Theses
Volume 4 Issue 2 - 2022
Assesment of Community Knowledge, Attitude and Practice on Rabies in Nejo Woreda
Jimma University By Doctor of Veterinary Medicine 2018, G:C. Now I am student of Msc Veterinary Microbiology at Jimma University in this year 2022, Ethiopia
*Corresponding Author: Gemechis Biratu, Jimma University By Doctor of Veterinary Medicine 2018, G:C. Now I am student of Msc Veterinary Microbiology at Jimma University in this year 2022, Ethiopia.
Received: August 17, 2022; Published: December 15, 2022
Abstract
Rabies comes from Latin word meaning for madness. It's an invariably fatal neurologic disease affecting most warm blooded animals and humans. However, it can be prevented via vaccination and community awareness. Therefore, this study was conducted to measure the level of community knowledge, attitudes and practices (KAP) related to rabies. A cross-sectional study was conducted from November, 2017 to April, 2018 in Nejo district, west wollega zone that found in Oromia regional state, west Ethiopia. Multistage sampling procedures were employed to select households for this study. The data were collected from 220 households through face to face interview using pretested and structured questionnaires. Descriptive statics techniques and Pearson’s chi squares analysis were used to manage the data and the association between outcome (KAP) and explanatory variables. Out of 220 respondents interviewed, 123 (55.9%) of them were males and 97 (44.1%) females and 111 (50.5%) were between 15-30 years old. The majority of the respondents 118 (53.6%) were protestants. Over 121(55%) of respondents owned domestic dogs and 206(93.6%) knew dogs are the most rabies transmitter than other animals. Greater than half of the study participants 124(56.4%) had good level of KAP. There was not significantly association between KAP scores and sex (χ²= 9.361, p =0.245), religion (χ²= 15.15, p =0.462) and age (χ²= 13.479, p =0.322), but there is significant association between Educational status and KAP score (χ²=336.99, p=0.001). Generally these findings indicate that the Nejo community has good knowledge about rabies. But it need for educational outreach in Nejotown to raise accurate knowledge on ways of infection, symptoms and appropriate prevention and treatment measures.
Keywords: Nejo; Attitude; Ethiopia; Knowledge; Practice; Rabies
Introduction
Rabies is an acute encephalitis illness caused by rabies virus. Rabies virus is the prototype species of the genus Lyssavirus in the family of Rhabdoviridae. The virus affects virtually all mammals and infected species invariably die from the disease once clinical signs are manifested [15]. Globally, human mortality from endemic canine rabies was estimated to be 55,000 deaths per year and 56% of the estimated deaths occur in Asia and 44% in Africa. About 98% of the human rabies cases occur in developing countries that possess large number of dogs, many of which are stray [38].
Rabies is endemic in developing countries of Africa and Asia, and most human deaths from the disease occur in these endemic countries [37]. The annual cost of rabies in Africa and Asia was estimated at US$ 583.5 million most of which is due to cost of post exposure prophylaxis (PEP) [19]. Ethiopia being one of the developing countries is highly endemic for rabies. Approximately 10, 000 people were estimated to die of rabies annually in Ethiopia which makes it to be one of the worst affected countries in the world [10]. Dogs are the principal source of infection for humans and livestock. In Ethiopia many households own dogs usually for guarding property. Although there are no formal studies, it is estimated that there is one owned dog per five household nationally (Deressaet al; 2010). Dog management is often poor and dog vaccination is limited to few dogs in urban centers. High population of dogs with poor management contributes for high endemicity of canine rabies in Ethiopia. Apart from its effects to human beings in endemic countries like Ethiopia, rabies has also significant economic implications by affecting livestock. For example, in Africa and Asia, the annual cost of livestock losses as a result of rabies is estimated to be US$ 12.3Million [19].
In Ethiopia, individuals who are exposed to rabies virus often see traditional healers for the diagnosis and treatment of the disease. These widespread traditional practices of handling rabies cases are believed to interfere with timely seeking of PEP. Rabies victims especially from rural areas seek PEP treatment after exhausting the traditional medicinal intervention and usually after a loss of life from family members [8].
The available information on rabies in Ethiopia is largely based on passive reports to the Ethiopian Health and Nutrition Research Institute zoonoses laboratory [28,9], the only rabies diagnostic laboratory in the country. Passive reports usually underestimate incidence and are poor indicator of the status of the disease in countries where human and animal health information systems are inadequate [16,17]. There is lack of accurate quantitative information on rabies both in humans and animals and little is known about the awareness of the people about the disease to apply effective control measures in Ethiopia.
Therefore, the objective of this study is to determine the level of knowledge, attitudes and practices (KAP) of the community in Nejo woreda about rabies.
Literature Review
Etiology
Rabies virus (RABV) is the prototype virus of the genus Lyssavirus (from the Greek lyssameaning 'rage') in the family Rhabdoviridae (from the Greek rhabdosmeaning 'rod'). The prototype RABV is a genotype 1 virus (formerly recognized as serotype 1). Lagos bat virus (LBV, genotype 2/serotype 2), Mokola virus (MOKV, genotype 3/serotype 3), Duvenhage virus (DUVV, genotype 4/serotype 4), European bat lyssavirus type 1 (EBL-1, genotype 5), European bat lyssavirus type 2 (EBL-2, genotype 6) and Australian bat lyssavirus (ABLV, genotype 7) are rabies-related lyssaviruses that reflect the genotypic diversity of the genus Lyssavirus (Kuzminet al., 2005). It is a disease of mammals, but the sensitivity to the virus can vary between different mammal hosts [21].
Etiology
Rabies virus (RABV) is the prototype virus of the genus Lyssavirus (from the Greek lyssameaning 'rage') in the family Rhabdoviridae (from the Greek rhabdosmeaning 'rod'). The prototype RABV is a genotype 1 virus (formerly recognized as serotype 1). Lagos bat virus (LBV, genotype 2/serotype 2), Mokola virus (MOKV, genotype 3/serotype 3), Duvenhage virus (DUVV, genotype 4/serotype 4), European bat lyssavirus type 1 (EBL-1, genotype 5), European bat lyssavirus type 2 (EBL-2, genotype 6) and Australian bat lyssavirus (ABLV, genotype 7) are rabies-related lyssaviruses that reflect the genotypic diversity of the genus Lyssavirus (Kuzminet al., 2005). It is a disease of mammals, but the sensitivity to the virus can vary between different mammal hosts [21].
Epidemiology
Distribution of rabies
Rabies occurs in most countries of the world except island countries, where it excluded by quarantine measures or prohibition of dog entry. Australia and New Zealand have never had the disease, and Britain, Hawaii and Scandinavian countries are currently free from the disease. The disease is enzootic in Yugoslavia, Turkey and much of the United States of America particularly the eastern and southern states [31]. In some parts of Europe, North America, Southern and Eastern Asia, Africa and Latin America the disease is widely distributed. Vampire bat transmitted bovine paralytic rabies, which is endemic in tropical regions extending from Northern Mexico to Southern Argentina and Island of Trinidad and Tobago [31].
