Research Article
Volume 1 Issue 1 - 2018
Chromatographic Evaluation of Leaves and Stem Extracts of Ventilago Maderaspatana Graetn.
1KYDSCT’s College of Pharmacy, Sakegaon
2JSS College of Pharmacy, Ooty
2JSS College of Pharmacy, Ooty
*Corresponding Author: Sanjay Nagdev, KYDSCT’s College of Pharmacy, Sakegaon.
Received: December 11, 2018; Published: December 26, 2018
Abstract
Chromatographic studies were carried out on leaves and stem extracts of Ventilago maderspatana Gaertn belonging to the family Rhamnaceae. It is an important and well known plant in the Indian system of medicine. Leaf and stem samples of V. maderspatana were subjected to maceration by using distilled water and ethanol. Aqueous and ethanolic extract were prepared from the leaf, stem of Ventilago maderspatana. Preliminary phytochemical analysis was carried out for all extracts. All extract were found to contain different phytoconstituents such as alkaloids, glycosides, steriods and sterols, saponins, flavonoids, carbohydrates, and tri- terpenoids and showed the absence of amino acids, proteins and acidic compounds. Extract were subjected to TLC and HPTLC analysis.
Keywords: Metastatic Adenocarcinoma; Fine needle aspiration cytology; Proximal tibia; Fine needle aspiration cytology; Computed Tomography
Introduction
V. maderspatana (Rhamnaceae) is commonly known as Red creeper in English, Raktavalli in Sanskrit and pitti in Hindi [1]. It is climbing shrub and identified by it dark grey bark branch lets with brownish pubescent. It flowers in winter with an offensive odour [2,3]. It is found in Indonesia, Malaysia, Shri Lanka, Bhutan and throughout India [4,5]. The powdered root bark is carminative, stomachic, tonic and stimulant; useful in atomic dyspepsia, debility and slight cases of fever. The powdered bark (mixed with gingelly oil) is used in South India as external application for itch and other skin diseases [6]. The root bark is a valuable source of reddish dye (Ventilagin), used for colouring mordanted cotton, wool and tasar silk In combination with the root of Hedyotis umbellate, the root bark yields a beautiful chocolate colour. The bark also yields fibres, used for cordage. The pale yellow wood may be used as fuel. The long climbing stems are sometimes used by fishermen as substitute for ropes. The Seeds are eaten when cooked, and the oil from them is used for cooking [4]. Five isofuranonaphthoquinones have been isolated from the root bark [7]. Two new naphthalene derivatives and three naphthoquinones from the root bark have been isolated [8]. Antibacterial, Nitric Oxide In-Vitro and Ex-Vivo scavenging, anti-denaturation property and anti-oxidant activity of stem-bark of V. maderspatana was reported [9-11]. However, a pharmacognostic study on leaf of V. maderspatana was reported [12]. Pharmacognostic studies of the leaves and stem of Ventilago maderspatana Gaertn[13]. There are no literatures supporting antioxidant properties on leaves and stem extract of Ventilago maderspatana Gaertn. Therefore, the present study was carried out to evaluate antioxidant activity of the plant.
Materials and Methods
Materials
Petroleum ether, Chloroform, ethyl acetate, methanol etc. all the chemical were procured from sigma Aldrich (Germany).
Petroleum ether, Chloroform, ethyl acetate, methanol etc. all the chemical were procured from sigma Aldrich (Germany).
Plant material
The Plant V. maderspatana (Stem and Leaves separately)was collected in the month of July from Tirupati district, Andhra Pradesh, India.The Plant V.maderspatana was authenticated by the botanist, Dr. K. Mahadavachetty, Assistant Professor, Department of Botany, Sri Venkateswara University, Tirupati. Andhra Pradesh, India. Voucher No S.V.U./SC/10/26/10-11, and the specimen were deposited at the department of Pharmacognosy, JSSCP. Ooty. The plant material was subjected for garbling and cleaning processing to ensure the plant quality [14].
