Research Article
Volume 1 Issue 1 - 2019
Caloric Restriction and Longevity
1Area of Environmental Toxicology, Institute of Health Carlos III, 28220-Majadahonda, Madrid, Spain
2Department of Biochemistry, Faculty of Pharmacy, Complutense University and CSIC, Madrid, Spain
3Royal National Academy of Pharmacy. Street Farmacia 9-11, 28004-Madrid, Spain
2Department of Biochemistry, Faculty of Pharmacy, Complutense University and CSIC, Madrid, Spain
3Royal National Academy of Pharmacy. Street Farmacia 9-11, 28004-Madrid, Spain
*Corresponding Author: Bartolomé Ribas Ozonas, Area of Environmental Toxicology, Institute of Health Carlos III, 28220-Majadahonda, Madrid, Spain.
Received: March 18, 2019; Published: April 19, 2019
Abstract
Caloric restriction (CR) in normal human bodies means to maintain a low-caloric diet, balanced in nutrients, vitamins and catalytic mineral ions (ca. 1750 Kcal/day), in order to benefit aging in good health, an active longevity preventing emerging diseases, and avoiding not genetic but certain kinds of obesity.
A correct caloric intake for the energy needs of the human body for normal activity is necessary. Every organism has a particular metabolism that depends on its genetic and epigenetic characteristics, diet and habits (activity, stress, and toxics) and we must adapt its diet, hoping to enjoy good health and achieve an active longevity. Food that we consume our body metabolizes, excretd or accumulated. If it is too much can be stored in the form of fat. The excess of diet leads to cell damage and shortening of life. The mitochondria is like a metabolic turbine of energy production, and eliminates the last steps of nutrients as CO2 and H2O. It’s functioning and its good physiological condition is cause of longevity. In humans, caloric restriction (CR) is beneficial, and prevents a long list of diseases of the elderly, which we quote in the text. It protects against the causes of aging, prevents production of free radicals, and the accumulation of fat and damage.
The researcher Guarente discovered that caloric restriction activated the transcription of a gene called Sirtuin2 (SIR2), with capacity to delay aging. Nowadays, as will be mentioned the Spanish researcher Maria Blasco, whose work focuses on the loss of the protective telomere of chromosome ends, which kept young the cells. Telomeres become worn down during cell division, while the enzyme telomerase repairs and lengthens the telomeres and obtains, according to the mentioned research in mouse and rats, increasing active longevity.
The circadian rhythm day-night activity is important in the regulation of psychic, physical activity and obesity. The light of the sun which control our hormonal rhythms (circadian rhythm) determines that the evening rises several hormones (growth hormone (HGH), melatonin and serotonin). HGH uses our fat reserves as fuel. Everyone’s rhythm is different. Melatonin helps maintain the body’s circadian rhythm, and there are reciprocal connections of the serotonin and circadian systems likely have importance for neurobehavioral disorders.
The mitohormetic hypothesis of CR, proposes a mitochondrial organic defense response to the genetic level that induces a new epigenome, which has led several scientist to propose that "we are what we eat". A fact that has opened a new scientific discipline, Nutrigenomics, which studies the effect of the diet on the expression of the genome of our cells.
Introduction
Caloric restriction for active longevity is to adapt the diet to individual body needs. Each human being has a certain metabolism that depends on its genetic and epigenetic characteristics, and they must adapt their diet, that is to say, to follow an adequate and balanced caloric restriction [1-4]. It should limit the energy intake of the diet, to prevent obesity, an actual pandemic disease of wellbeing that affects most families today. Sufficient amounts of vitamins, minerals, and other important nutrients are needed and essential amino acids [5]. In humans, calorie restriction (CR) has been shown to reduce cholesterol, fasting glucose, and blood pressure.
On the other hand, experiments with animal species so far exposed to CR, including primates, rats, mice, spiders, Drosophila flies, earthworms and rotifers, have shown an increase in their longevity. CR is a dietary measure capable of increasing maximum longevity, comparing with average longevity [6-10].
Everything that we consume in excess, our organism responds according to our physiology and molecular biochemistry, it is metabolized obtaining energy (ATP), or stored in the form of fat or excreted (feces, urine, exhalation H2O and Co2, and sweat). The ATP is synthesized in the mitochondria converts’ energy for intellectual, mechanical, muscular or nervous physical activity. The number of mitochondria in each organ or tissue is the function of the needs of each one.
Epidemiological studies suggest that lifestyle, such as sedentariness (reduced physical activity), high or excessive food consumption and adiposity by hereditary predisposition are responsible for overweight, and a 70% of chronically diseases, its manifestation or aggravation, as well as the decrease in longevity [11].
Overweight is a clear risk factor for disease development, such as type 2 diabetes, cardiovascular disease, and metabolic syndrome. The latter is a group of different entities whose common factor is oxidative stress. Caloric restriction also prevents respiratory disorders, high blood pressure, osteoarthritis, reproductive abnormalities, hepatitis, and some types of cancer [4].
In persistent obesity, it is not yet known specifically for each of them, the precise caloric intake, which equivalent to optimal health and longevity. In a series of experimental works initiated by Guarente and followed by other researchers, was found that the caloric restriction or a diet low in calories activated the transcription of a gene called sirtuin-2 (SIR2), with the ability to delay aging [12-14]. The light of the sun, that controls our hormonal rhythms (circadian rhythm), determines that at nightfall the hormones which use our fat reserves as fuel are elevated. One of these hormones is the growth hormone (HGH), which rises at dusk and reaches its maximum secretion at midnight. In addition to stimulating growth and activity in children, it mobilizes our fat deposits during night sleep.
The Hormética or mitohormética hypothesis of caloric restriction (CR) is known, due to the involvement of mitochondria, in the process of aging and active longevity. It proposes that the reduced diet imposes a biological stress although of low intensity to the organism, which raises a response of organic defense to the mitochondria and at the genetic level that induces a new epigenome, because we are what we eat. Fact that has opened a new scientific discipline, the Nutrigenomics, which studies the diet effect on the genome of our cells. We have 4 million of genes that are exposed to the infinite food compounds that affect their microRNAs that control important aspects of our organism [6,15]. The microRNAs are small fragments of 18 to 25 ribonucleotides capable of controlling the expression of genes coding for proteins, for example through the maternal feeding, both in pregnancy and in maternal nutrition. The diet of adults and environmental pollutants also regulate the expression of microRNAs, and these are contained in animal and plant foods exerting their action, which should be taken into account to diversify our diet [16, 17].