Distribution of rabies
Rabies occurs in most countries of the world except island countries, where it excluded by quarantine measures or prohibition of dog entry. Australia and New Zealand have never had the disease, and Britain, Hawaii and Scandinavian countries are currently free from the disease. The disease is enzootic in Yugoslavia, Turkey and much of the United States of America particularly the eastern and southern states [31]. In some parts of Europe, North America, Southern and Eastern Asia, Africa and Latin America the disease is widely distributed. Vampire bat transmitted bovine paralytic rabies, which is endemic in tropical regions extending from Northern Mexico to Southern Argentina and Island of Trinidad and Tobago [31].
Host Range and Susceptibility
Rabies occurs in all warm-blooded animals including cattle, sheep, pigs, and horses in most countries. The most important animal families in maintaining rabies cycles are Canidae (dogs, foxes, jackals, wolves etc.), Mustelidae (skunks, martens, weasels, ferrets stoats etc.), Viverridae (mongooses, meerkat etc.), Procyonidae (raccoon etc.), and Chiroptera (> 1,200 species of bats) [32]. Many animal species can be regarded as accidental hosts or ‘dead end’ hosts, and these species have no epidemiological Significance in sustaining rabies epidemics. These include humans and other primates, horses, cattle, sheep and pigs [26].
Rabies occurs in all warm-blooded animals including cattle, sheep, pigs, and horses in most countries. The most important animal families in maintaining rabies cycles are Canidae (dogs, foxes, jackals, wolves etc.), Mustelidae (skunks, martens, weasels, ferrets stoats etc.), Viverridae (mongooses, meerkat etc.), Procyonidae (raccoon etc.), and Chiroptera (> 1,200 species of bats) [32]. Many animal species can be regarded as accidental hosts or ‘dead end’ hosts, and these species have no epidemiological Significance in sustaining rabies epidemics. These include humans and other primates, horses, cattle, sheep and pigs [26].
Pathogenesis
After inoculation of infectious saliva by bite, the virus may either persist and replicate in the striated muscles of inoculation site for two weeks or follow a relatively rapid centripetal to the central nervous system, with replication and dissemination prior to the development of a significant immune response. After peripheral nerve entry, virus moves centripetally with in axons to the CNS at the estimated rate of 3 mm/hour. Once virus reaches the brain, it spread centrifugally to a variety of organs, the spread in to the salivary gland, which represents the final phase of infection, is important from animal to animal and from animal to human transmission [18]. Hematogenous spread can occur but is rare. Although the disease usually is fatal once clinical signs appear, recovery has been recorded in several animals, including man [12]. The primary lesions produced are in the CNS, and spread from the site of infection occurs only by way of the peripheral nerves. This method of spread accounts for the extremely variable incubation period, which varies to a large extent with the site of the bite [32].
After inoculation of infectious saliva by bite, the virus may either persist and replicate in the striated muscles of inoculation site for two weeks or follow a relatively rapid centripetal to the central nervous system, with replication and dissemination prior to the development of a significant immune response. After peripheral nerve entry, virus moves centripetally with in axons to the CNS at the estimated rate of 3 mm/hour. Once virus reaches the brain, it spread centrifugally to a variety of organs, the spread in to the salivary gland, which represents the final phase of infection, is important from animal to animal and from animal to human transmission [18]. Hematogenous spread can occur but is rare. Although the disease usually is fatal once clinical signs appear, recovery has been recorded in several animals, including man [12]. The primary lesions produced are in the CNS, and spread from the site of infection occurs only by way of the peripheral nerves. This method of spread accounts for the extremely variable incubation period, which varies to a large extent with the site of the bite [32].
Bites on the head usually result in a shorter incubation period than bites on the extremities. The severity and the site of the lesions will govern to a large extent whether the clinical picture is primarily one of iritative or paralytic phenomena. The two extremes of the paralytic or dumb form and the furious form are accompanied by many cases that lie somewhere between the two. Gradually ascending paralysis of the hindquarters may be followed by severe signs of mania, which persist almost until death. Destruction of spinal neurons results in paralysis, but when the virus invades the brain, irritation of higher centers produces manias, excitement, and convulsions. Death is usually due to respiratory paralysis. The clinical signs of salivation, indigestion and pica, paralysis of bladder and anus, and increased libido all suggest involvement of the autonomic nervous system, including endocrine glands [32].
Clinical signs and symptoms
The clinical course in domestic carnivores, which usually lasts for days or for a few weeks, may encompass prodromal, furious (exitative) and dumb (paralytic) phases. In certain rabid animals, some of these phases may not be observed. In the prodromal phase affected animals are often confused and disorientated; wild animal may lose their natural fear of humans. The furious phase is characterized by an increase in aggressiveness and hyperexitability, and there is a tendency to bite at inanimate objects and at other animals. Affected animal may roam over long distances. The furious form is observed more often in cats than in dogs. Foxes rarely exhibit this form of the disease. In dumb phase muscle weakness, difficulty in swallowing, profuse salivation and dropping of the jaw are the usual features. Those clinical signs may be mistaken for those caused by a foreign body in the throat or mouth [21].
The clinical course in domestic carnivores, which usually lasts for days or for a few weeks, may encompass prodromal, furious (exitative) and dumb (paralytic) phases. In certain rabid animals, some of these phases may not be observed. In the prodromal phase affected animals are often confused and disorientated; wild animal may lose their natural fear of humans. The furious phase is characterized by an increase in aggressiveness and hyperexitability, and there is a tendency to bite at inanimate objects and at other animals. Affected animal may roam over long distances. The furious form is observed more often in cats than in dogs. Foxes rarely exhibit this form of the disease. In dumb phase muscle weakness, difficulty in swallowing, profuse salivation and dropping of the jaw are the usual features. Those clinical signs may be mistaken for those caused by a foreign body in the throat or mouth [21].
Diagnosis
Clinical diagnosis
Clinical diagnosis is difficult, especially in areas where rabies is uncommon, and should not be relied on when making public health decisions. In the early stages, rabies can easily be confused with other diseases or with normal aggressive tendencies. Therefore, when rabies is suspected and definitive diagnosis is required, laboratory confirmation is indicated. Suspect animals should be euthanized, and the head removed for laboratory shipment [12].
Clinical diagnosis
Clinical diagnosis is difficult, especially in areas where rabies is uncommon, and should not be relied on when making public health decisions. In the early stages, rabies can easily be confused with other diseases or with normal aggressive tendencies. Therefore, when rabies is suspected and definitive diagnosis is required, laboratory confirmation is indicated. Suspect animals should be euthanized, and the head removed for laboratory shipment [12].
A presumptive diagnosis of rabies, an acute, progressive encephalomyelitis, with the highest case fatality rate of any infectious disease, is simple in a person presenting with a compatible illness after documented exposure to a laboratory-confirmed rabid animal. Specific clinical signs of hydro- or aerophobia in humans provide a strong suspicion of rabies, if they are well documented. In the absence of a history of exposure or paramount signs, however, the diagnosis of rabies on clinical grounds alone is difficult and often unreliable. For example, some patients can present with a paralytic or Guillain-Barré-like syndrome or other atypical features. Atypical or non-classical rabies is increasingly recognized and may be responsible for underreporting of cases [29].
