure 14: Scanning electron micrograph showing short epimastigote of Trypanosoma evansi in the blood of Camelus dromedaries x bar = 1 µm.
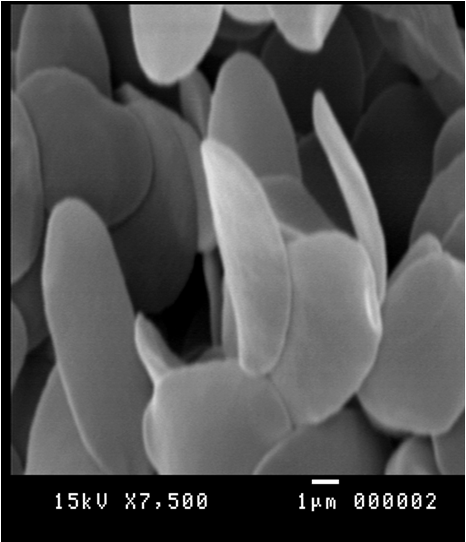
Figure 15: Scanning electron micrograph showing metacyclic form of Trypanosoma evansi in the blood of Camelus dromedarius x bar = 1 µm.
Discussion
The incidence of the infection with Tr. evansi in the present study was found (2.5%). It was a lower as compared to (Shah, et al., 2004) who reported that, the infection rate (13.7%). The lower incidence may due to the high awarness of Tr. evansi effects and treatment means in slaughter houses.
Trypanosomes of the section Salivaria might or might not possess a free flagellum, their kinetoplast is terminal or subterminal in position and the posterior end of the parasite is usually rounded (Hoare, 1964), but in the present study the posterior end vary between rounded to truncated or pointed. The polymorphism of the subgenus Trypanozoon has been indicated in some studies (OMS, 1969), which agree with these present study but some authors considered that, T. evansi has been monomorphic and exclusively in the slender intermediate form (Gill, 1977).
Several studies have observed significant morphological differences in some T. evansi isolate (Ramirez, et al., 1997; Davila, et al., 1998; Gonzalez, et al., 2003); some have even reported a relationship between the parasite’s measurable morphology and the host’s physical condition (Tejero, et al., 2008). Hoare, (1972) was described all isolates of T. evansi ranged 15-34 µm in length. The mean morphological parameters (Length, Posterior end to nucleus, Nucleus to anterior end, Flagellum width and nuclear index) were compatible with the data obtained for T. evansi by other authors (Ramirez, et al., 1997; Silva, et al., 2002; Sarataphan, et al., 2007). However, the presence of several morphological types observed in the present study could suggest presence of different trypanosome species. However, the total length of T. brucei varies from 14 to 39 µm, width mean values between 27 and 29 µm (Hoare, 1972). The longest mean length values obtained in some types (as form 5) which in the range of T. brucei. Also there are some information on the morphological differences of T. evansi from different hosts in the same area (Ramirez, et al., 1997; John, et al., 1992), as well as differences observed in the same animal hosts but located in different geographical areas (Silva, et al., 2002; Gonzalez, et al., 2003).
References
- Higgins, A. J., Allen, W. R., Mayhew, I. G. and Snbow, D. H., (1992). An introduction to the camel in health and disease. Proc. Ist. Int. Camel Conf., Dubai. pp: 9- 17.
- Ibadullaev, F. I. and Khalikov, F. R., (1972). Pathological changes caused by experimental trypanosomiasis in mice, rats and guinea pigs. Trudy Uzbekskogo Nauchno issledovatel’ skog. Vet. Inst., 20: 70–72.
- Boid, R., Jones, T. W. and Luckin, A. G., (1985). The camel in health and disease. Protozoal. diseases of camels. Br Vet J 141: 87–105.
- Boid, R., Jones, T.W. and Luckins, A. G., (1985): Protozoal. Diseas. Camel. Brit. Vet. J., 13: 6- 141.
- Lohr, K. F., Pholpark, S., Siriwan, P., Leesirikul, N., Srikitjakarn, L. and Staak, C., (1986). Trypanosoma evansi infection in buffaloes in north–east Thailand. II. Abortions. Trop. Anim. Hlth. Prod., 18: 103–8.
- Luckins, A. G., (1992). Protozoal disease of camel. Proc. Ist int. Camel Conf., Feb. 2–6, held at Dubai. pp: 23–27.
- Singh, R. P., Vashishta, M. S. and Kala, C., (1980). Diseases of Camels. Seven Seas, Hyderabad, India, p. 9.
- Raisinghani, P. M; Bhatia, J. S., Vyas, U. K., Arya, P. L. and Lodha, K. R., (1980). Pathology of experimentally induced trypanosomiasis in camels. Ind. J. Anim. Sci. 50: 966.
- Dias, J. C. P. & Coura, J. R., (1997). Epidemiologia. In: Clínica e Terapêutica da Doença de Chagas. Uma Abordagem Prática para o Clínico Geral. J. C. P. 33- 66.
- Elamin, E. A., El Bashir, M. O. and Seed, E. M., (1998). Prevalence and infection pattern of Trypanosoma evansi in camels in mid-eastern Sudan. Trop. Anim. Health Prod. 30 (2): 107–114.