A diversified CR diet helps protect against the causes of aging and elderly diseases by slowing mitochondrial activity, quickly neutralizing free radicals, improving health and longevity. This situation can be controlled by the genes of longevity that induce the synthesis of sirtuins (see below).
Although the hypothesis of mitohormesis (mitochondrial excitation) was a purely hypothetical concept until the end of 2007, later works carried out in animals and flies, earthworm (Caenorhabditis elegans) and other species showed that the restriction caloric diet and glucose metabolism in mitochondria increases longevity, reducing oxidative stress, and damage by oxygen free radicals (ROS) and the damage of by free radicals of oxygen and other oxidizing forms of chemical compounds [9].
Caloric Restriction
Clive McCay and Mary Crowell of Cornell University [18,19] observed that laboratory rats, fed a very low calory diet with vital nutrients, increased their longevity twice as long as expected. These observations were examined in detail by a series of experiments with mice performed by Walford and his pupil Weindruch [1], reported that the restriction of caloric intake of laboratory mice increased proportionately their longevity compared to a group of mice with a normal diet. These maintained a more youthful appearance, increased their level of activity and showed delay in the onset of diseases associated with ageing.
Longevity
A study conducted at the Salk Institute for Biological Studies and published in 2007 in the journal Nature [9] determined that the gene PHA-4 was responsible for longevity under the effect of caloric restriction in animals, with similar effects in humans. The discovery has given hope for the synthesis of future drugs that increase human longevity; however, a drug treatment should not be a substitute for a healthy lifestyle.
A study conducted at the Salk Institute for Biological Studies and published in 2007 in the journal Nature [9] determined that the gene PHA-4 was responsible for longevity under the effect of caloric restriction in animals, with similar effects in humans. The discovery has given hope for the synthesis of future drugs that increase human longevity; however, a drug treatment should not be a substitute for a healthy lifestyle.
A new study published in June 2012 by the University of Washington in St. Louis [20,21], explained that the limitation in calorie intake results in an improvement of the cardiovascular system, more potent pumping of the heart and better adaptation to physical activity. All of this suggests an improvement in overall health.
Other data in the scientific literature report that dehydroepiandrosterone (DHEA) [22] is an endogenous prohormone precursor of androgens and estrogens, which is effective in preventing aging and stimulating active longevity. The secretion of dehydroepiandrosterone (DHEA) and its sulfated ester decreases with age. Its inclusion as a dietary supplement has not shown a noticeable improvement compared to placebos in the regeneration of hormonal activities during old age. However, their low blood levels of this hormone are associated with poor health status and signs of ageing, which coincide with the human physiology of the elderly [21].
The Spanish researcher Maria Blasco in recent mice studies explains that longevity depends on the length of telomeres at the ends of the chromosomes, the longer the telomere length at birth would be translated into a longer life and youthful longevity. And that longevity would depend on the accumulation of cellular damage that is reflected in the wear and shortening of telomeres, and these are repaired and lengthened by telomerase, whose administration could increase longevity [5].
Mitochondria and longevity
The mitochondria is an intracellular organelle that is considered totally integrated in the cellular functions, so much so that, when the mitochondria are damaged, they have an impact on the whole organism and on the general health condition. Mitochondria affects nuclear gene expression this is a coordinated response to the causes that affect the organism. By means of glycolysis (lysis of glucose) energy is obtained for the cell, and consists of 10 consecutive enzymatic reactions. It is performed inside the plasma membrane or cytoplasm, under anaerobic conditions, and the glucose molecule is degraded into 2 molecules of pyruvic acid. Its overall reaction is:
Glucose + 2NAD+ + 2ADP + 2Pi + 2Pyruvate + 2NADH + 2ATP + 2H + 2H2O.
The mitochondria is an intracellular organelle that is considered totally integrated in the cellular functions, so much so that, when the mitochondria are damaged, they have an impact on the whole organism and on the general health condition. Mitochondria affects nuclear gene expression this is a coordinated response to the causes that affect the organism. By means of glycolysis (lysis of glucose) energy is obtained for the cell, and consists of 10 consecutive enzymatic reactions. It is performed inside the plasma membrane or cytoplasm, under anaerobic conditions, and the glucose molecule is degraded into 2 molecules of pyruvic acid. Its overall reaction is:
Glucose + 2NAD+ + 2ADP + 2Pi + 2Pyruvate + 2NADH + 2ATP + 2H + 2H2O.
The two molecules of pyruvate, are able to follow other metabolic pathways and thus continue releasing energy to the organism. Through the Krebs cycle or cellular respiration, the pyruvic acid formed in the glycolysis is completely oxidized to CO2 and H2O in the presence of oxygen. It is developed in two successive stages: the Krebs cycle and the respiratory chain, associated with oxidative phosphorylation (Figure 1).
Currently, among the different theories of aging, there are two more important, as the theory of free radicals and the theory of the glycation, being able to explain both the mechanism of the caloric restriction.
An excessively high calorie diet affects the mitochondria that saturated cannot neutralize the excess of free radicals, and the enzymatic reactions of defense either, which has an unfavorable effect on the cell and consequently on the systemic organs and tissues and to the organism in general. In this situation mitochondria are damaged, and degenerate. In a situation of caloric restriction, mitochondria work loosely, and neutralize free radicals that do not damage cell structures and membranes. The mitochondrial efficiency and efficacy of a calorie-restricted organism will have less fatty content and will need less energy to maintain its weight, which also means that it will have less glucose in the bloodstream. Less blood glucose means less glycation of adjacent proteins and less fat than oxidizing that may cause atherosclerosis in the bloodstream. Diabetics suffering from Type 2 diabetes mellitus have insensitivity to insulin caused by long-term exposure to high blood glucose levels. Obesity leads to type 2 diabetes. This type of diabetes and the uncontrolled type 1 produce an accelerating effect of aging due to the above effects [22, 23].
