Research Article
Volume 3 Issue 1 - 2021
A Drug Design Perspective of Metal Ions in Medicine
1Assistant Professor, Department of Chemistry, Prabhu Jagatbandhu College, Howrah, West Bengal, India
2Ex-student, Department of Chemistry, Prabhu Jagatbandhu College, Howrah, West Bengal, India
2Ex-student, Department of Chemistry, Prabhu Jagatbandhu College, Howrah, West Bengal, India
*Corresponding Author: Dr. Santarupa Thakurta, Assistant Professor, Department of Chemistry, Prabhu Jagatbandhu College, Howrah, West Bengal, India.
Received: August 30, 2021; Published: September 10, 2021
Abstract
Metal ions play many critical functions in humans. The use of metal salts for healing purposes has been present from ancient times. Medicinal inorganic chemistry can utilize the unique properties of metal ions for the design of new drugs. When a particular metal deficiency happens in our body, we need that metal in the form of medicine.
Moreover, metals have important role as therapeutic and diagnostic agents. This review mainly focuses on the medical use of some approved metal-based drugs.
Keywords: Therapeutic drugs; Cisplatin; Anti-arthritic Drugs; Diagnostic Agents; MRI
Introduction
A significant number of metal ions such as Ca, Mg, Na, K, Fe, Co, Ni, Cu, Zn are found to be essential for functioning of cells and organisms. These essential elements, either bulk or trace, are required for growth and survival of human biological system. Deficiency of these metal ions can lead to disease like pernicious anaemia resulting from iron deficiency, growth retardation arising from insufficient dietary zinc, and heart disease in infants owing to copper deficiency. The nutritional deficiency due to the insufficient intake of essential minerals can be easily met by eating a variety of foods from the different food groups. Metal ions can also induce toxicity in humans, classic examples being heavy-metal poisons such as mercury, lead and arsenic. Even essential metal ions can be toxic when present in excess.
It is only in the 1980s that research on the role of metal ions in the biological system started gaining momentum. Recent research highlights the multidimensional role of metal ions in biological systems. Metals possess unique characteristics like redox activity, variable coordination modes, and reactivity towards organic substrates. These properties of metals lead to the development of metal-based drugs with promising pharmacological application and unique therapeutic opportunities. Especially, coordination complexes, either as drugs or pro-drugs, become very attractive probes in medicinal chemistry. Considerable efforts are made for the development of transition metal complexes as anti-inflammatory, anti-infective and anti-diabetic compounds. A lot still remains to be unraveled though. Main challenge of inorganic-pharmacology is to determine the mechanism of action at the molecular level. The metal complexes are usually more susceptible to the hydrolysis than organic molecules. In general, the mechanism of action is proposed by extrapolation of in vitro studies.
In this review, we will discuss some important metal complexes as clinically approved drugs, or drug candidates that are in clinical trials. We will highlight the main features including structure and primary mechanism of action of these metal-based therapeutic drugs and imaging agents.
Therapeutic drugs developed from some essential metal ions
Hydrotalcite
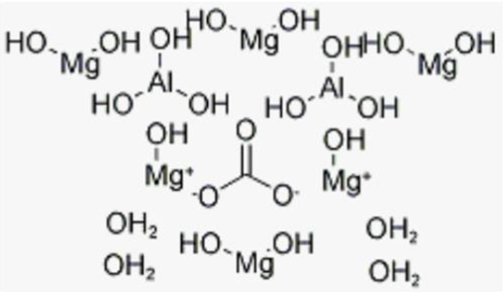
It is a layered double hydroxide (LDH) of general formula Mg6Al2CO3(OH)16•4H2O (Figure 1). This Magnesium based Antacid is used in treatment of heartburn and gastric acid [1].
Hydrotalcite
It is a layered double hydroxide (LDH) of general formula Mg6Al2CO3(OH)16•4H2O (Figure 1). This Magnesium based Antacid is used in treatment of heartburn and gastric acid [1].