- El Sawalhy, A. and Seed, J. R., (1998). Diagnosis of trypanosomiasis in experimental mice and field-infected camels by detection of antibody to trypanosome tyrosine amino-transferase. J. Parasitol. Dec; 84(6): 1245-1249.
- Olaho-Mukani, W., Nyang’ao, J. M. and Ouma, J. O., (1996). Use of Suratex for ?eld diagnosis of patent and non-patent Trypanosoma evansi infections in camels. Br. Vet. J. 152 (1): 109 -111.
- Pathak, K. M., Singh, Y., Meirvenne, N. V. and Kapoor, M., (1997). Evaluation of various diagnostic techniques for Trypanosoma evansi infections in naturally infected camels. Vet. Parasitol. 69 (1–2): 49–54.
- Nyang’ao, J. M., Olaho-Mukani, W., Maribei, J. M. and Omuse, J. K., (1995). Evaluation of the ef?cacy of melarsenoxyde cysteamine (Cymelarsan) in treatment of camels experimentally infected with Trypanosoma evansi using antigen trapping enzyme-linked immunosorbent assay. J. Vet. Pharmacol. Ther. 18 (6): 468–470.
- Yagoub, I. A., (1989). Haematological studies in dromedary camels with single or concurrent natural infections of Trypanosoma evansi and Haemonchus longistipes. Act. Vet. 39 (2–3): 109–119
- WHO. (2006). Human African trypanosomiasis (sleeping sickness). Weekly Epidemiological Record 81: 71-80.
- Budd, L. T., (1999). Dfid-funded tsetse and trypanosomiasis research and development since 1980. Economic analysis. London: Department for International Development.
- Jordan, A. M., (1986). Trypanosomiasis Control and African Rural Development. London: Longman.
- Kabayo, J. P., (2002). Aiming to eliminate tsetse from Africa. Tren. in Parasit. 18: 473 - 475.
- Aksoy, S., Gibson, W. C. and Lehane, M. J., (2003). Interactions between tsetse and trypanosomes with implications for the control of trypanosomiasis. Advances in Parasit. 1-83.
- Tamarit, A., Gutierrez, C., Arroyo, R., Jimenez, V., Zagalá, G., Bosch, I., Sirvent, J., Alberola, J., Alonso, I. and Caballero, C., (2010). Trypanosoma evansi infection in mainland Spain. Vet. Parasitol. 167, 74–76.
- Shah, S. R., Phulan, M. S., Memon, M. A., Rind, R. and Bhatti, W. M., (2004). Trypanosomes infections in camels. Pakist. Vet. J., 24: (4). 209- 210.
- Hoare, C. A., (1964). Morphological and taxonomic studies on mammalian trypanosomes.V. Revision of the system. J. Protozool. 11: 200–207.
- Oms, A., (1969). Studios comparatives sober las Tripanosomiasis Americana y Africana. Ser. Inf. Técn. N? 411. WHO TRS. 411.
- Gill, B. S., (1977). Trypanosomes and Trypanosomiases of Indian Livestock. Indian Council of Agriculture Research, New Delhi.
- Ramirez, L., Dávila, A. M. R., Victorio, A. M., Silva, R. A. M. S., Trajano, V. and Cansen, A. M., (1997). Measurements of Trypanosoma evansi from the Pantanal. Mem. Inst. Oswaldo Cruz. 92: 483-484.
- Gonzalez, J.A., Gonzalez, A.O., Santa Cruz, A.C., Ortiz, J.C., Comolli,J.A., Poux., J.P.,Toccaline, P.A.Navias, J.C.,Cayo, (2003). (Hydrochaeris hydrochaeris ) en cautiverlo, de la Provin de comunicationes Cientificas y TechnologicasPN Unisersiteded Nacional del Nordest. Cyt/ /comunicaciones/04 – Veterinarias / V- 033.
- Hoare, C.A., (1972). The Trypanosomes of Mammals. Blackwell Scientific Publications, Oxford, p. 749. Infectius disease & International Medicine parasitology volume 1 for web, 2008.
- Tejero, F., Roschman-Gonzalez, A., Perrone-Carmona, T.M., Aso, P.M., (2008). Trypanosoma evansi: a quantitative approach to the understanding of the morphometry–hematology relationship throughout experimental murine infections. J. Protozool. Res. 18: 34–47.
- Silva, R.A.S., Seidl, A., Ramirez, L., Davila, A.M.R., (2002). Trypanosoma evansi e Tripanosoma vivax. Biologia, Diagn?stico e Controle. EMBRAPA, Pantanal, Corumbà, 141 pp.
- Sarataphan, N., Vongpakorn, M., Nuansrichay, B., Autarkool, N., Keowkamkah, T., Rodtian, P., Stich, R.W., Jittapalapong, S., (2007).Diagnosis of a Trypanosoma lewisi-like (Herpetosoma) infection in a sick infant from Thailand. J. Med. Microbiol. 56: 1118–1121.
Citation: Barakat Shehata Abd-Elmaleck and Gama Hassan Abed. (2022). Description for Five Different Forms of Trypanosoma Evansi Infecting Blood of Camelus Dromedarius. Archives of Veterinary and Animal Sciences 4(1).
Copyright: © 2022 Barakat Shehata Abd-Elmaleck. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