Glycolysis and Krebs Cycle
NADH and FADH2 (sinthetized during glycolysis containing high energy electrons. In the mitochondria NADH and FADH2 transfer their electrons to O2 and synthetized ATP.
NADH and FADH2 (sinthetized during glycolysis containing high energy electrons. In the mitochondria NADH and FADH2 transfer their electrons to O2 and synthetized ATP.
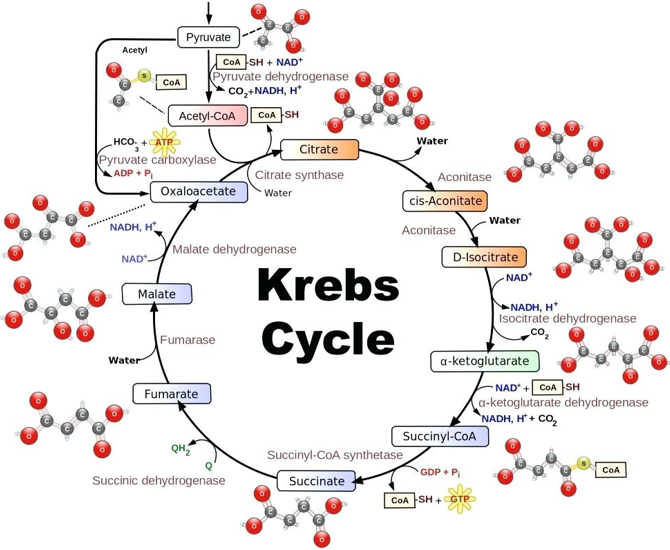
Figure 1: Energy production from glucose through glycólysis and Krebs cycle. (https://en.wikipedia.org/wiki/Citric_acid_cycle).
Currently the numerous and recent publications have shown that the mechanisms of intracellular and mitochondrial caloric reduction favor the neutralization of free radicals inhibiting oxidative stress, through numerous molecules reducing the organism's defense system.
If we control a balanced energy supply, our diet, in relation to our caloric needs, achieve better health, delay aging, help reduce cholesterol and other lipids, reduce the resistance to insulin, we benefit in a word our organism, physiological and biochemical state, and ultimately we increase our quality of life and longevity. Balanced diets mean that all immediate principles, vitamins and the necessary mineral elements must be included. A change of diet is not enough; it must be accompanied by a change in lifestyle, in which we are aware of what is best or ideal for our health.
Glycolysis and Krebs cycle
We are aware that the genetic map in the mitochondria lies the age, and the subjective time of life, which is transmitted precisely by the maternal mitochondria. The energetic power of the food goes through the glycolysis and the Krebs cycle. The latter, aerobic metabolism is the most efficient way to obtain energy (ATP) from nutrients and calories’ formation (1 ATP = 7.3 kcal; 1 ATP produces 10,300 calories per mol).
We are aware that the genetic map in the mitochondria lies the age, and the subjective time of life, which is transmitted precisely by the maternal mitochondria. The energetic power of the food goes through the glycolysis and the Krebs cycle. The latter, aerobic metabolism is the most efficient way to obtain energy (ATP) from nutrients and calories’ formation (1 ATP = 7.3 kcal; 1 ATP produces 10,300 calories per mol).
This process occurs in mitochondria and includes the conversion of pyruvate into acetyl coenzyme-A, in the tricarboxylic acid cycle (Krebs cycle, figure 1), and then oxidative phosphorylation or electron transport chain (Figure 2). The basic source of energy for muscle contraction is adenosine-triphosphate, ATP, whose empirical formula is: Adenosine-PO3-PO3-PO3. The last two phosphate bonds are of high energy. Each link stores 7.3 kcal of energy per mol of ATP, under normal conditions. Therefore, when a molecule radical phosphate is released, 7.3 kcal can be used, and provided to muscle contraction or to other energy expenditure.
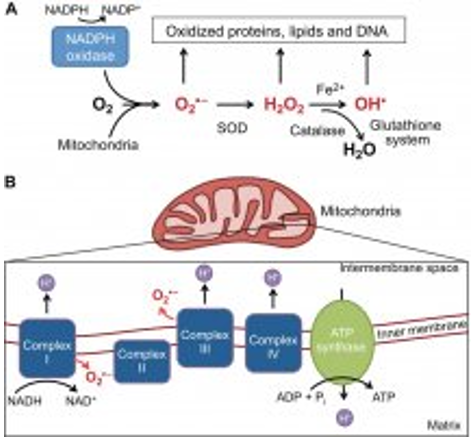
Figure 2: Electron transport chain (oxidative phosphorylation) in the internal mitochondrial membrane, and a scheme for the production of oxygen-free radicals. Molecular O2 acts as acceptor of electrons. Superoxide radicals, hydrogen peroxide, and hydroxyl radical are represented. (http://www.robertlfurler.com/2013/04/03/global-climate-change-finding-an-equilibrium -between-oxidation-and-reduction/)
To keep us in our weight is essential to adjust our consumption of food to our needs. The recommendations of the WHO (World Health Organization) establish a caloric intake of 2,000 to 2,500 kcal/day for an adult male and 1,500 to 2,000 kcal/day for women. These needs decrease as we get older. A man of 65 years of average constitution will need about 1,900-2,100 kcal/day while a woman 65 years of average constitution oscillates between 1,500 – 1,700 kcal/day. However, the experience with obese and patients, recommends a calorie restriction for the man 1,500 kcal/day and for the woman 1,200 kcal/day, to maintain a calorie restriction and for a suitable weight.
The "free radicals" and other oxidizing forms of numerous native or polluting compounds, damage the nuclear DNA causing the organic or germ cell to reproduce defectively, do not or be sterile. Mitochondria, which are the sources of cellular and organic energy, to synthesize ATP, also suffer, not only in their DNA molecules but in their membranes, whose oxidative effects lead to cell death. If the action of free radicals is avoided, mitochondria perform their task without detrimental function. Also many fatty acid, proteins and hormones suffer the oxidative effect of free radicals.