Calcium carbonate
Formula: CaCO3
Calcium carbonate is a dietary supplement used when the amount of calcium taken in the diet is not enough. Calcium is needed by the body for muscles, nervous system and heart. Calcium carbonate also is used as an antacid to relieve heartburn, acid indigestion, and upset stomach [2].
Formula: CaCO3
Calcium carbonate is a dietary supplement used when the amount of calcium taken in the diet is not enough. Calcium is needed by the body for muscles, nervous system and heart. Calcium carbonate also is used as an antacid to relieve heartburn, acid indigestion, and upset stomach [2].
Zinc Gluconate
Chemical formula: C12H22O14Zn
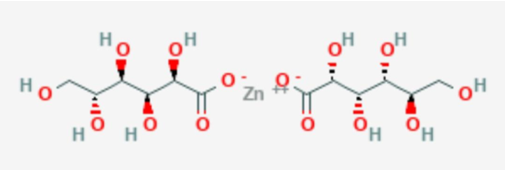
Zinc is an essential trace element required for immune function, wound healing, protein synthesis, DNA synthesis and cell division. Zinc gluconate (Figure 2) is mainly used in conditions like zinc deficiency. It is often used in cold remedies, such as lozenges and nasal sprays and can also be administered in adjunctive therapy as an alternative drug of choice in diarrhea [3].
Chemical formula: C12H22O14Zn
Zinc is an essential trace element required for immune function, wound healing, protein synthesis, DNA synthesis and cell division. Zinc gluconate (Figure 2) is mainly used in conditions like zinc deficiency. It is often used in cold remedies, such as lozenges and nasal sprays and can also be administered in adjunctive therapy as an alternative drug of choice in diarrhea [3].
Although the mechanism of action is not completely known, zinc supplementation may be used to increase immunity against viruses or may interfere with the replication of certain viruses, such as the human papillomavirus.
Polaprezinc
Chemical Formula: C9H12N4O3Zn
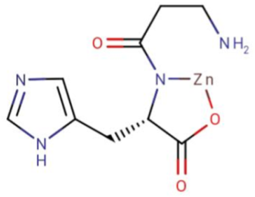
Polaprezinc is a chelated form of zinc and L-carnosine (Figure 3). It is used to in treatment of the disease of Peptic ulcer disease, dyspepsia [4].
Chemical Formula: C9H12N4O3Zn
Polaprezinc is a chelated form of zinc and L-carnosine (Figure 3). It is used to in treatment of the disease of Peptic ulcer disease, dyspepsia [4].
Polaprezinc increases the expression of various antioxidant enzymes, including superoxide dismutase 1 (SOD-1), SOD-2, heme oxygenase-1 (HO-1), glutathione S-transferase (GST), glutathione peroxidase (GSH-px), peroxidredoxin-1 (PRDX1; PRXI) and PRXD5 (PRXV). This process occurs in the gastric mucosa, defending mucosal cells against reactive oxygen species. This drug inhibits the activity of the transcription factor nuclear factor-kappaB (NF-kB) and decreases the expression of various inflammatory cytokines.
Ferric Maltol
Chemical formula: C18H15FeO9
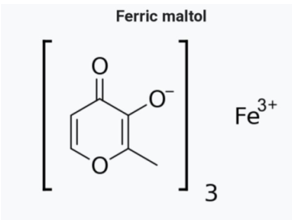
In this complex, an iron (III) atom is coordinated to three maltol molecules to increase the bioavailability compared to iron (II), without depositing it in the duodenum as insoluble ferric hydroxide and phosphate. This is an oral iron replacement that delivers iron for uptake across the intestinal wall and transfer to transferrin and ferritin. It is indicated for iron deficiency in adults [5] (Figure 4).