The Plant V. maderspatana (Stem and Leaves separately)was collected in the month of July from Tirupati district, Andhra Pradesh, India.The Plant V.maderspatana was authenticated by the botanist, Dr. K. Mahadavachetty, Assistant Professor, Department of Botany, Sri Venkateswara University, Tirupati. Andhra Pradesh, India. Voucher No S.V.U./SC/10/26/10-11, and the specimen were deposited at the department of Pharmacognosy, JSSCP. Ooty. The plant material was subjected for garbling and cleaning processing to ensure the plant quality [14].
Extraction of selected plant material
The coarse powdered materials of the plant parts were subjected to the cold maceration. 500 gm.of leaf and stem of V. maderaspatana was extracted by cold maceration process in 3 liter round bottom flasks. For extraction, two solvents were used, ethanol and distilled water. The nature and yield of the extracts were noted. two extracts each of different parts of plant, were stored in a refrigerator at 40C until further used and the extracts were labelled as V. maderspatana leaf ethanolic (VMLE), V.maderspatana leaf aqueous (VMLA), V. maderspatana stem ethanolic (VMSE), V. maderspatana stem aqueous (VMSA), respectively, for the purpose of convenient identification. Preliminary phytochemical analysis was carried out for all extracts [15-17]. All extract were found to contain different phytoconstituents such as alkaloids, glycosides, steroids and sterols, saponins, Flavonoids, carbohydrates, and tri- terpenoids and showed the absence of amino acids, proteins and acidic compounds, Results were mentioned in table 1.
The coarse powdered materials of the plant parts were subjected to the cold maceration. 500 gm.of leaf and stem of V. maderaspatana was extracted by cold maceration process in 3 liter round bottom flasks. For extraction, two solvents were used, ethanol and distilled water. The nature and yield of the extracts were noted. two extracts each of different parts of plant, were stored in a refrigerator at 40C until further used and the extracts were labelled as V. maderspatana leaf ethanolic (VMLE), V.maderspatana leaf aqueous (VMLA), V. maderspatana stem ethanolic (VMSE), V. maderspatana stem aqueous (VMSA), respectively, for the purpose of convenient identification. Preliminary phytochemical analysis was carried out for all extracts [15-17]. All extract were found to contain different phytoconstituents such as alkaloids, glycosides, steroids and sterols, saponins, Flavonoids, carbohydrates, and tri- terpenoids and showed the absence of amino acids, proteins and acidic compounds, Results were mentioned in table 1.
Fractionation of aqueous and ethanolic leaves and stem extract of Ventilago maderaspatana Graetn.
The column chromatographic technique is for separation, isolation and purification of the natural products. The principle involved in this is the adsorption towards the adsorbent packed in the column. By changing the polarity of the mobile phase, the separation can be achieved in the column chromatography technique. Here fractions were prepared using solvent extraction techniques. Selection of the solvent was carried out based on their polarity.
The column chromatographic technique is for separation, isolation and purification of the natural products. The principle involved in this is the adsorption towards the adsorbent packed in the column. By changing the polarity of the mobile phase, the separation can be achieved in the column chromatography technique. Here fractions were prepared using solvent extraction techniques. Selection of the solvent was carried out based on their polarity.
- Petroleum ether
- Petroleum ether: Chloroform (1:1)
- Chloroform
- Chloroform: Ethyl Acetate (1:1)
- Ethyl acetate
- Ethyl acetate: Methanol (1:1)
- Methanol
- Water
The aqueous and ethanolic leaves and stem extracts of Ventilago maderaspatana Graetn, was subjected for fractionation using above mentioned solvents to separate the phytoconstituents. 8 fractions were prepared. The extracts were triturated with activated silica gel using pestle and mortar. After that the mixture was dissolved in the solvent of low polarity, stirred, heated moderately (luke warm), and filtered. The filtrated solution was collected in a lebelled conical flask, and the marc was air dried, which was used for the next solvent system of high polarity. The process was followed according to polarity of solvent system as mentioned above.