Rabies should be included in the differential diagnosis of all patients who present with unexplained, acute, progressive viral encephalitis, even in areas where the disease is rare, as it can occur locally in wildlife, such as bats, can be acquired during travel to enzootic areas and because imported cases of human and animal rabies continue to occur [29]. In addition, rabies may be misdiagnosed and death ascribed to another cause (e.g. cerebral malaria), without adequate epidemiological scrutiny and laboratory confirmation [22]. As transmission of rabies virus to recipients of solid organ transplants has been described, all potential organ donors who present with a compatible encephalitis should be screened and tested to determine whether they present an infectious risk, by examining suitable ante- or post-mortem specimens by sensitive, specific laboratory methods [27].
Laboratory techniques
A definitive diagnosis of rabies can be made only with the appropriate laboratory methods. The direct fluorescent antibody technique is a rapid, sensitive, specific method for diagnosing rabies in animals and humans and is the gold standard for rabies diagnosis. The accuracy of the test depends, however, on variables such as the expertise of the examiner, the quality of the anti-rabies conjugate and basic equipment, including the fluorescence microscope. The test is based on microscopic examination of impressions or smears of brain tissue after incubation with anti-rabies polyclonal globulin or broadly cross-reactive monoclonal antibodies conjugated with fluorescence isothiocyanate [33].
A definitive diagnosis of rabies can be made only with the appropriate laboratory methods. The direct fluorescent antibody technique is a rapid, sensitive, specific method for diagnosing rabies in animals and humans and is the gold standard for rabies diagnosis. The accuracy of the test depends, however, on variables such as the expertise of the examiner, the quality of the anti-rabies conjugate and basic equipment, including the fluorescence microscope. The test is based on microscopic examination of impressions or smears of brain tissue after incubation with anti-rabies polyclonal globulin or broadly cross-reactive monoclonal antibodies conjugated with fluorescence isothiocyanate [33].
Other methods for the detection of lyssavirus antigens, such as enzyme-linked immune sorbent assays (ELISAs) and direct rapid immunohistochemistry tests have provided consistently reproducible results in several laboratories [27]. Virus might have to be isolated to confirm the results of antigen detection tests and for further amplification or characterization of an isolate. Virus can be isolated in cell cultures, such as neuroblastoma cells, or by intracranial inoculation into mice. Virus isolation in animals should be replaced by alternative methods, whenever possible. [25]. Molecular methods, such as the reverse transcription polymerase chain reaction (RT-PCR) and other amplification techniques, are playing an increasingly important role in many countries but are not recommended currently for routine post-mortem diagnosis of rabies if brain tissue is available, when the direct fluorescent antibody test should be used [25].
Situation of Rabies in Ethiopia
Rabies is one of the most severe infectious diseases in Ethiopia; many cases diagnosed in various parts of the country. The dog is the species most responsible for human exposure; over 98% of human cases and vaccinations are due to the bite of rabid or suspected rabid dogs. Peaks in canine rabies reports associated with seasonal reproductive pattern of dogs, measured by household surveys or observation of the proportion of pups in the canine population. In Ethiopia seasonal peak of rabies incidence in rainy season, June to July was recorded [2]. Most of the treatments are due to stray dogs that bite and escape and are not available for observation. Most of the people who die of rabies are under 40 years of age. Among adults, the majority are males [10].
Rabies is one of the most severe infectious diseases in Ethiopia; many cases diagnosed in various parts of the country. The dog is the species most responsible for human exposure; over 98% of human cases and vaccinations are due to the bite of rabid or suspected rabid dogs. Peaks in canine rabies reports associated with seasonal reproductive pattern of dogs, measured by household surveys or observation of the proportion of pups in the canine population. In Ethiopia seasonal peak of rabies incidence in rainy season, June to July was recorded [2]. Most of the treatments are due to stray dogs that bite and escape and are not available for observation. Most of the people who die of rabies are under 40 years of age. Among adults, the majority are males [10].
Persistence, and to some extent expansion of the overall rabies situation in the regions of the country indicate inadequacy of control activities [24]. The various constraints that are responsible for the situation include: Not a priority disease, insufficient surveillance systems, inadequate resources due to lack of political support, lack of a national policy and a comprehensively coordinated national rabies control program, weak inter-sectarian coordination, and dog population management programs, non-implementation of technically sound strategies, inadequate research and development and absence of health education as well as lack of public awareness and cooperation [40].
Rabies in Nejo
There is no study on KAP of rabies in Nejo community before but dog bites were 'most common’. Now a day many people in the area attend traditional medication and under estimating the reports, Nejo Hospital and few health center based reports shows currently the disease is endemic in the area. According to data from Nejohospital from 2006-2009E.c, around 480 people bitten by animal, from this 80% are children under the age of 18 and elder above 60 ages. Out of this 465were bitten by dogs.
There is no study on KAP of rabies in Nejo community before but dog bites were 'most common’. Now a day many people in the area attend traditional medication and under estimating the reports, Nejo Hospital and few health center based reports shows currently the disease is endemic in the area. According to data from Nejohospital from 2006-2009E.c, around 480 people bitten by animal, from this 80% are children under the age of 18 and elder above 60 ages. Out of this 465were bitten by dogs.
Material and Methods
Study Area
Cross-sectional study was conducted in Nejotown, west wollegazone of Oromia region from November, 2017 to April, 2018to assess the knowledge, attitudes and practices of the Communities on rabies Nejoworeda was one of the districts of West Wollega Administrative Zone, Oromia region and the 2007 national census reported a total population for this woreda of 130,909 in 25,336 households, of whom 64,654 were men and 66,255 were women; 24,505 or 18.72% of its population were urban dwellers. The majority of the inhabitants observed Protestantism, with 63.72% reporting that as their religion, while 33.69% observed Ethiopian Orthodox Christianity, and were 1.72% Muslim [5,6].
Cross-sectional study was conducted in Nejotown, west wollegazone of Oromia region from November, 2017 to April, 2018to assess the knowledge, attitudes and practices of the Communities on rabies Nejoworeda was one of the districts of West Wollega Administrative Zone, Oromia region and the 2007 national census reported a total population for this woreda of 130,909 in 25,336 households, of whom 64,654 were men and 66,255 were women; 24,505 or 18.72% of its population were urban dwellers. The majority of the inhabitants observed Protestantism, with 63.72% reporting that as their religion, while 33.69% observed Ethiopian Orthodox Christianity, and were 1.72% Muslim [5,6].
It is 498km far from the capital city, Addis Ababa and It shares the boundary with Manasibu district to the west; southeast by Boji, Jarso district to the south-west, Bila district to east and Benishangulgumuz region to the northeast direction. This town has latitude and longitude of 9.30°N 35.30°E with elevation 1821m.a.s.l. The rain fall and temperature vary from 1350-2300mm and 18°c-28°c respectively with humidity 25%-83.5%. It has a livestock population of cattle (210317), equine (9711), shoat (57150), poultry (48259) and 350 owned dog in the town.