Electrons (e-) and protons (H+) derived from the oxidation of food and toxic agents intervene in a series of oxidation-reduction reactions through which they are transported to oxygen. These processes befall in the inner membrane of the mitochondria where the system sits. The transporting molecules of e-and H+ are NADH (Nicotinamin-adenine-dinucleotide reduced) and FADH2 (Flavin-adenine-dinucleotide reduced), reduced coenzymes that when losing an electron and a proton, go to oxidized, according to the following reactions:
NADH + H+ + ½ O2 = NAD+ + H2O
FADH2 + H+ + ½ O2 = FAD+ + H2O
NADH + H+ + ½ O2 = NAD+ + H2O
FADH2 + H+ + ½ O2 = FAD+ + H2O
The cellular metabolism (oxidation) of the food, generates reduced compounds in all the cellular compartments, which transfer their reducing equivalents to the coenzymes in their oxidized form NAD+ and FAD+, transforming in their reduced form, before cited. The reoxidation of the reduced electronic transporters, NADH and FADH2, produce ATP, through the electron transport chain enzymes in the mitochondrial internal membrane. The “Quimiosmótic hypothesis” of Mitchell (1961) proposes that the transport of electrons and the oxidative phosphorylation producing ATP, are two events coupled by a gradient of protons (H+) through the internal mitochondrial membrane (Figure 2).
As the high-energy electrons run in the respiratory chain, the liberated energy pumps protons from the matrix into the inter-membrane space resulting in an electrochemical gradient between the negative charges of the matrix and the positive charges of the intermembrane space. The energy stored in that gradient is used for ATP synthesis by ATP-synthase, when protons return to the matrix and form from one ADP molecule to ATP.
In the biosphere, matter is limited so that its recycling is a key point in the maintenance of life on Earth; otherwise, the nutrients would be exhausted and life would disappear. All living beings need oxygen to generate energy; however, they have to cope with their waste products, including free radicals (reactive oxygen species, ROS).
These are mainly formed in the mitochondria causing oxidative damage to other cellular components and induce a whole series of pathologies expressed in the scientific literature. The key enzyme in the organism's defense system is nicotinamin-nucleotide-transhydrogenase (TH) (Figure 3).
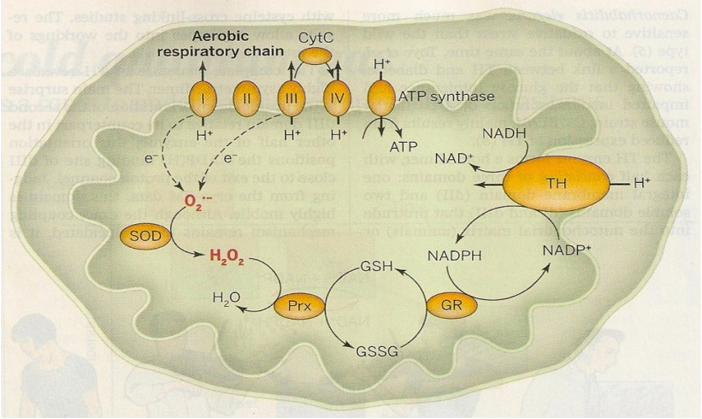
Figure 3: The mitochondrial defense system transforms the free radicals of oxygen to H2O2 which is detoxified by peroxidase to water with the help of reduced glutathione (GSH) as substrate. The elevated levels of mitochondrial glutathione (GSH) are maintained by Nicotinamin-adenine-dinucleotide-phosphate (NADPH)-dependent on glutathione-reductase, and this is where transhydrogenase acts (TH). Krengel U. and Törnroth-Horsefield A. 2015; Coping with oxidative stress. Science vol 347, 125-126.
The mitochondrial enzyme transhydrogenase (TH) produces NADPH that contributes to the defense against free radicals of oxygen and other molecules. Electrons migrate from complexes I and III to molecular oxygen (O-2) and are formed superoxide anions O-2 which by superoxide-dismuntase (SOD) become H2O2, and finally by peroxidase (PRX) and glutathione (GSH) form water (H2O) [22, 23].
Benefits of caloric restriction
In mammals rodent and also in humans, caloric restriction increases longevity by preventing or delaying the onset of chronic diseases such as diabetes, atherosclerosis, cardiomyopathy, autoimmune disorders, renal and respiratory pathologies, and cancer. Also, caloric restriction decreases cerebral neurodegeneration and increases neurogenesis in animal models of Alzheimer's disease, Parkinson's, Huntington's and stroke although it could be deleterious in cases of amyotrophic lateral sclerosis [1,11,24].
In mammals rodent and also in humans, caloric restriction increases longevity by preventing or delaying the onset of chronic diseases such as diabetes, atherosclerosis, cardiomyopathy, autoimmune disorders, renal and respiratory pathologies, and cancer. Also, caloric restriction decreases cerebral neurodegeneration and increases neurogenesis in animal models of Alzheimer's disease, Parkinson's, Huntington's and stroke although it could be deleterious in cases of amyotrophic lateral sclerosis [1,11,24].
The caloric restriction, defined as the reduction of the caloric intake below the habitual voluntary consumption without malnutrition, delays the ageing and increases the maximum life duration in different species such as yeasts, flies, worms, fish and rodents. The magnitude of life prolongation depends on the earliest age of onset of caloric restriction, the intensity of such restriction, and the own genome.
With a caloric restriction diet, the decrease in the production of reactive oxygen species is promoted; and activation of antioxidant systems that reduce oxidative stress and free radical-induced tissue damage. There is also a decrease in levels of T3 and the sympathetic activity that induces the decrease of body temperature and energy expenditure of rest, reduction of the levels of inflammatory cytokines and modest increase of the level of cortisol, with a decrease in systemic inflammation; protection against deterioration of the immune function associated with aging, and increased expression of neurotrophic factors.
In relation to aging, restriction in calories ingestion simultaneously affects multiple processes involved in aging, such as improving DNA repair processes, in relation to the damaged cell proteins and oxidized lipids. It also promotes the reduction of protein glycation and the formation of PFGA (accumulation of final products of advanced glycation, and collagen crosslinking. Many of the effects of caloric restriction are mediated by the regulation of the genetic expression of high significance in cellular repair and survival, stress resistance and protection against oxidative damage, and other genes involved in the inflammation [25,26].
Caloric restriction and aging in humans.