Chromatographic studies
Thin Layer Chromatography [18]
TLC is a very effective technique for the separation of chemical constituents of an extract and for their identification. TLC profile developed for an extract and its fractions using a defined solvent system and other parameters could be used as fingerprints in comparative qualitative evaluation of herbal drugs. The trend of evaluation by this method is becoming popular in view of its simplicity and reproducibility.
TLC is a very effective technique for the separation of chemical constituents of an extract and for their identification. TLC profile developed for an extract and its fractions using a defined solvent system and other parameters could be used as fingerprints in comparative qualitative evaluation of herbal drugs. The trend of evaluation by this method is becoming popular in view of its simplicity and reproducibility.
TLC is an important analytical tool in the separation, identification and estimation of different classes of natural products. In this technique, the different components are separated by the differential migration of solute between two phases – a stationary phase and a mobile phase. Here, the principle of separation is adsorption and the stationary phase acts as an adsorbent. Depending on the particular type of stationary phase, its preparation and use with different solvent, separation can be achieved on the basis of partition or a combination of partition and adsorption.
Separation of components
The extract was dissolved in respective solvents separately and spotted using a calibrated capillary tube on a prepared TLC plate 1 cm above from the bottom of the plate. The spot was equally sized and had a diameter ranging from 2-3 mm. The method was followed for the crude extracts.
The extract was dissolved in respective solvents separately and spotted using a calibrated capillary tube on a prepared TLC plate 1 cm above from the bottom of the plate. The spot was equally sized and had a diameter ranging from 2-3 mm. The method was followed for the crude extracts.
Selection of mobile phase:
The selection of solvent or mobile phase depends upon various factors as mentioned below.
Nature of substance to be separated
Nature of stationary phase (polar/non polar)
Mode of chromatography (normal/reverse phase)
Extent of separation to be achieved (analytical/preparative)
The selection of solvent or mobile phase depends upon various factors as mentioned below.
Nature of substance to be separated
Nature of stationary phase (polar/non polar)
Mode of chromatography (normal/reverse phase)
Extent of separation to be achieved (analytical/preparative)
Based on the chemical tests and nature of phytoconstituents present, the solvent system was selected. The different spots developed in each system were detected by means of specific spray reagents and iodine staining. For this study, many solvent systems were used to detect the number of phytoconstituents present in the extracts. Precoated TLC plate of E – Merck was used for the study.
High performance thin layer chromatography (HPTLC) [19]
HPTLC is a modern chromatographic technique in which the principle of TLC is sophisticated and automated by which the samples are accurately and precisely estimated which can be utilized for both qualitative and quantitative purpose.
HPTLC is a modern chromatographic technique in which the principle of TLC is sophisticated and automated by which the samples are accurately and precisely estimated which can be utilized for both qualitative and quantitative purpose.
General procedures of HPTLC
Preparation of sample: The different solvent fractions of the alcoholic and aqueous crude extracts of the plant were filtered using whatman filter paper and used for study.
Preparation of sample: The different solvent fractions of the alcoholic and aqueous crude extracts of the plant were filtered using whatman filter paper and used for study.
Application of sample: Commercially available precoated HPTLC plates (Merck) can be used for the study. The solutions of various concentrations should be applied on the respective HPTLC plates using Linomat IV applicator. The plates were dried after application. For this study Silica gel GF254 was used.
Application and development of plates: The 6µl sample solutions of the fractions were applied on the HPTLC plate using Linomat IV applicator. The plates were then dried after application and used.
Solvent system: The solvents were different for each fraction.
Detection: The developed plates first were observed under day light and UV light for the detection of constituents.
Densitometric scanning: The developed plates were scanned at a suitable wavelength for the qualitative analysis. Peak areas and peak heights were recorded from which the unknown concentration of the samples have been determined.
Results and Discussion
Phytochemical studies
The ethanolic and aqueous crude extracts of different parts of V.maderspatana and Z. xylopyrus were subjected to preliminary phytochemical screening for the detection of phytoconstituents. The results obtained were given below
The ethanolic and aqueous crude extracts of different parts of V.maderspatana and Z. xylopyrus were subjected to preliminary phytochemical screening for the detection of phytoconstituents. The results obtained were given below
Abbreviations used for different prepared extracts and are as follows
VMLE- V. maderspatana leaf ethanolic extract.