Study Population
A cross-sectional study design and multi-stage sampling procedures were employed to select households for this study. Four kebeles were randomly selected using lottery method from list of 6 kebeles by using simple random method. Communitiesabove age of fifteen and both sexes were asked and also they are those lived in the district for at least six months as permanent resident of the town.
A cross-sectional study design and multi-stage sampling procedures were employed to select households for this study. Four kebeles were randomly selected using lottery method from list of 6 kebeles by using simple random method. Communitiesabove age of fifteen and both sexes were asked and also they are those lived in the district for at least six months as permanent resident of the town.
Sample size
Community based cross-sectional quantitative study design was used to assess the knowledge, attitudes and practices (KAP) on rabies community of Nejo town. The communities of Nejo town who are above 15 years and lived in the district for at least six months were included in this study. The required number of population to be sampled was calculated using the formula given by Arsham (2005). N = 0.25 / SE2, Where N = sample size, SE = standard error of 5%. Accordingly, the required sample size was 100. However, to increase the precision and representativeness, the sample size was increased, 220. The calculated sample was proportionally distributed to the selected kebele.
Community based cross-sectional quantitative study design was used to assess the knowledge, attitudes and practices (KAP) on rabies community of Nejo town. The communities of Nejo town who are above 15 years and lived in the district for at least six months were included in this study. The required number of population to be sampled was calculated using the formula given by Arsham (2005). N = 0.25 / SE2, Where N = sample size, SE = standard error of 5%. Accordingly, the required sample size was 100. However, to increase the precision and representativeness, the sample size was increased, 220. The calculated sample was proportionally distributed to the selected kebele.
Sampling methodology and Sample collection
Sampling Method, Data Collection Tools and Procedures: A multi-stage sampling technique was employed for the selection of the sampling units. From the entire Primary sampling unit, i.e. 6 kebeles, 4 kebeles of the town were selected by lottery method. The number of households to be included in each kebele was determined as equal number house hold from all kebele. From the entire secondary sampling unit, Individual household, in the selected kebeles was selected using a systematic random sampling technique. From each selected household individual was further selected by simple random sampling technique and interviewed. A pretested structured questionnaire consisting of closed ended questions was used for this study. The data were collected via interview. The questionnaire was first developed in English and then translated in to Afan Oromo language (native language) for appropriateness and easiness in approaching the study participants.
Sampling Method, Data Collection Tools and Procedures: A multi-stage sampling technique was employed for the selection of the sampling units. From the entire Primary sampling unit, i.e. 6 kebeles, 4 kebeles of the town were selected by lottery method. The number of households to be included in each kebele was determined as equal number house hold from all kebele. From the entire secondary sampling unit, Individual household, in the selected kebeles was selected using a systematic random sampling technique. From each selected household individual was further selected by simple random sampling technique and interviewed. A pretested structured questionnaire consisting of closed ended questions was used for this study. The data were collected via interview. The questionnaire was first developed in English and then translated in to Afan Oromo language (native language) for appropriateness and easiness in approaching the study participants.
Data analysis
After collecting, the data was cleaned and checked for its completeness. Those incomplete and inconsistent were corrected when possible. After complete check-up the data was coded and entered to Microsoft Excel and transport to SPSS version 20 statistical packages for windows and analysis made. The frequency distribution of both dependent and independent variables were worked out by using descriptive statics techniques (Frequencies, mean and percentage). Association between independent variables and KAP scores on rabies was calculated using Pearson’s Chi square and a 95% confidence interval of the p-values were used to describe statistical significance associations. The association is judged as significant when p- value is less than 0.05.
After collecting, the data was cleaned and checked for its completeness. Those incomplete and inconsistent were corrected when possible. After complete check-up the data was coded and entered to Microsoft Excel and transport to SPSS version 20 statistical packages for windows and analysis made. The frequency distribution of both dependent and independent variables were worked out by using descriptive statics techniques (Frequencies, mean and percentage). Association between independent variables and KAP scores on rabies was calculated using Pearson’s Chi square and a 95% confidence interval of the p-values were used to describe statistical significance associations. The association is judged as significant when p- value is less than 0.05.
Ethical Clearance
The study protocol was reviewed and approved by Institutional Review Board of University of Jimma School Veterinary Medicine and support from Community Service Office Nejo town. Oral informed consents were obtained from each participant after informing them about the purpose of the study as well as the risks, benefit and rights of the study participants. Only voluntary participants were involved in the study. All the information obtained from the study participants was kept confidential.
The study protocol was reviewed and approved by Institutional Review Board of University of Jimma School Veterinary Medicine and support from Community Service Office Nejo town. Oral informed consents were obtained from each participant after informing them about the purpose of the study as well as the risks, benefit and rights of the study participants. Only voluntary participants were involved in the study. All the information obtained from the study participants was kept confidential.
Results
Socio-Demographic Characteristics
A total of 220 community members were interviewed during the study period and all respondents were responded to the questioner. The majority of the respondents in this study were males 123 (55.9%), while the number of females was 97 (44.1%). Regarding age group, 111(50.5%) of the study participants were between 15-30 years old. The majority of the respondents 118 (53.6%) were Protestants followed by Orthodox 83 (37.7%) and 19(8.6%) where Muslims. Concerning educational status, 87(39.5%) of the participants were secondary school, 71(32.3%) higher education, 44(20%) were primary school and 18(8.2%) of the respondent were illiterate. Socio demographic characters of the participant were summarized in Table 1 below.
A total of 220 community members were interviewed during the study period and all respondents were responded to the questioner. The majority of the respondents in this study were males 123 (55.9%), while the number of females was 97 (44.1%). Regarding age group, 111(50.5%) of the study participants were between 15-30 years old. The majority of the respondents 118 (53.6%) were Protestants followed by Orthodox 83 (37.7%) and 19(8.6%) where Muslims. Concerning educational status, 87(39.5%) of the participants were secondary school, 71(32.3%) higher education, 44(20%) were primary school and 18(8.2%) of the respondent were illiterate. Socio demographic characters of the participant were summarized in Table 1 below.
| Characteristics | Frequency | Percept |
| Sex | ||
| Male | 123 | 55.9 |
| Female | 97 | 44.1 |
| Religion | ||
| Protestant | 118 | 53.6 |
| Muslims | 19 | 8.6 |
| Orthodox | 83 | 37.7 |
| Age | ||
| 15-30 | 111 | 50.5 |
| 30-50 | 63 | 28.6 |
| >50 | 46 | 20.9 |
| Educationalstatus | ||
| Illiterate | 18 | 8.2 |
| Primary school | 44 | 20 |
| Secondary school | 87 | 39.5 |
| Higher education | 71 | 32.3 |
Table 1: Socio-demographic information of the study participants in Nejo (N= 220), 2017-2018.
Knowledge of Participants Related to Cause, Transmission, Clinical Signs and Fatal Nature of Rabies.