Epidemiological studies suggest that caloric restriction may have beneficial effects on the factors involved in the pathogenesis of primary and secondary aging in humans.
Epidemiological studies suggest that caloric restriction may have beneficial effects on the factors involved in the pathogenesis of primary and secondary aging in humans.
Recently, data from several studies carried out in members of the "Calorie Restriction Society" have been published; a group that practices this restriction voluntarily believing that it will prolong their lives. Why do we age? What are the data so far known for aging?: a).- oxidative stress; b).- alteration of mitochondria; c).- oxidation of macromolecules (deterioration); d).- DNA injuries (genetic instability and epigenetic alterations); e).- Glucation, of proteins (increment of age); f).- lipoperoxidation of membranes; g).- shortening of telomeres in replicative cells [5,7,10].
The experimental calorie restriction group consisted of people of both sexes with a median body mass index (BMI) of 19.6 kg/m² that ingested an average of 1,800 kcal (30% less than usual) from nutrient-rich foods such as vegetables, fruit, nuts, dairy, egg white, wheat and soybean proteins for an average of 6.5 years, compared to controls that consumed a typical Western diet. This group showed lower percentage of body fat, lower blood pressure, improved lipid profile, increased sensitivity to insulin (SI), decreased inflammatory markers, growth factors and T3, and diastolic function of the left ventricle similar to people 16 years younger.
In several randomised studies, the effect of caloric restriction on ageing-related variables in non-obese adults has been assessed. In one study it was observed that 25% reduction in caloric intake over 6 months reduced visceral fat, insulin resistance, body temperature and oxidative stress marker levels. Another study also showed that caloric restriction reduced bone mass as well as muscle mass and strength in the lower extremities.
Excessive caloric restriction
The excessive CR is the decrease of the caloric contribution to a level that carries harmful effects on health, like anemia, muscular detriment, neurological deficit, edema in inferior limbs, weakness, dizziness, depression and irritability. The subject becomes a patient with anorexia nervosa (opposite to bulimia), and also has altered the regulation of body temperature, skin, bone, cardiovascular, blood, pulmonary, immune and reproduction system.
The excessive CR is the decrease of the caloric contribution to a level that carries harmful effects on health, like anemia, muscular detriment, neurological deficit, edema in inferior limbs, weakness, dizziness, depression and irritability. The subject becomes a patient with anorexia nervosa (opposite to bulimia), and also has altered the regulation of body temperature, skin, bone, cardiovascular, blood, pulmonary, immune and reproduction system.
It has been shown that the reduction of 45% of the normal energy supply for 24 weeks is harmful in thin men. A parameter to assess the safety of calorie restriction is BMI: a BMI lower than a 18.5 is associated with an increase in the death rate in adults and a BMI of 13 in males and 11 in women is associated with possible death by starvation.
Gens and Sirtuins in caloric restriction and longevity
It is estimated that protein coding genes are 20,344. Of these each cell type uses on average about 10,000 and there are 400 different cell types. That's why the organism has 4 million. If we also consider those that do not code for proteins would be like those that encode for RNA circular 38,000, the IncRNA 30,000 and the mirRNA 2000. In this category we would have about 70,000. Then there are the nuclear, nucleolar, ribosomics, and of transfer. If we add all and assume the concept of gene as a fragment of DNA that is transcribed to RNA, there are around 100,000 [6,16].
It is estimated that protein coding genes are 20,344. Of these each cell type uses on average about 10,000 and there are 400 different cell types. That's why the organism has 4 million. If we also consider those that do not code for proteins would be like those that encode for RNA circular 38,000, the IncRNA 30,000 and the mirRNA 2000. In this category we would have about 70,000. Then there are the nuclear, nucleolar, ribosomics, and of transfer. If we add all and assume the concept of gene as a fragment of DNA that is transcribed to RNA, there are around 100,000 [6,16].
In the year 2000, Leonard Guarante, of the Massachusetts Institute of Technology (MIT, Massachusetts, USA), discovered that caloric restriction or a low-calorie diet activated the transcription of a gene called SIR2 (SIRTUIN2), with the ability to delay the aging. This gene, which encodes the protein SIR2, was detected in greater concentration in the fly Drosophila melanogaster, when it was subjected to a diet of lower caloric intake. The SIR2 gene plays a central role in the cell metabolic cycle. From this finding, these authors created a mutant fly that overexpressed the gene Sirtuin, and discovered that with this over-expression, these flies, could live up to 60% more than normal (control). They also demonstrated that SIR2 is related to greater life expectancy, also in yeast and nematodes, and that in humans there is a SIRT2 analogue gene [12,13,27]. The enzymes Sirtuins are protein molecules called NAD-dependent deacetylases that connect the metabolism to longevity (figures 4 and 5).
Environmental changes, stress and diet regulate the activity of sirtuins altering the quotient NAD/NADH, the intracellular concentration of nicotinamide and those of other sirtuins. The sirtuins of Mammals (SIRT1 – 7) have different meanings and objectives in the nucleus, cytoplasm and mitochondria, and can exert an impact on metabolism, DNA repair or cell survival (figure 4).

Figure 5: Deacetylation and ADP-ribosyl-reactions by sirtuins. Both reactions involve the break of the NAD to free nicotinamide.
As mentioned above, its presence and mechanism of action has been established in Saccharomyces cerevisiae, Caenorhabditis elegans and Drosophila melanogaster (Figure 6). The mammals contain seven counterparts of the yeast SIR2, SIRT1 to 7 [8,28]. It has been demonstrated the meaning of these sirtuins as regulators of aging or longevity, which makes them potential pharmacological targets for the treatment of age-related diseases such as metabolic and degenerative diseases, and with the metabolic syndrome.
SIR (silent, information regulator) proteins regulate longevity in many organisms. In yeast, an extra copy of the gene SIR2 increases life expectancy, while eliminating the gene shortens it. The protein SIR silences chromatin, increases the ability to repair DNA and is involved in chromosome fidelity during meiosis. The SIR promotes longevity by suppressing the formation of extra-chromosomal circles of RDNA (ERC) in yeast. The Ortologo of the Caenorhabditis elegans Sir 2.1 also broadens life expectancy, although by a different mechanism. The protein Sir 2.1 requires the protein DAF-16 to induce a greater longevity in these species [12,13,27].
