Research Article
Volume 2 Issue 1 - 2020
Colorectal Cancer in Niger Delta, Nigeria: Multi-centre lower Gastrointestinal Endoscopy Study.
1Digestive Disease Unit, Oak Endoscopy Centre Port Harcourt, Rivers State, Nigeria
2Department of Surgery, University of Port Harcourt Teaching Hospital Port Harcourt, Rivers State, Nigeria
3Shawsand Medical Centre Port Harcourt, Rivers State, Nigeria
4Gastroenterology Unit, Federal Medical Centre Yenagoa, Bayelsa State, Nigeria
5Department of Anatomical Pathology, University of Port Harcourt Teaching Hospital Port Harcourt, Rivers State, Nigeria
2Department of Surgery, University of Port Harcourt Teaching Hospital Port Harcourt, Rivers State, Nigeria
3Shawsand Medical Centre Port Harcourt, Rivers State, Nigeria
4Gastroenterology Unit, Federal Medical Centre Yenagoa, Bayelsa State, Nigeria
5Department of Anatomical Pathology, University of Port Harcourt Teaching Hospital Port Harcourt, Rivers State, Nigeria
*Corresponding Author: Emeka Ray-Offor, Department of Surgery, University of Port Harcourt Teaching Hospital, P.M.B.6173, Port Harcourt, Rivers State, Nigeria.
Received: January 28, 2020; Published: February 06, 2020
Abstract
Introduction: Colorectal cancer (CRC) is a disease of global importance. The practice and formulation of effective health policies in developing countries is marred by lack of appropriate colorectal cancer screening and up-to-date cancer registry.
Aims: To study the frequency and presentation pattern of CRC in two metropolitan cities in a region of Nigeria.
Materials and Method: An observational study of lower gastrointestinal (GI) endoscopy performed from April 2013 to April 2018 at private/public health facilities with endoscopy service in 2 major cities in the Niger Delta area, Nigeria. A questionnaire was distributed for completion to all practicing endoscopists in the area. The variables collated included demographics, indications, endoscopic findings and histopathology. Analysis was done using IBM SPSS Statistics for Windows, version 20 Armonk, NY.
Results: A total of 466 flexible lower GI endoscopies were recorded: 434 colonoscopies; 32 sigmoidoscopies. Histologically confirmed cases of CRC were 45 (44 adenocarcinoma and 1 non-Hodgkin’s lymphoma) with age range from 28 years to 79 years; mean age of 52.3±13.7 years. There were 30 males and 15 females; predominant site was the rectum with 25 (55.6%) cases recorded.
Conclusion: Colorectal cancer is not uncommon in this Nigerian population. A targeted screening policy is recommended.
Keywords: Colorectal cancer; Colonoscopy; Nigeria
Introduction
Global emerging trend has non-communicable diseases leading to nearly two-third of annual mortality with 70% estimated to occur in low– and middle–income countries (LMIC) [1]. This is double jeopardy as communicable diseases still pose an unresolved challenge in these countries. Globally, colorectal cancer is the third most common cancer responsible for over 600, 000 deaths annually. [2] In Nigeria, colorectal cancer (CRC) is the most common gastrointestinal cancer diagnosed. [3] It is the second most common cancer in males and third most common in females according to two population-based cancer registries., [4] An adenoma-carcinoma sequence is well documented thus an early detection and removal of adenomatous polyps is an effective preventive measure. Flexible lower gastro-intestinal endoscopy is an effective screening, diagnostic and therapeutic modality. [5]
The risk factors for colorectal cancer are modifiable and non-modifiable. The former includes increased intake of red meat, high-fat diet, inadequate intake of fibre, obesity, sedentary lifestyle, smoking and high consumption of alcohol. These factors are rife with urbanization and increasing life expectancy. Evidence to this fact is the increasing incidence of CRC in countries that recently transited from a low to high -income economy like Japan, Singapore and Eastern European countries. [6,7] The non-modifiable risk factors include age, gene-related and personal history of chronic inflammatory bowel disease. A worrisome prevalence of adenomatous polyps in asymptomatic individuals in Niger delta region of Nigeria has been reported. [8] However, there is yet paucity of literature on the incidence and prevalence of colorectal cancer in this region which is experiencing rapid urbanization from oil exploration revenue. [9] The practice and formulation of effective health policies in developing countries is marred by lack of up-to-date cancer registry and appropriate colorectal cancer screening.