In this study all participants 220(100%) were heard rabies before. Among 220 respondents, 125 (56.8%) were know that virus is the cause of rabies. One hundred (45.5%) of the respondent were answered biting and contact with rabid animal is way of getting rabies. 167 (75.9%) respondent were aware or knows rabies is not curable after onset of clinical sign and 145 (65.9) were responded the human to human transmission of rabies is possible.206 (93.6%) were aware that dog is the most common animal transmit rabies followed by cat 14 (6.4%) (Table 2). Eighty two (37.3%) of respondents were answered that paralysis is symptoms in a rabid animals. While 32(14.5%) of respondents were mentioned that hyper salivation is symptoms in a rabid animals.
In this study all participants 220(100%) were heard rabies before. Among 220 respondents, 125 (56.8%) were know that virus is the cause of rabies. One hundred (45.5%) of the respondent were answered biting and contact with rabid animal is way of getting rabies. 167 (75.9%) respondent were aware or knows rabies is not curable after onset of clinical sign and 145 (65.9) were responded the human to human transmission of rabies is possible.206 (93.6%) were aware that dog is the most common animal transmit rabies followed by cat 14 (6.4%) (Table 2). Eighty two (37.3%) of respondents were answered that paralysis is symptoms in a rabid animals. While 32(14.5%) of respondents were mentioned that hyper salivation is symptoms in a rabid animals.
Eighty-nine (40.5%) of the respondentwere answered Puppy movement in abdomen is the symptoms of rabies in human, while eighty (36.4%) were answered the paralysis is the symptoms of rabies in human. One hundred and twenty-eight (58.2%) of the respondent were knows the seasonal occurrences of rabies and from those 46.8% of the respondent were knows the summer occurrences of rabies, but 43.2% are answered as the autumn occurrences of rabies. One hundred and ninety-one (86.8%) of the respondent were knows rabies is fatal disease if not treated on time and 67.3% of respondent were knows as it curable if post exposure prophylaxis is given on right time, while 26.8% were knows herbal medication for curing of rabies.
One hundred and thirty-six (61.8%) of the respondent were knows vaccination is the means of prevention of rabies in animal, while 12.3% are indicates eliminate stray dogs is also means for prevention of rabies in animals. 66.4% of the respondent were knows that the vaccinating pets is essential for preventing human from getting rabies, but 33.6% of the respondent were knows avoiding being bitten prevent human from getting rabies. Knowledge of Participants Related to Cause, ways of getting rabies, Clinical Signs, prevention method, seasonality of rabies and Fatal Nature of Rabies are summarized in table 2 below.
| Characteristics | Freq. | % | Characteristics | Freq. | % |
| Heard rabies before 220 100 Causes of rabies | If not treated | ||||
| Virus | 125 | 56.8 | The person survive | 9 | 4.1 |
| Spiritual | 52 | 23.6 | The person dies | 191 | 86.8 |
| I Don’t know | 43 | 19.5 | Healed but not as before | 20 | 9.1 |
| Ways of getting rabies | Symptom of rabies in human | ||||
| Biting and Contact with rabid animal | 100 | 45.5 | Paralysis | 80 | 36.4 |
| Eating rabid animal meat | 26 | 11.8 | Hydrophobia | 6 | 2.7 |
| Living with rabid animal | 59 | 26.8 | Hyper salivation | 45 | 20.5 |
| ALL | 35 | 15.9 | Puppy movement in abdomen | 89 | 40.5 |
| Treatable or curable after onset of symptom | |||||
| Yes | 53 | 24.1 | |||
| No | 167 | 75.9 | |||
| Animal involved in transmission | How soon symptoms appear | ||||
| Dog | 206 | 93.6 | Immediately | 57 | 25.9 |
| Cat | 14 | 6.4 | 1week | 22 | 10 |
| Donkey | 0 | 0 | 1-2 weeks | 62 | 28.2 |
| Sheep and Goats | 0 | 0 | 1month | 79 | 35.9 |
| Human to human transmission | Rabies signs in animals | ||||
| Yes | 145 | 65.9 | Paralysis | 82 | 37.3 |
| No | 75 | 34.1 | Hyper salivation | 32 | 14.5 |
| Rabies seasonal occurrences | Hydrophobia | 23 | 10.5 | ||
| Yes | 128 | 58.2 | All | 83 | 37.7 |
| No | 92 | 41.8 | |||
| If yes; season of occurrence | Rabies prevention in animal | ||||
| Summer | 103 | 46.8 | Eliminate stray dogs | 27 | 12.3 |
| Autumn | 16 | 7.5 | Vaccination | 136 | 61.8 |
| Spring | 6 | 2.7 | Herbal medicine application | 57 | 25.9 |
| Winter | 2 | 0.9 | |||
| Curing rabies | How a person prevented from getting rabies? | ||||
| Herbal medicine | 59 | 26.8 | Avoiding being bitten | 74 | 33.6 |
| Praying | 13 | 5.9 | Vaccinating pets | 146 | 66.4 |
| PEP | 148 | 67.3 | Via good nutrition | 0 | 0 |
Table 2: Knowledge of Participants Related to Cause, ways of getting rabies, Clinical Signs and Fatal Nature of Rabies.
Attitude of Participants Related to action to be taken after exposure, whether cured after onset of symptoms, where should go if bitten by rabid animal and constraints of control rabies
The attitude of participants regarding rabies was assessed. Attitudes related to action to be taken after exposure, whether cured after onset of symptoms, constraints of control rabies and where should go if bitten by rabid animal were included for the purpose. From all respondents 75.9%, knows the fatal nature of rabies after onset of clinical signs.
The attitude of participants regarding rabies was assessed. Attitudes related to action to be taken after exposure, whether cured after onset of symptoms, constraints of control rabies and where should go if bitten by rabid animal were included for the purpose. From all respondents 75.9%, knows the fatal nature of rabies after onset of clinical signs.
Concerning were the person should go if bitten by rapid animal, healthycenter for post exposure vaccination was responded by 160(72.7%) of the participants whereas, traditional treatment was responded to by 60 (27.3%) of the participants. Regardingconstraints of control rabies 35.9% of respondent said that lack of veterinary professional is constraints for control rabies in Nejo, 33.2% were consider insufficient budget and 28.6% said that lack of awareness. (Figure 1).