When cells undergo calorie restriction, a cascading process is initiated through the cell membrane. A signal activates a gene called NAMPT, and the resulting enzyme builds up inside the cell. NAMPT accumulation also causes another small molecule called NAD (Nicotinamin-adenine-dinucleotide; NAD). This is singular, because the accumulation of NAD only occurs within the mitochondria; in the cellular cytoplasm the concentration of NAD plummets when the calories are missing. Returning to mitochondria, NAD accumulation has a posterior effect: it increases the activity of two other mitochondrial proteins produced by the genes called SIRT3 and SIRT4.
The combined effect of what we are dealing with is clear. The mitochondria enhance, increase their energy production, and the process of cellular aging is slows: it's as if the cell said: Die? Not at all! Summarizing, then, a low calorie diet increases the concentrations of NAMPT, NAD, SIRT3 and SIRT4, and as a whole makes the cell live longer, and with more energy. The following idea immediately arose: what would happen to induce, at the molecular level, and increase the concentrations of NAD, SIRT3 and SIRT4, without having to diet? This is, again, the significant importance and necessity of research for the treatment and cure of the disease.
The physiological regulation by SIRT1 in mammals is a fact. SIRT1 regulates the survival of neurons, gluconeogenesis, lipolysis, the survival of beta cells of insulin secretion by interacting with a series of proteins [29,30]. The activation of AMPK during the glucose restriction induces the activation of SIRT1, and blocks the induction of the Biogenic program, subject that is treated in another lesson of the course. However, activation of SIRT1 by AMPK may also result in the regulation of other regulation objectives by SIRT1, such as p53, the coactivator of the gamma receptor activated by the Peroxisomal proliferator (PGC-1 β), or the FOXO family of factors of transcription, which leads to a plethora of possible effects that perhaps go beyond the regulation of biogenesis.
Caloric restriction and prevention of pathologies
Habit and circadian rhythm.
a) Metabolites of tryptophan in caloric restriction.
Many obese have a certain gap between their hormonal levels during the day-night rhythm of the circadian cycle or rhythm. An example is the high concentration of cerebral serotonin that appears in blood when awakening and induces satiety. Serotonin is a metabolite of the essential amino acid tryptophan. And precisely many obese present at dawn elevated levels of serotonin, whose metabolite is melatonin, both related to sleep and the last as a drug (Figure 7).
Habit and circadian rhythm.
a) Metabolites of tryptophan in caloric restriction.
Many obese have a certain gap between their hormonal levels during the day-night rhythm of the circadian cycle or rhythm. An example is the high concentration of cerebral serotonin that appears in blood when awakening and induces satiety. Serotonin is a metabolite of the essential amino acid tryptophan. And precisely many obese present at dawn elevated levels of serotonin, whose metabolite is melatonin, both related to sleep and the last as a drug (Figure 7).
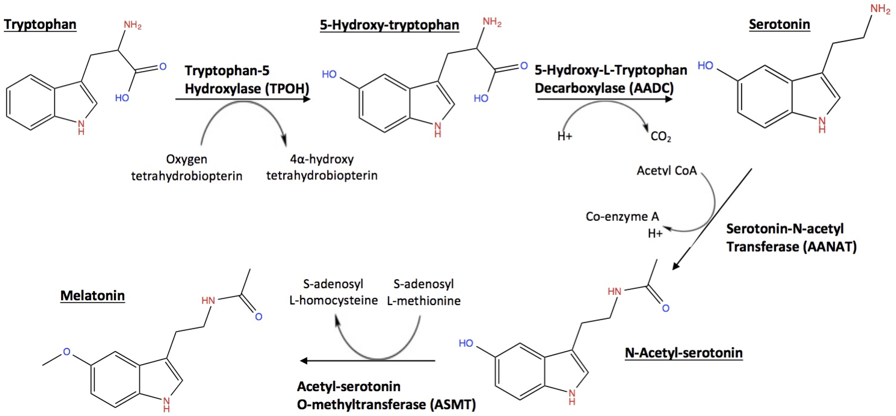
Figure 7: Some interesting metabolites of Tryptophan. (https://sites.tufts.edu/sleep/biochemical-pathway/)
Serotonin is a neurotransmitter secreted by the pineal gland that carries signals (stimulus) along and between (synapsis) neurons, the cells of the brain and nervous system. Contributing to sleep as its metabolite the melatonine and to happiness and wellbeing. It plays an important role also in the regulation of mood and appetite [31]. It structure as chemical compound is synthesized from the amino acid tryptophan in the brain and gastro-intestinal system.
High levels of serotonin cause a reluctance and rejection for breakfast. On the other hand in many obese at dusk befall decreases in serotonin, which increase the appetite that causes addictive impulses to ingest food. In frequent cases the obese ones incline towards flours, bread with jam, candies and chocolates. These sudden oscillations of cerebral and blood serotonin, promote in the obese, the modification of their meal schedule. A tendency to eat more at night before bedtime, than during breakfast before work and energy expenditure, is the so-called "inverted diet".
b) Circadian rhythm and hormones.
When applying calorie restriction, habits should be overcome if they are inverse to hormonal rhythms, normal day-night circadian rhythm. Food Ingestion hours: breakfast-lunch-dinner, must coincide with both: with the circadian rhythm day-night, with the level hormones that govern a normal body, and with the schedule of their physical activity or work. Deregulation leads to obesity. The lack or poor breakfast, and an abundant dinner favor the obesity. Some obese do not have breakfast, while others make a poor protein breakfast.
When applying calorie restriction, habits should be overcome if they are inverse to hormonal rhythms, normal day-night circadian rhythm. Food Ingestion hours: breakfast-lunch-dinner, must coincide with both: with the circadian rhythm day-night, with the level hormones that govern a normal body, and with the schedule of their physical activity or work. Deregulation leads to obesity. The lack or poor breakfast, and an abundant dinner favor the obesity. Some obese do not have breakfast, while others make a poor protein breakfast.