Chemical formula: C18H15FeO9
In this complex, an iron (III) atom is coordinated to three maltol molecules to increase the bioavailability compared to iron (II), without depositing it in the duodenum as insoluble ferric hydroxide and phosphate. This is an oral iron replacement that delivers iron for uptake across the intestinal wall and transfer to transferrin and ferritin. It is indicated for iron deficiency in adults [5] (Figure 4).
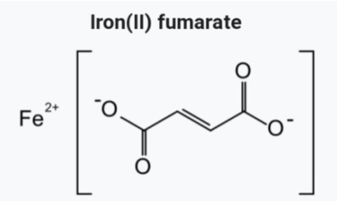
Ferrous Fumarate
Chemical Formula: C4H2FeO4
Ferrous fumarate (Figure 5) is a replacement of iron stores found in hemoglobin, myoglobin, and enzymes; works to transport oxygen via hemoglobin. It is indicated in the prevention and treatment of iron-deficiency anemia.
Chemical Formula: C4H2FeO4
Ferrous fumarate (Figure 5) is a replacement of iron stores found in hemoglobin, myoglobin, and enzymes; works to transport oxygen via hemoglobin. It is indicated in the prevention and treatment of iron-deficiency anemia.
Anti-Cancer Agents
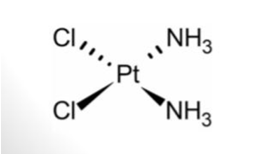
Cisplatin
Cancer is a disease in which some of the body's cells grow uncontrollably and spread to other parts of the body. Chemotherapy is the one of the best ways of treatment of cancer. Cis-diaminedichloroplatinum (II) is one of the best anticancer agents. Cisplatin is useful against lung cancer, bladder, head, testicular cancer and cervix. Cisplatin is injected every few weeks with saline. Cisplatin was first synthesized by M. Peyrone in 1844 and its chemical structure was first elucidated by Alfred Werner in 1893. At 1965, the research of Rosenberg at Michigan State University created much interest in the possible use of electrolysis products of platinum in cancer chemotherapy. Cisplatin was accepted by FDA in 1978 as chemotherapeutic agent [6]. Its structure is square planner with the metal in the +2-oxidation state (Figure 6).
Cisplatin
Cancer is a disease in which some of the body's cells grow uncontrollably and spread to other parts of the body. Chemotherapy is the one of the best ways of treatment of cancer. Cis-diaminedichloroplatinum (II) is one of the best anticancer agents. Cisplatin is useful against lung cancer, bladder, head, testicular cancer and cervix. Cisplatin is injected every few weeks with saline. Cisplatin was first synthesized by M. Peyrone in 1844 and its chemical structure was first elucidated by Alfred Werner in 1893. At 1965, the research of Rosenberg at Michigan State University created much interest in the possible use of electrolysis products of platinum in cancer chemotherapy. Cisplatin was accepted by FDA in 1978 as chemotherapeutic agent [6]. Its structure is square planner with the metal in the +2-oxidation state (Figure 6).