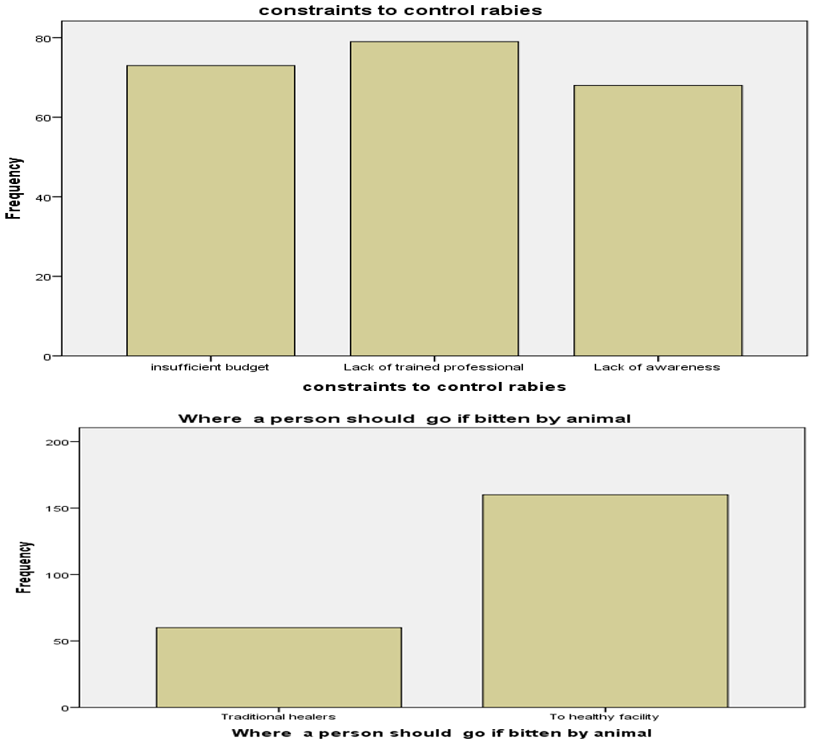
Figure 1: Frequency of respondent to constraints to control rabies and where the person should go if bitten by rabid animal Nejo Town, 2017-2018.
Community practice on immediate action for dog bites wound, Dog vaccination and when they take for vaccination in Nejo woreda, 2017-2018
In this study out of 121(55%) dog owner, 83(37.7%) were taken their dog for vaccination and from those taken their pet to vaccination 27(32.5%), 40(48.2%), 15(18.1%) of participant were vaccinated after sickness, regularly and after one month respectively.
In this study out of 121(55%) dog owner, 83(37.7%) were taken their dog for vaccination and from those taken their pet to vaccination 27(32.5%), 40(48.2%), 15(18.1%) of participant were vaccinated after sickness, regularly and after one month respectively.
The health facility of treatment 135(61.4%) was mentioned as the best option in most of respondents as immediate action for dog bites but others 22(10%) and 63(28.6%) were mentioned wash the wound and traditional medication respectively. Community practice on immediate action for dog bites wound, Dog vaccination and when they take for vaccination were summarized in figure 2 below.
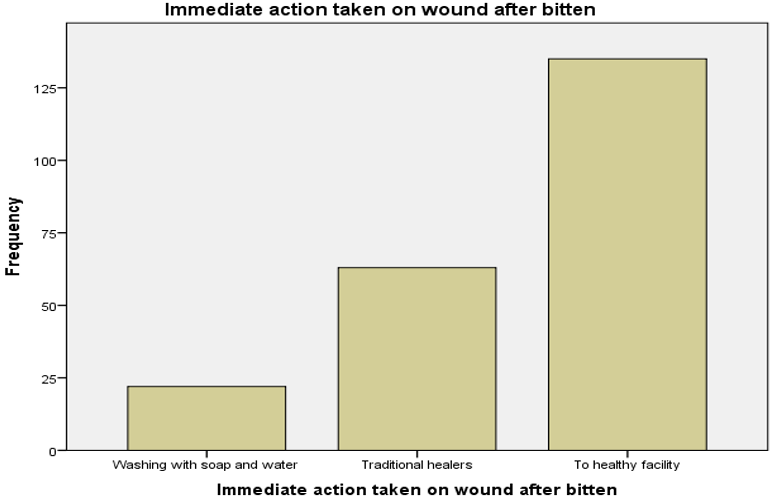
Figure 2: Frequency of respondents to immediate action on wound after bitten in Nejo town, 2017-2018.
Community KAP about Rabies in Nejo
Twenty-one questions were asked for each respondent regarding cause, transmission, clinical sign and prevention practices, treatment measures of rabies and when they vaccinate their pet which was resulted in a response of either, choose the correct answer were counted for each question. The number of questions or the KAP for which the respondent gave correct responses was counted and scored and also calculated by using the frequency of the distribution of each respondents score. The mean score of the respondents was 15.7±1.8 SD. Good KAP was considered as those respondents who score greater than or equal to the mean value (Mean≥15.7) and poor KAP was those who score less than mean value. Accordingly 124(56.4%) and 96(43.6%) respondents were have Good and Poor KAP score respectively. Means the respondents were compared with in the each variable. But there was no significant difference in means in each category of the variables except for educational status (table3) below. Association between independent variables and KAP scores on rabies was calculated using Pearson’s Chi square (Table 3). There was not significantly association between KAP scores and sex (χ²= 9.361, p = 0.245), religion (χ²= 15.15, p = 0.462) and age (χ²= 13.479, p = 0.322) ,but there is significant association between the KAP score and Educational status (χ²=336.99, p= 0.001).
Twenty-one questions were asked for each respondent regarding cause, transmission, clinical sign and prevention practices, treatment measures of rabies and when they vaccinate their pet which was resulted in a response of either, choose the correct answer were counted for each question. The number of questions or the KAP for which the respondent gave correct responses was counted and scored and also calculated by using the frequency of the distribution of each respondents score. The mean score of the respondents was 15.7±1.8 SD. Good KAP was considered as those respondents who score greater than or equal to the mean value (Mean≥15.7) and poor KAP was those who score less than mean value. Accordingly 124(56.4%) and 96(43.6%) respondents were have Good and Poor KAP score respectively. Means the respondents were compared with in the each variable. But there was no significant difference in means in each category of the variables except for educational status (table3) below. Association between independent variables and KAP scores on rabies was calculated using Pearson’s Chi square (Table 3). There was not significantly association between KAP scores and sex (χ²= 9.361, p = 0.245), religion (χ²= 15.15, p = 0.462) and age (χ²= 13.479, p = 0.322) ,but there is significant association between the KAP score and Educational status (χ²=336.99, p= 0.001).
| Variables | Good | Poor | χ² | P-value |
| Sex Male Female |
73(59.3%) 51(52.6%) |
46(40.7%) 46(47.4%) |
9.361 | 0.245 |
| Age 15-30 30-50 >50 |
66(59.5%) 37(58.7%) 21(45.7%) |
45(40.5%) 26(41.3%) 25(54.3%) |
13.479 | 0.322 |
| Religion Orthodoxy Protestants Muslims |
44(53.01%) 70(59.3%) 10(52.6%) |
39(46.9%) 48(40.7%) 9(47.4%) |
15.15 | 0.462 |
| Educational status Illiterate Primary school Secondary school Higher education |
9(12.5%) 73(82.9%) 68(80.96%) 49(81.7%) |
63(87.5%) 15(17.04%) 16(19.04%) 11(18.3%) |
33.699 | 0.001 |
Table 3: Relationships between KAP scores about rabies and some key independent variables among study respondents of Nejo town (N=220) 2017-2018.
Discussion
Rabies virus is a serious pathogenic agent that has ability to infect wide range of species and cause major host mortality. It is estimated that 55,000 people die from dog-mediated rabies annually in Africa and Asia [38]. Rabies in Ethiopia is a neglected zoonotic disease but major public health problem especially in regions where stray dogs are ineffectively controlled. In reality people in developing countries, may not receive lifesaving treatments either because of people may not visit the hospital for treatment owing to lack individual's depth of rabies knowledge or, there is a lack of understanding in the response to dog bites, people may contact with local traditional healers for treatment or apply herbal medication on the dog bite wound, or perform folk remedies at home rather than treatment from health facilities [8, 10, 40].