This study aims to evaluate the frequency and presentation pattern of CRC in two metropolitan cities in Niger delta region of Nigeria.
Materials and Method
Study setting
This collaborative study was conducted in Endoscopy referral centres in two cities in the Niger delta region of Nigeria- Port Harcourt and Yenagoa. Port Harcourt, is the capital of Rivers state and a major industrial centre lying along the Bonny River which is located in the Niger Delta area. According to the National Bureau of Statistics, Rivers State has a population of 7, 303,924 with Port Harcourt, its capital, being the fifth largest city in Nigeria- Africa’s most populous country. [10] The second city is Yenagoa which is the capital of Bayelsa state and has a population of 2,277,961 with one fifth of this residing in the capital city.
This collaborative study was conducted in Endoscopy referral centres in two cities in the Niger delta region of Nigeria- Port Harcourt and Yenagoa. Port Harcourt, is the capital of Rivers state and a major industrial centre lying along the Bonny River which is located in the Niger Delta area. According to the National Bureau of Statistics, Rivers State has a population of 7, 303,924 with Port Harcourt, its capital, being the fifth largest city in Nigeria- Africa’s most populous country. [10] The second city is Yenagoa which is the capital of Bayelsa state and has a population of 2,277,961 with one fifth of this residing in the capital city.
Study design
This was an observational study from April 2013 to April 2018 of patients undergoing flexible gastro-intestinal endoscopy. A study questionnaire was sent for completion to all endoscopists in centres with functional endoscopy facilities in the two states of Nigeria. Data from two Surgeon endoscopists and a Gastroenterologist were available representing the 5 centres with gastro-intestinal endoscopy facility in the two cities at the inception of study. The variables collated from centre records included: demographics, indications, endoscopic findings and histopathology.
This was an observational study from April 2013 to April 2018 of patients undergoing flexible gastro-intestinal endoscopy. A study questionnaire was sent for completion to all endoscopists in centres with functional endoscopy facilities in the two states of Nigeria. Data from two Surgeon endoscopists and a Gastroenterologist were available representing the 5 centres with gastro-intestinal endoscopy facility in the two cities at the inception of study. The variables collated from centre records included: demographics, indications, endoscopic findings and histopathology.
Statistical analysis
The statistical analysis was done using IBM SPSS Statistics for Windows, version 20 Armonk, NY. The mean age and standard deviation were calculated. The categorical variables were analysed in simple percentages. An association between the variables was made using the chi-square test with a value of P < 0.05 considered statistically significant.
The statistical analysis was done using IBM SPSS Statistics for Windows, version 20 Armonk, NY. The mean age and standard deviation were calculated. The categorical variables were analysed in simple percentages. An association between the variables was made using the chi-square test with a value of P < 0.05 considered statistically significant.
Results
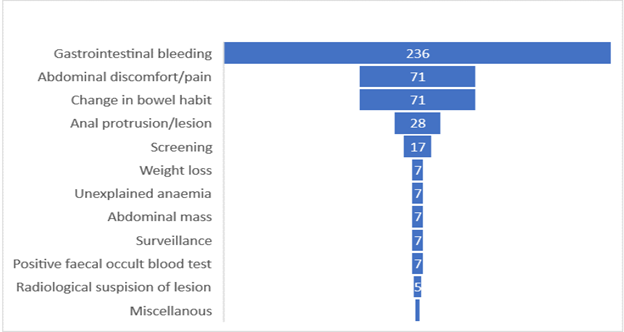
A total of 466 flexible lower GI endoscopies were reported during the study period: 434 colonoscopies; 32 sigmoidoscopies. The age ranges were from 4 years to 79 years. The leading primary indication for the lower GI endoscopy was gastrointestinal bleeding in 236 (50.6%)-Figure 2.