VMLA-V. maderspatana leaf aqueous extract.
VMSE- V. maderspatana stem ethanolic extract.
VMSA- V. maderspatana stem aqueous extract
VMLE- V. maderspatana leaf ethanolic extract.
VMLA-V. maderspatana leaf aqueous extract.
VMSE- V. maderspatana stem ethanolic extract.
VMSA- V. maderspatana stem aqueous extract
| Sr. No. | Phytochemical tests | Ventilago maderspatana | |||
| Leaf extract | Stem extract | ||||
| VMLE | VMLA | VMSE | VMSA | ||
| 1 | Alkaloids | + | + | + | + |
| 2 | Carbohydrates | + | + | + | + |
| 3 | Steroids and sterols | + | - | + | - |
| 4 | Glycosides | + | + | + | + |
| 5 | Saponins | + | + | + | + |
| 6 | Protein and amino acids | - | - | - | - |
| 7 | Flavonoids | + | + | + | + |
| 8 | Phenolic | + | + | + | + |
| 9 | Acidic | - | - | - | - |
| 10 | Fixed oils | - | - | - | - |
| 11 | Triterpenoids | - | - | + | + |
Table 1: Preliminary phytochemical screening of the prepared extracts.
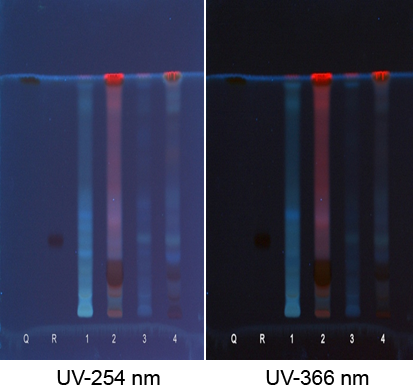
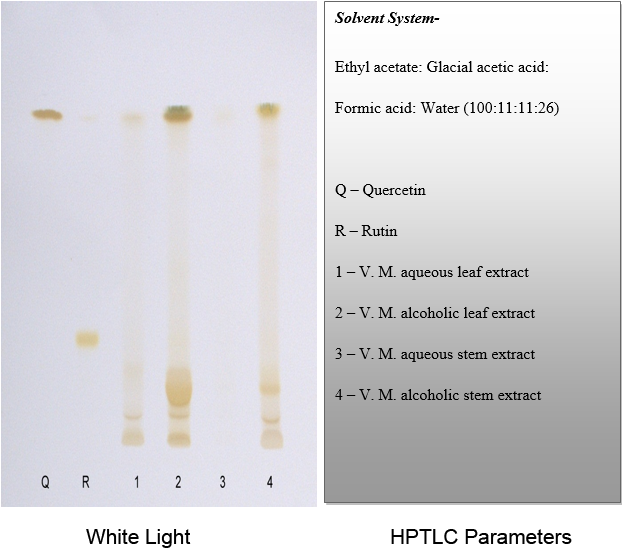
TLC of different extracts of Ventilago maderaspatana Graetn.