It is important to know the metabolism and calorie expenditure. Even in the case of a subject with normal weight and balanced energy expenditure, when breakfast is lacking or it is a very scarce breakfast, during his physical activity there is an expense and lowering of the blood glucose levels that detects the brain. The lack of blood glucose and therefore cerebral, diminishes the faculties of the brain, which feeds on glucose.In the absence of breakfast, a consumption or destruction of muscle proteins, collagen joints, skin and ligaments begins.
These are transformed into amino acids and leave the tissues, as you all know, to be converted into glucose in the liver and mitochondria, through the glycolysis and the Krebs cycle mentioned above (Figure 1), restoring the levels of blood glucose and in the brain. This general pathology and specifically dermal translates into some elderly, although not obese, to suffer itching, itching in the skin, which come from the toxins of their muscles and tendons.
c) Circadian rhythm and cortisol
In this context, cortisol (Figure 8), is one of the hormones that affects the transformation of proteins into energy, and determines that throughout the morning proteins are used to keep blood glucose levels stable. This situation causes muscular and joint pain, as well as a generalized weakness. The skin loses collagen, slimming, loses its turgor and damages its seven cell layers of various embryonic origin.
In this context, cortisol (Figure 8), is one of the hormones that affects the transformation of proteins into energy, and determines that throughout the morning proteins are used to keep blood glucose levels stable. This situation causes muscular and joint pain, as well as a generalized weakness. The skin loses collagen, slimming, loses its turgor and damages its seven cell layers of various embryonic origin.
The brain starts up a saving system, which allows to lower the dietary energy expenditure as a mechanism to survive without eating. Of this it turns out that if in a hike preceded by the breakfast we spend some 100 calories food, the lack of breakfast will cover them with the own reserves. Researchers quantify the intensity of physical activity in a unit of measurement called MET, as a metabolic equivalent. A MET equals the number of calories a body consumes in the unit of time while it is resting. The foods are usually expressed in calories and values of "indicative daily amounts, CDO" of an adult (2,000 kcal). Nutritional needs vary according to age, sex, genetics, physical and intellectual activity, and other factors.
d) Circadian rhythm and growth hormone.
The sunlight that directs our hormonal rhythms (circadian rhythm) determines that at nightfall the growth hormone (HGH) that uses our reserve fat as fuel is elevated. A generous dinner accompanied by no activity, prevents fat mobilization. At dusk begins the rise of growth hormone (HGH), involving in addition to other hormones such as epinephrine, norepinephrine, ghrelin, testosterone, and cortisol). HGH, also called hormone somatotropin, is a peptide hormone. HGH stimulates growth, cell reproduction and regeneration in humans (children) and other species.
The sunlight that directs our hormonal rhythms (circadian rhythm) determines that at nightfall the growth hormone (HGH) that uses our reserve fat as fuel is elevated. A generous dinner accompanied by no activity, prevents fat mobilization. At dusk begins the rise of growth hormone (HGH), involving in addition to other hormones such as epinephrine, norepinephrine, ghrelin, testosterone, and cortisol). HGH, also called hormone somatotropin, is a peptide hormone. HGH stimulates growth, cell reproduction and regeneration in humans (children) and other species.
The growth hormone is a polypeptide of 191 amino acids of a single chain synthesized, stored and secreted by the somatótropas cells inside the lateral wings of the adenohypophysis, is the one that uses the fat of reserve as fuel and the one responsible for Weight loss or thinning that occurs during night sleep. However, excessive ingestion of food uses that contribution, rather than body fat deposits.
It is estimated that a person slims between 500 and 800 grams during night sleep (during the day increases in weight, while slimming when sleeping). You should avoid flours and starches, and the over feeding just at night, when the HGH begins to increase.
Otherwise in the night will not lose weight, and dawn with more fat and heavier than the night before. The HGH hormone, in addition to mobilizing fat, also activates the immune system by promoting the white blood cells to attack the bacteria and malignant cells thereby facilitating the formation of antibodies.
e) Caloric restriction and diabetes.
During the night, in fasting, the levels of insulin are low, however at the beginning of the digestion by food intake appear in the blood two peaks of secretion of insulin, the first corresponding to the reserve of the beta cells of the Langerhans islets of the endocrine pancreas, and the second to the rapid new synthesis, caused by the ingestion of food and elevation of the blood glucose level.
During the night, in fasting, the levels of insulin are low, however at the beginning of the digestion by food intake appear in the blood two peaks of secretion of insulin, the first corresponding to the reserve of the beta cells of the Langerhans islets of the endocrine pancreas, and the second to the rapid new synthesis, caused by the ingestion of food and elevation of the blood glucose level.
Obesity s also important a risk factor of diabetes. CR minimice the levels of glucose and the action of glucolisis, Krebs cycle and electron transport chain, can act easily reducing free radicals formation and diminishing cell damage in mitochondrial membranes that cause cellular death, which results in inducing premature aging. Obesity intervenes in the development of type 2 diabetes, is known as adult onset or non-insulin-dependent diabetes. It is the most common form of this disease that affects 90% of diabetics. Therefore, calorie restriction is advised in obese people to avoid inheriting other pathologies [32,33].
f) Other data of interest
Experimental data on the interrelationship between obesity and periodontitis are available, both of which involve a hyper inflammatory state and an aberrant lipid metabolism prevalent in obesity, as well as insulin resistance, with loss of support tissue. Periodontal adipocytes secrete important adipoquines in the control of appetite and body weight [33]. One of these cytokines is leptin, derives from the Greek root leptos which means thin, which is due to its evident role in the control of body weight through the regulation of appetite and thermogenesis (process by which fat is burned). Besides the hormone of the thinness, it is protective against the obesity and involved in the metabolic syndrome and in the periodontitis, considered the latter as the sixth complication of the diabetes. Adipocytes also segregate other cytokines, such as adiponectin and resistine; the first presents low levels in the aforementioned pathologies, while the second, the resistine, is proinflammatory and is associated with insulin resistance. However, some researchers point out that the most important mediator associated with obesity and insulin resistance is TNF-α, mostly expressed in adipose tissue of obese.