Polyps were detected in 90 cases (polyp detection rate PDR of 19.3%) and 10 cases of adenoma recorded (adenoma detection rate ADR of 2.1%). There were 2 cases of ulcerative colitis recorded but no case of Crohn’s disease.
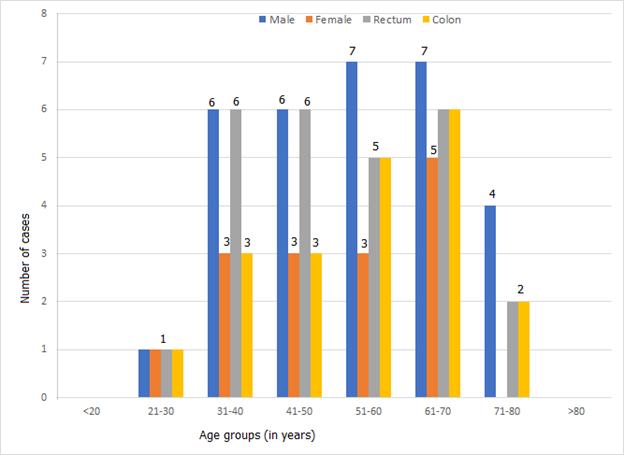
A total of 45 cases had biopsy-confirmed colorectal cancer. The least age was 28 years and the oldest was 79years (Figure 3). The mean age of CRC patients was 52.3 ± 13.7 years; mode 65 years and median age as 53 years. Eleven cases (24.4%) of CRC cases were below 40 years in age. There were 30 males and 15 females with a male to female ratio of 2:1. The male sex was predominantly affected across the various age groups irrespective of colon or rectal origin.
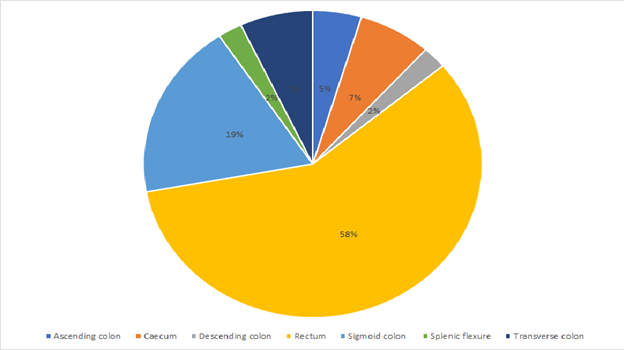
The site distribution of colorectal cancer cases is as shown in Figure 4. The predominant site being the rectum with 25 (55.6%) cases recorded.
Adenocarcinoma was the histologic diagnosis in all cases of colorectal cancer but for one case of non-Hodgkin lymphoma recorded.
Discussions
Cancer of the colon including the rectum is a leading cause of morbidity and mortality. [1] There is strong evidence that the removal of polyps and adenomas by colonoscopy lowers colorectal cancer (CRC) incidence and mortality. [5] However, comprehensive studies in epidemiology of cancer are limited in developing countries by the dearth of tools for disease control and cancer surveillance. [11] In this flexible lower gastro-intestinal endoscopy based study, a detection rate of 7.5% per annum over a 6-year period (2013-2018) was recorded. This is an increase from a prevalence of 3.75% reported over a 12-year period (1990- 2001) from a tertiary hospital record in Port Harcourt, Rivers State, Nigeria. [12] Reports from other regions of Nigeria equally report a rising prevalence of colorectal cancer. [13] The reasons attributed to this trend are westernization of diet especially in cosmopolitan population, increased awareness and availability of diagnostic facilities (laboratory, endoscopy and radiology).