This study revealed that community in Nejo town is familiar with general information on rabies as 100% of the respondent had heard about rabies. This is in agreement with study by Singh and Choudhary (2005) in rural community of Gujarat, India, Digafe et al. (2015) [7] in Gondar Zuria District and Yalemebrat et al. (2016) [39]in Debark district, North Gondar, Ethiopia who reported 98.6, 99.3 and 100% awareness about rabies, respectively. This KAP analysis in the study area revealed that 75.9% of respondents recognize rabies as danger and fatal disease if there is no on time treatment. While this result is lower than a study conducted in the city of New York, USA, report which is 94.1% [14]. The difference may be due to knowledge difference between USA and Ethiopia and also due to difference in Educational status of the community.
On the etiology of rabies 56.8%, 23.6% and 19.5% responded Virus, spiritual and don’t know respectively. This result is higher than study done by [1] 13.3% in Dedo on those respondents who said virus. The majority of respondent in this study 160(72.7%) responded as they go healthy center Post exposure prophylaxis and this result higher than study done by [1] 65.9% in Dedo. The variation could be explained by the fact that majority of study participants were from rural area in Abdela’s study and Urban in this study and also the educational level of respondent in Dedo district is lower than Nejo town. Ninety-three-point six percent of respondent know that dogs are the most transmitters (source) of rabies. This result is almost consistent with a study conducted in the Gondar district reported that almost all respondent knows that dogs are most source of rabies followed by cats [7]. The little difference may be due to the presence of rabies control in Nejo town with collaboration with Wollega University for dog vaccination in this year. In this study no respondents knew that rabies could be transmitted by other species of animals other than domestic dogs and cats. This is consistent with findings from Thailand only 16% of participants knew all mammals suffer from rabies [20]. This may be because of health education differences between Thailand and Ethiopia.
This study revealed that, 22% of the respondents know wound washing as an immediate action to mitigate the unnecessary outcomes after a dog bite. This result is highly lower than studies done by [1] 49.6% and in Bhutan where majority of respondents were aware about wound washing with soap and water after animal bite [36]. This difference could be due to the difference in level of knowledge of respondents in study area. From total of participants 60(27.3%) in Nejoarea showed strong belief on traditional medicine which is somewhat lower than study done in Gondar district in which 35% respondents prefer traditional medicine [7]. This difference might be due to respondents believe, cultural set up and lack of awareness. This difference might be due to respondents believe, cultural set up and lack of awareness.
Most of the respondent indicated that practicing regular dog vaccination as an effective measure to control rabies. This finding was not consistence with results recorded in Sir Lanka and Bahir Dar in which the majority of the participants were in favor of rabies control programs that mainly focused on stray dog population control [23,35]. The difference may be due to increased health extension activities and the role of mass media in utilization and importance of dog vaccinations compared to mass killing.
The findings of this study indicated that, about 124(56.4%) of the respondents had good level of knowledge, attitude and practices about rabies. This result is higher than [1] who reported 51.9% in Dedo District, south west Oromia. But lower when compared with the study by Guadu et al. (2014) [13] who reported about 64.1% among the community of Bahir Dar town and Yalemebrat et al. (2016) [39] who reported 60.3% in Debark District, North Gondar. This difference might be due to the difference in level of awareness of community about rabies in the study area. The current study indicated an association between KAP and Sex (p=0.245), age (p=0.322) religion (p=0.462) and educational status (p=0.001). This result showed that there is significant association among independent variable educational status and KAP value. All respondents with primary, secondary and higher education levels had good KAP of rabies. The possible explanation could be educated person would have better information access and can easily understand the disease. This result is also supported by the result of the studies conducted in Flagstaff and Bahir Dar [3,35].
Conclusion and Recommendations
In conclusion, despite being entirely preventable, canine rabies still kills 55,000 people per year in developing countries and rabies was considered as the disease of both a veterinary and public health importance in the study area. Information about local beliefs and practices can identify knowledge gaps that may affect prevention practices and lead to unnecessary deaths. This study reveals important knowledge gaps related to, and factors influencing the prevention and control of rabies in Nejo town, so in order to increase knowledge regarding wound washing, seeking post-exposure prophylaxis and the need to vaccinate dogs; veterinary and medical sectors are needed to ensure the availability of preventative services.
Therefore, based on the above conclusion the following recommendations are forwarded:
- The Nejo Health Office Administration should provide education to raise community knowledge on rabies and provide accurate information targeted to people who have lower educational level, housewives or females more commonly present at home and small number of children in the household.
- The Oromia Regional Health Bureau should also design accurate and urgent Community based rabies education program with collaboration of veterinarians should emphasis on mode of transmission, clinical signs and immediate benefits of wound management and need for Anti-rabies vaccine following dog bite.
- The Federal Ministry of Health and Ministry of Agriculture should work in cooperation with information sources like radio, television programs and newspapers to forward information related to rabies for enhancing the level of knowledge of the community about the deadly nature of the disease and the availability of preventive measures like vaccinations both for human and animals.
- Close collaboration and integration of public health, veterinary sector and local authorities is a key element for preventing this fatal incurable disease.
References
- Abdela, N., Midekso, B., Jabir, J. and Abdela, W., (2017). Knowledge, attitudes and practices towards rabies in Dedo district of Jimma zone, southwestern Ethiopia: A community based cross-sectional study. International Journal of Medicine and Medical Sciences, 9(5), pp.61-71.
- Ali, A., Mengistu, F., Hussen, K., Getahun, G., Deressa, A., Yimer, E. and Tafese, K., (2010). Overview of Rabies in and around Addis Ababa, in Animals Examined in EHNRI Zoonoses Laboratory Between, 2003 and 2009. Ethiopian Veterinary Journal, 14(2), pp.91-101.
- Andrea,M. and Jesse D., (2012). Community Survey after Rabies Outbreaks, Flagstaff, Arizona, USA.
- Arsham, H., (2005). Questionnaire design and survey sampling 9th edition. http://home.ubalt.edu/ntsbarsh/Business-stat. 2002.
- Central Statistical Authority (CSA), (2007). Sample enumeration report on livestock and farm implementIV, Addis Ababa, Ethiopia, pp: 26-136.
- Central Statistical Agency (CSA), (2007). Population and Housing Census of Ethiopia: Results for Oromia Region, Vol. 1, Tables 2.1, 2.5, 3.4 (accessed 13 January 2012)
- Degafe, T. Reta., Legesse G. Kifelew and Abraham F. Mechesso., (2015). Knowledge, attitudes and practices towards rabies: questionnaire survey in rural household heads of Gondar Zuria District, Ethiopia.
- Deressa, A., Ali A., Beyene M., Newaye B. and Yimer E., (2010). The status of rabies in Ethiopia: A retrospective record review. Ethiopian Journal of Health Development, 24: 12.