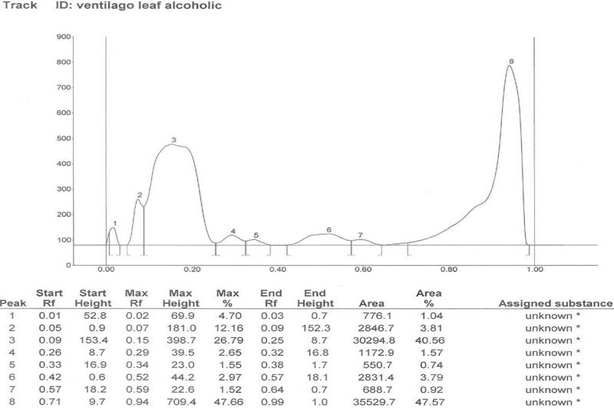
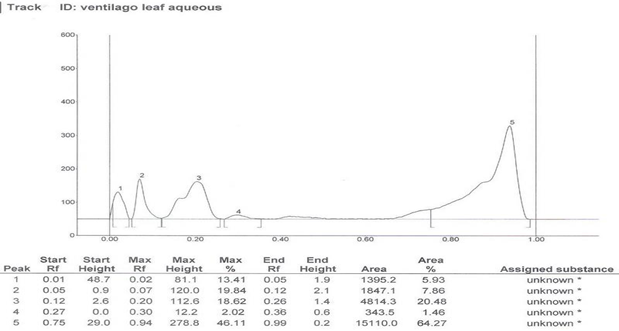
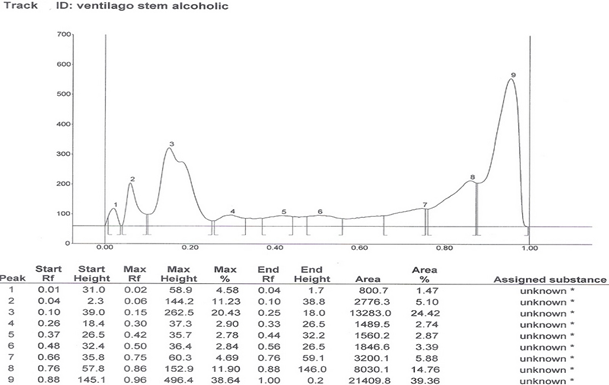
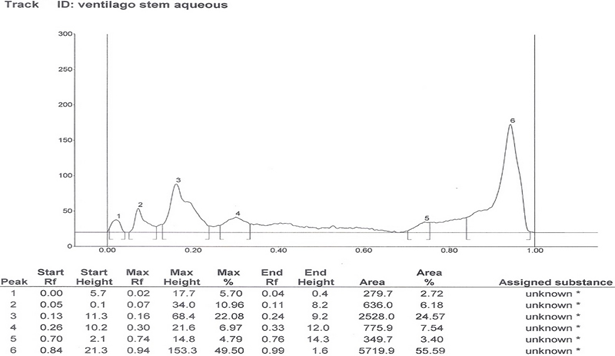
HPTLC Finger print of the different extracts of Ventilago maderaspatana Graetn. Were taken and data’s are recorded below in table 2
HPTLC Finger print of the different extracts of Ventilago maderaspatana Graetn. Were taken and data’s are recorded below in table 2
Solvent system which showed good separation in case of TLC was optimized and HPTLC work was carried out. On the basis of phytochemical tests, the solvent system of flavonoids was used for the HPTLC fingerprinting of the different extracts of Ventilago maderaspatana Graetn.
Solvent System- Ethyl Acetate: Glacial Acetic Acid: Formic Acid: Water (100:11:11:26)
| S. No. | Name of extract | No. of peaks/ spots | Rf values |
| 1. | Alcoholic leaf extract | 8 | 0.02, 0.07, 0.15, 0.29, 0.34, 0.52, 0.59, 0.94 |
| 2. | Aqueous leaf extract | 5 | 0.02, 0.07, 0.20, 0.30, 0.94 |
| 3. | Alcoholic stem extract | 9 | 0.02, 0.06, 0.15, 0.30, 0.42, 0.50, 0.75, 0.86, 0.96 |
| 4. | Aqueous stem extract | 4 | 0.02, 0.07, 0.16, 0.30, 0.74, 0.94 |
Table 2: HPTLC fingerprinting data for the different extracts of Ventilago maderspatana Graetn.
Conclusion
These parameter which reported for the first time, could be useful in isolation and identification of new lead molecule from the V. maderspatana plant. The results of the study can serve as a valuable source of information and provide suitable standards for identification of this plant material in future investigation and application.
Acknowledgements
Authors thanks to J. S. S. University Mysore, (India) for providing facilities to conduct this research work. Authors also acknowledge Dr. K. Mahadavachetty, Assistant Professor, Department of Botany, Sri Venkateswara University, Tirupati, Andhra Pradesh (India) in collection and authentification of the plant material.