No early stage colorectal cancer (malignant polyp) was recorded. This is relevant as over 90% of CRC cases arise from an adenoma and take about 10 years for polyp greater than 1 cm in size to become an invasive malignancy. [14] The detection of pre- malignant or early malignant stage is crucial to survival rate as the 5-year survival rate for patients with early disease (limited to the muscularis mucosa without involvement of adjacent organs, lymph nodes or distant sites) is 90%. For regional disease with lymph node involvement this drops to 70%, and that for metastatic disease is only 10%. [15,16] The polyp and adenoma detection rates from this study were 19.3% and 2.1% respectively. A multi-centre colonoscopy study in South-West Nigeria revealed a lower polyp detection rate of 6.8%. [17] In all, these rates are low in comparison to Western developed countries with higher prevalence of CRC. The possible reasons for this low rate of adenoma detection range from low incidence, quality of bowel preparation and inter-observer variability.
Adenocarcinoma was the common histopathology of CRC observed (97.8%). Notably, one-quarter of CRC patients (24.4%) in this study were below 40 years of age; no case of over 100 polyps in a patient as seen in familial adenomatous polyposis (FAP). Yet, this is lower than a 34.4% report for similar subset of study population from a systematic review of colorectal cancer from different parts of Nigeria. [13] These patients (below 40years) are candidates for molecular studies including tests for mutations in DNA repair genes- MLH1 and MSH2, responsible for hereditary non-polyposis colorectal cancer HNPCC.
A low acceptance level of screening colonoscopy by family members of colorectal cancer patients detected below 40 years of age was noted. This may be due to financial constraints or fear of the examination and results. There were only 2 cases of ulcerative colitis recorded and no case of Crohn’s disease. In comparison to a North African population, ulcerative colitis has been reported as a common colonoscopy finding. [18] As reported, the relative risk of colorectal cancer in patients with inflammatory bowel disease is estimated at between 4-to 20-fold. [7]
A serrated pathway is an alternative pathway in which serrated polyps replace the traditional adenoma as precursor lesions to CRC. [19] This results from an extensive methylation at the CpG island promoter site, which may demonstrate microsatellite instability. There was a sole case of serrated adenoma recorded in this study. Newer imaging technologies, such as narrow band imaging, autofluorescence and confocal endomicroscopic methods, may lead to higher diagnostic yield of premalignant lesions. [20] As white light endoscopy was used by the endoscopists in this study, it is highly probable that these newer more expensive imaging techniques will increase the yield of smaller adenomatous lesions in this population. The cost and traditional report of a low incidence of CRC discourage their ready availability. However, a withdrawal time for careful inspection of at least eight minutes on navigating white light endoscope to caecum is associated with a high diagnostic yield of colonoscopy as reported by one of the authors. [21]
The routine screening for CRC is associated with reduction of mortality. [22] Evidence from literature is in favour of colonoscopy as the most effective screening tool when compared to flexible sigmoidoscopy and air-contrast barium studies. [23] The guideline for screening by American College of Gastroenterologists recommends colonoscopy be offered first to all asymptomatic individuals aged 50 years or older with no personal history of CRC ,adenomas nor chronic inflammatory bowel disease and no family history of CRC or genetic syndrome. [24] This is the average-risk group. From this multi-centre study that shows a mean age of diagnosis of CRC patients at 52 years, a lower screening age of 45 years for medium risk patients is recommended in this population. The reason being the over 10-year transition period of adenomas and the consideration of an alternate pathway in the geographic variation of disease pattern.
There were limitations to this prevalence study of colorectal cancer from flexible GI endoscopy services. Some factors known to affect polyp/adenoma detection including quality of bowel preparation, caecal intubation rate and withdrawal time were not available for analysis. Another limitation was that endoscopy services are in the urban areas but there is significant rural population and a poor patient referral system. Hence, study findings may not truly reflect the prevalence of colorectal cancer. The cost of colonoscopy service which is about US $222 (N80, 000 at N360 per $1 USD), though cheap when compared to the cost in Western countries yet is unaffordable to a large population who are mostly out - of -pocket paying clients. Lastly, a vast pool of asymptomatic patients was not incorporated into this study as the practice of screening colonoscopy or any coordinated screening program was not in place.