Experimental data on the interrelationship between obesity and periodontitis are available, both of which involve a hyper inflammatory state and an aberrant lipid metabolism prevalent in obesity, as well as insulin resistance, with loss of support tissue. Periodontal adipocytes secrete important adipoquines in the control of appetite and body weight [33]. One of these cytokines is leptin, derives from the Greek root leptos which means thin, which is due to its evident role in the control of body weight through the regulation of appetite and thermogenesis (process by which fat is burned). Besides the hormone of the thinness, it is protective against the obesity and involved in the metabolic syndrome and in the periodontitis, considered the latter as the sixth complication of the diabetes. Adipocytes also segregate other cytokines, such as adiponectin and resistine; the first presents low levels in the aforementioned pathologies, while the second, the resistine, is proinflammatory and is associated with insulin resistance. However, some researchers point out that the most important mediator associated with obesity and insulin resistance is TNF-α, mostly expressed in adipose tissue of obese.
Both TNF-α and IL-6 are secreted by adipocytes after intracellular signaling, and induce insulin resistance and stimulate liver production of C-reactive protein.
Caloric restriction should be a habit, necessary for weight loss, to prevent various diseases and pathologies, such as the entities of the metabolic syndrome, and the improvement of senescence and to achieve an active longevity.
References
- Walford R, Weindruch R. (1988). Book Review: The retardation of aging and disease by dietary restriction. Springfield, Ill, U.S.A. 339-397. ISBN: 0-398-05496-7
- Santos Ruiz A, Cascales M. (2000). Restricción calórica y expectativa de vida. En: Alimentos y Salud, B Sanz (ed.) Real Academia Nacional de Farmacia. Madrid 413-453.
- Cascales Angosto M. (2001). La naturaleza del envejecimiento. En: Envejecimiento y Cultura (Eds P García Barreno y A Portera Sánchez) Instituto de España 173-224.
- Bishop KS, Ferguson LR. (2015). The interaction between epigenetics, nutrition and the development of cancer. Nutritients 7. 922-947.
- Blasco M.A.Salomone M.G. Morir joven a los 140 años (die young to 140 years old). 2016; (256 pages) Editorial Paidós Ibérica. ISBN: 9788449332067.
- De la Osada J (2016). Como consigue nuestra dieta que seamos lo que somos. Academia de Farmacia Reino de Aragón. Colegio Oficial de Farmacéuticos de Zaragoza (ed). 2016; Cometa S.A. 17-39.
- Heydary AR, Unnikrishnan A, Lucente LV et al. (2007). Caloric restriction and genomic stability. Nucleic Acid Res 35(22): 7485-7496.
- Rogina B y Helfand SL. (2004). Sir2 mediates longevity in the fly through a pathway related to caloric restriction. Proc Natl Acad Sci USA 101, 15998-16003.
- Panowski SH, Wolff S, Aguilaniu H et al. (2007). PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447, 550-555.
- Baur JA, et al (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006; 444 (7117): 337-42.
- Qin W, Chachich M, Lane M, et al. (2006). Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J Alzheimers Dis 10 (4): 417-422.
- Haigis MC, Guarente LP. (2008). Mammalian sirtuins-emerging roles in physiology, aging and calorie restriction. Genes & Dev 20(21): 2913-2921.
- Howitz KT, Bitterman KJ, Cohen HY, et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425 (6954): 191-196.
- Cohen HY, Miller C, Bitterman KJ et al. (2004). Caloric restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305 (5682): 390-2.
- Montoya Villarroya J. (2009). Biogénesis y patología mitocondrial. Lecture in Academy of Pharmacy of Reino de Aragón, on 01.12.2009. pp 13-72.
- Abente EJ, Subramanian M, Kamachandran V et al. (2016). MicroRNAs I n obesity-associated disorders. Arch Biochem Biophys Doi 10.1016/09.018.
- Yang J, Hirschi KD, Farmer LM. (2015). Dietary RNAs: New stories regarding oral delivery. Nutrients 7, 3184-3199.
- McCay CM, Crowell MF. (1935). Prolonging the life span. Sci Month 39, 405-414.
- McCay CM, Crowell MF, Maynard LA. (1935). The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr 10, 63-79.
- Stein Ph. K., Soare A., Meyer .E., Cangemi R., Holloszy J.O., Fontana L. (2012). Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell,; DOI: 10.1111/j.1474- 9726.2012.00825.x.
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, et al., (2000). Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. PNAS, 97(8): 4279-4284.
- Krengel U, Törnroth-Horsefield S. (2015). Coping with oxidative stress: A crystal structure helps to explain how cells detoxify. Sience 347, 125-126.
- Leung JH, et al. (2015). Division of labor in transhydrogenase by alternating proton translocation and hydride transfer. Science 347 (6218), 178-181.
- Milne Jill C, Denu JM. (2008). The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12(1): 1-17.
- Sinclair DA, Guarente L. (1997). Extrachromosomal rDNA circles-a cause of aging in yeast. Cell 26, 91(7), 1033-1042.
- Uhlen M, Fagerberg L, HallstromBM et al. Proteomics. Tissue-based map of the human proteome. Science. 2015; 347, 12604-19.
- Canto C, Auwerx J. (2008). Glucose restriction: Longevity SIRTainly, but without building muscle? Developmental cell 14(5): 642-644.
- Picard F, Kurtev M, Chung N, et al. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429 (6993): 771-776.
- Rogina B y Helfand SL. (2004). Sir2 mediates longevity in the fly through a pathway related to caloric restriction. Proc Natl Acad Sci USA 101, 15998-16003.
- Wood JG, Rogina B, Lavu S et al. (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430 (7000), 686-689, Erratum in Nature 431(7004): 107.
- Ciarleglio CM, Resuehr HE, McMahon D.G. (2011). Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience. Dec 1;197:8-16. doi: 10.1016/ J. Neuroscience. 2011.09.036.
- Marchel E et al. (2012). Periodontal disease: the influence of metabolic syndrome. Nutrition & Metabolism 9 (1): 88.
- Zimmermann G S, et al. (2013). Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. Journal Periodontology 84 (5): 624.
Citation: Bartolomé Ribas Ozonas and María Cascales Angosto. (2019). Caloric Restriction and Longevity. Journal of Pharmacy and Drug Development 1(1). DOI: 10.5281/zenodo.3373326
Copyright: © 2019 Bartolomé Ribas Ozonas. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