- David, M. K., Peter, M. K., Diane, E. G., Rober, A. L. and Malcom, A. M., (2001). Fields virology. 4thed. LippinCott Williams and Wikings, Philadelphia, 1, 1245-1258.
- Eshetu, Y., Bethlehem, N., Girma, T., Yared, M., Yoseph, B., (2000). Situation of rabies in Ethiopia: A retrospective study 1990–2000. EthiopianJournal of Health Development, 16, 105–112.
- Fekadu, M., (1997). Human rabies surveillance and control in Ethiopia. In, proceeding of the Southern and Eastern African rabies group meeting 1997 March 4–6; Nairobi, Kenya.
- Fekadu, M., (1982). Rabies in Ethiopia.Amere J. E. pidxemio l., 115 (2), 266-273.
- Fraser, C. M., Berqeron, T. A., Mays, A and Aiello, S. E., (1991). The Merck Veterinary Manual: A handbook of diagnosis, therapy, and disease prevention and control for the veterinarians. 7th ed. MERCK and Co., Inc: RAHWAY, N.T., U.S.A. Pp. 619-622.
- Guadu, T., Shite, A., Chanie, M., Bogale, B. and Fentahun, T., (2014). Assessment of knowledge, attitude and practices about rabies and associated factors: in the case of Bahir Dar town. Global Veterinaria, 13(3), pp. 348-54.
- Hosmer D.W, Lemeshow S., (2000). Applied Logistic Regression, 2nd ed. New York: Wiley. pp. 92–97.
- Jackson, A. C. and Wunner, W. H., (2007). Rabies. 2nd ed. San Diego: Academic press.
- Kayali, U., Mindekem, R., Yémadji, N., Oussiguéré, A. and Naýssengar, S., (2003). Incidence of canine rabies in N'Djaména, Chad. Preventive Veterinary Medicine, 61, 227–233.
- Kitalaa, P. M., McDermotta, J. J., Kyulea, M. N. and Gathuma, J. M., (2000). Community-based active surveillance for rabies in Machakos District, Kenya. Preventive Veterinary Medicine.
- Knipe, D. M., Mowley, P. M., Ggiffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. and Straus, S. E., (2001). Field virology. 4th ed., LippinCott Williams and Wikings, USA, 1, 1221-1244.
- Knobel, D. L., Cleaveland, S., Coleman, P. G., Fevre, E. M. and Meltzer, M. I., (2005). Re-evaluating the burden of rabies in Africa and Asia. Bulletin of the World Health Organization, 83, 360–368.
- Kongkaew W, Coleman P, pfeiffer DU, Antarasena C and Thiptara., (2004). Vaccination Covarage and Epidemiological parameters of the owned dog population in Thungsongdistrict, Thailand. Prev vet med. 65: 105-115.
- Quinn, P. J., Markey, B. K., Carter, M. E., Donnelly, W. J. & Leonard, F. C, (2002). Veterinary Microbiology and Microbial Disease, Oxford: Blackwell Science. Pp. 390-392.
- Mallawa, M., (2007). Rabies encephalitis in a malaria-endemic area of Malawi, Africa. Emerging Infectious Diseases,13, 136–139.
- Matibag G.C., Ohbayash.Y. KandaK., Yamashina H., Kumara W. R. and Perera I.N., 2009. A pilot study on the usefulness of information and education campaign materials in enhancing the knowledge, attitude and practice on rabies in rural Sri Lanka. J Infect Developing Countries. 3 (1): 55-64.
- Matter H.C., Wandeler A.I., Neunschwander B.E., Harischandra P.A.L. and Meslin F.X., (2000). Study of the dog population and the rabies control activities in the Mirigama area of Sri Lanka. Acta Tropica. 75: 95–108.
- Meslin, F.X., Kaplan, M.M., Koprowski, H. and World Health Organization, 1996. Laboratory techniques in rabies.
- Ministry of agriculture (MOA), (2011). Rabies Control Strategy. Addis Ababa, Ethiopia, Pp. 1-38.
- Orciari, L., Rupprecht, C. E. and Versalovic, J., 2011. Rabies.Manual of clinical microbiology, 10th ed. Washington DC, ASM Press, pp, 1470–1478.
- Paulos, A., Eshetu, Y., Bethelhem, N., Abebe, B. and Badeg, Z., (2002). A study on the prevalence of animal rabies in Addis Ababa during 1999–2002. Ethiopian Veterinary Journal, 7, 69–77.
- Petersen, B. W. and Rupprecht, C. E., (2011). Human rabies epidemiology and diagnosis. In: Tkachev S, ed. Non-flavivirus encephalitis. Rijeka, In Tech.
- Quinn, P. J., Markey, B. K., Carter, M. E., Donnely, W. J. and Leonard, F. C., (2002). Rabies. In: Veterinary Microbiology and Microbial diseases. 1st ed., Black well science Ltd, UK, Pp. 390 - 395.
- Radostits, O. M., Blood, D. C., Gay, C. C. and Hinch Cliff, K.W., (2002). Veterinary Medicine: A text book of the disease of cattle, sheep, pig, goats, and horses. 9thed., Sounders Company Ltd., New York, Pp. 1201-1208.
- Radostits, O. M., Gay, C.C., Hinchcliff, K. W. and Constable, P. D., (2007). Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats.10thed, Sounders Company Ltd., New York, Pp. 1201-1208.
- Rudd, R. J., Smith, J. S., Yager, P. A, Orciari, L. A andTrimarchi, C. V., (2005). A need for standardized rabies-virus diagnostic procedures: Effect of cover-glass mountant on the reliability of antigen detection by the fluorescent antibody test. Virus Research, 111(1), 83–88.
- Singh SU, Choudhary SK., (2005). Knowledge, attitude, behavior and practice study on dog-bites and its management in the context of prevention of rabies in a rural community of Gujarat. Indian J. Community Med. 30(3): 81-83.
- Tadesse G, Anmaw S, Mersha C, BasazinewB, Tewodros F., (2014). Assessment of Knowledge, Attitude and Practice about Rabies and Associated Factors: In the Case of Bahir Dar Town. Global Veterinarian 13(3): 348-354.
- Tenzin, N. Dhand N.K., Gyeltsheb T., Firestone S., Zangmo C., Dema C., Gyeltshen R. and Ward M.P., (2012). Community based study on knowledge, attitudes and perception of rabies Bhutan. Inthealth. Oxford journals. 210-219.
- World Health Organization (WHO), (1998). World survey of rabies, Geneva. 32.
- World Health Organization (WHO), (2004). First Report of Expert the WHO Consultation on Rabies: Technical Series No; 79. Geneva, Switzerland.
- Yalemebrat N, Bekele T, Melaku M., (2016). Assessment of public knowledge, attitude and practices towards rabies in Debark Woreda, North Gondar, Ethiopia. J. Vet. Med. Anim. Health, 8(11): 183-192.
Citation: Gemechis Biratu and Baisa Fakensa. (2022). Assesment of Community Knowledge, Attitude and Practice on Rabies in Nejo Woreda. Archives of Veterinary and Animal Sciences 4(2).
Copyright: © 2022 Gemechis Biratu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