Conclusion
Colorectal cancer is not uncommon in this Nigerian population with a male predominance across all ages and sites of presentation. A targeted screening policy in asymptomatic individuals commencing from 45 years and further molecular studies on microsatellite instability status of colorectal cancer in Nigeria are hereby recommended.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5): E359-386.
- Hill LB, O'Connell JB, Ko CY (2006). Colorectal cancer: epidemiology and health services research. Surg Oncol Clin N Am 15(1):21-37.
- Abdulkareem FB, Faduliye FA, Daramola AO, Rotimi O, Banjo AA, Elesha SO, et al (2009). Malignant gastrointestinal tumours in south western Nigeria: a histopathologic analysis of 713 cases. West Afr J Med 28(3):173-6.
- Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F et al (2012). Cancer Incidence in Nigeria: A Report from Population-based Cancer Registries. Cancer Epidemiol 36(5):e271-e278.
- Jacob BJ, Moineddin R, Sutradhar R, Baxter NN, Urbach DR (2012). Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointest Endosc 76(2): 355-364.e1.
- Boyle P, Ferlay J (2000). ABC of colorectal cancer: Epidemiology. BMJ 321(7264): 805-8.
- Janout V, Kollarova H (2001). Epidemiology of colorectal cancer. Biomed Pap Med Fac Uni Palacku Olomouc Czech Repub 145(1):5-10.
- Ray-Offor E, Abdulkareem FB (2019). Screening colonoscopy in Port Harcourt, Nigeria. Gastroenterology Insights 10(1):7987.
- Adotey JM, Jebbin NJ (2008). Colorectal carcinoma in Port Harcourt. PH Med J 2(3): 198-203.
- Nigeria population. National Bureau of Statistics. Available on http://www.nigerianstat.gov.ng/. Accessed October 23, 2018
- Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M et al (2003). Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer 107(1): 113-118.
- Seleye-Fubara D, Gbobo I (2005). Pathological study of colorectal carcinoma in adult Nigerians: a study of 45 cases. Niger J Med 14(2): 167-172.
- Rotimi O, Abdulkareem FB (2014). Fifty-three years of reporting colorectal cancer in Nigerians-a systematic review of the published literature. Niger Post Med J 21(1):68-73.
- Saha D, Roman C, Beauchamp D (2002). New Strategies for Colorectal Cancer Prevention and Treatment. World J Surg 26(7):762-66.
- Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM et al (2004). Annual report to the nation on the status of cancer,1975-2001, with a special feature regarding survival. Cancer 101(1):3-27.
- Hugh James Freeman (2013). Early stage colon cancer. World J Gastroenterol 19(46): 8468-8473
- Onyekwere CA, Odiagah JN, Ogunleye OO, Chibututu C, Lesi OA (2013). Colonoscopy Practice in Lagos, Nigeria: A Report of an Audit. Diagn Ther Endosc 2013:798651.
- Elbatea H, Enaba M, Elkassas G, El-Kalia F, Elfert AA (2011). Indications and outcome of colonoscopy in the middle of Nile delta of Egypt. Dig Dis Sci 56(7): 2120-2123.
- Leggett E, Whitehall V (2010). Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 138(6):2088-2100.
- Dik VK, Moons LM, Siersema PD (2014). Endoscopic innovations to increase the adenoma detection rate during colonoscopy. World J Gastroenterol 20(9): 2200-11.
- Ray-Offor E, Ibeanusi SEB. Diagnostic yield of colonoscopy. J Clin Gastroenterol Hepatol 2018; 2(2):11.
- Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM (1992). Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 84(20): 1572- 5.
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G (2000). Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 343(16): 162-8.
- Rex DK, Johnson DA, Anderson JC, Schoenfield PS, Burke CA, Inadomi JM (2009). American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol 104(3): 739-750.
Citation: Emeka Ray-Offor., et al. (2020). Colorectal Cancer in Niger Delta, Nigeria: Multi-centre lower Gastrointestinal Endoscopy Study. Journal of Medical Research and Case Reports 2(1).
Copyright: © 2020 Emeka Ray-Offor. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.