Authors thanks to J. S. S. University Mysore, (India) for providing facilities to conduct this research work. Authors also acknowledge Dr. K. Mahadavachetty, Assistant Professor, Department of Botany, Sri Venkateswara University, Tirupati, Andhra Pradesh (India) in collection and authentification of the plant material.
References
- Varier PS. (2006). Indian medicinal plants. Volume 5, orient Longman, Hyderabad, 352.
- RDA-Gene bank, Republic of Korea. 2010 [cited 2010 Nov 29].
- Dinesavalli. Ayurvedic medicinal plants. 2010 [cited 2010 Nov 29].
- Wealth of India: Raw Materials. V0l X. New Delhi: NISCAIR, CSIR; 2009. p442.
- Pullaiah’s Encyclopaedia of World Medicinal Plants. Regency publication, anantpur; 2006. p2023, 2024.
- Kirtikar KR, Basu BD. Indian Medicinal Plants, Vol-1st, 2nd edition. Derhadun: International Book Distributors; 1987, p 585.
- Hanumaiah T, Rao GSR, Rao CP, Rao KVJ, Heather JC, Philip JC, Howe RA, David SM, Thomson RH. (1985). Isofuranonaphthoquinones from V. maderspatana crystal structure of Ventilone - c. Tetrahedron. 41(3): 635-642.
- Hanumaiah T, Rao GSR, Rao CP, Rao KVJ, Heather JC, Philip JC, Howe RA, David SM, Thomson RH. (1985). Naphthalenes and naphthoquinones from Ventilagospecies. Phytochemistry. 24(8): 1811-1815.
- Subhalakshmi B, Abhijit G, Banasri H. (2005). Evaluation of the Antibacterial Activity of Ventilago maderspatana Gaertn, Rubia cordifolia Linn. And Lantana camara Linn: Isolation of Emodin and Physcion as Active Antibacterial Agents. Phytother. Res. 19: 888–894.
- Subhalakshmi B, Banasri H. (2006). Evaluation of Nitric Oxide Scavenging Activity, In Vitro And Ex Vivo, of Selected Medicinal Plants Traditionally Used in Inflammatory Diseases. Phytother. Res. 20: 896–900.
- Duganath N, Kumar SR, Kumanan R, Jayaveera KN. (2010). Evaluation of anti-denaturation Property and anti-oxidant activity of traditionally used medicinal plants. Int J Pharm. Bio. Sci. 1(2): 1-7.
- Syeda S, Sandhya S, Vinod KR, David B, Rao KNV, Narender PD, Nema RK. (2010). Pharmacognostic Studies on The Leaf of Ventilago maderspatana Gaertn. International Journal of Pharmaceutical and Clinical Research 2(1): 51-53.
- Gandagule UB., B. Duraiswamy et.al. (2018). Pharmacognostic studies of the leaves and stem of Ventilago maderspatana Gaertn. Inventi rapid:planta activa [4]: 1-7.
- Chaudhary RD. Herbal Drug Industry, 1st edition. New Delhi: Eastern Publication (1996). P 373-375,473.
- Kokate CK, Purohit AP, Gokhale SB. The Text Book of Pharmacognosy. 3rd ed. New Delhi: Vallabh Prakashan; 1991, p. 606-611. Khandelwal KR. Practical Pharmacognosy. Pune: Nirali Prakashan; 1998, p. 149-156.
- Raaman N. Qualitative phytochmeical screening In: Phytochemical techniques.Ne Delhi: New India publishing agency (2006): 19-24.
- Wagner H, Bladt S. Plant Drug Analysis, A Thin Layer Chromatography Atlas, 2nd edition. New York: Springer; (1996).
- Eike R, Anne S. HPTLC for the Analysis of Medicinal Plants. New York: Thieme Medical Publishers; (2007).
Citation: Upendra Gandagule, B. Duraiswamy, Mayur Bhurat, Sanjay Nagdev., et al. (2018). Chromatographic Evaluation of Leaves and Stem Extracts of Ventilago Maderaspatana Graetn. Journal of Pharmacy and Drug Development 1(1)
Copyright: © 2018 Sanjay Nagdev., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.