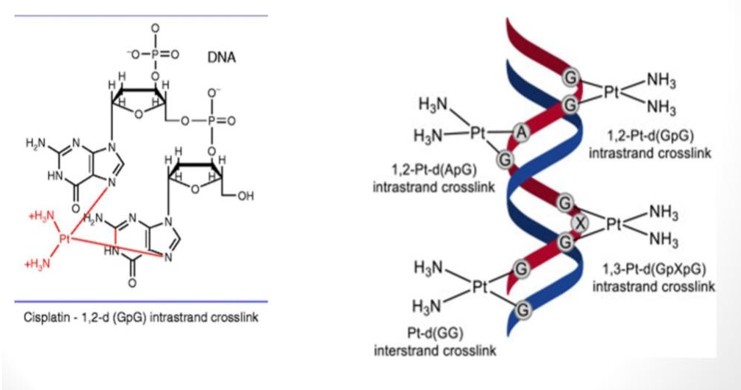
Mechanism of cisplatin is based on Pt-DNA interaction inhibiting DNA replication. The activity of cis DDP is suppressed in blood where [Cl]- is relatively high. It diffuses across the cytoplasmatic membrane of the cell where [Cl]- is low. Hydrolysis occurs inside the cell due to a much lower concentration of chloride ion. The aquo-complexes are kinetically more reactive. The hydrolyzed product then interacts with target DNA. Studies in vitro as well as in vivo indicate that Pt binds covalently with N7 of guanine bases of DNA forming interstrand crosslink and 1,2 or 1,3 intrastrand crosslinks (Figure 7). This binding changes the active conformation of DNA and has blocked the action of DNA polymerase, an enzyme necessary for replication. In particular, 1,2-intrastrand adducts of cisplatin with DNA has stopped polymerases from doing their job. This leads to inhibiting of DNA replication or cell division and thus inhibits the growth of tumor cells. The cytotoxic activity of cisplatin may arise from the cell’s inability to repair DNA damage caused by cisplatin. Indeed, in vitro studies on cell extracts suggest that the most common cisplatin–DNA adducts (that is, 1, 2-intrastrand adducts) are not readily repaired by the repair system. However, the damage recognition protein binds the damaged site and is expelled by excretion. Thus, the body becomes drug resistant with continuous use.
Cis- as well as trans- platin alter the double helical structure of DNA and its replication, but only cis is active. The trans isomer is resorbed more rapidly than cisplatin. But the concentration of DNA coordinated trans complex begins to decrease after six hours whereas the cis-isomer is then still accumulated in the cell nucleus. Only very little trans compound is still coordinated to DNA after 24 hours. Due to its geometry, trans-DDP cannot form 1,2-intrastrand adducts with DNA. Since trans-DDP is inactive in killing cancer cells, it is believed that 1,2-intrastrand adducts formed between cisplatin and DNA are important for the anticancer activity of cisplatin [7,8].
Changes in the DNA structure caused by the trans isomer are sensed differently by the endogenous repair mechanism from those caused by coordination of cisplatin. A structure specific recognisition protein (SSRP) recognises the bending of DNA containing cis platinum and binds DNA. These proteins all contain a common portion called a high mobility group (HMG); proteins in this class are called HMG-domain proteins. This protein binds specifically to DNA containing cisplatin and not with trans. Though the mechanism is not clear but this binding of SSRP may be the reason activity of cisplatin and inactivity of trans.
The most common side effects of a cisplatin therapy include kidney and gastrointestinal problems, including nausea, this may be attributed to the inhibition of enzymes through coordination of the heavy metal platinum to sulfhydryl groups in proteins. Accordingly, a treatment with sulfur compounds such as sodium diethyldithiocarbamate or thiourea. Kidney toxicity is can also be avoided by injecting water with osmotic diuretic agents, D-mannitol.
Second Generation Platinum Based Anti-Cancer Drugs
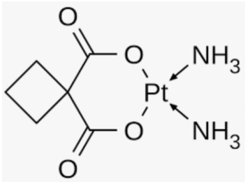
Carboplatin
Carboplatin (cis-diaminedicyclobutane-1, 1-dicarboxylatoplatinum (II)) (Figure 8) is used to treat a number of forms of cancer. This includes ovarian cancer, lung cancer, head and neck cancer, brain cancer. It may be used for some types of testicular cancer but cisplatin is generally more effective. It has also been used to treat triple-negative breast cancer [9]. It was approved by FDA in 1989 [10]. The chelate effect of the six-member ring in carboplatin is thought to reduce the chemical reactivity of the platinum drug; thus, lowering its nephrotoxicity and ototoxicity.
Carboplatin
Carboplatin (cis-diaminedicyclobutane-1, 1-dicarboxylatoplatinum (II)) (Figure 8) is used to treat a number of forms of cancer. This includes ovarian cancer, lung cancer, head and neck cancer, brain cancer. It may be used for some types of testicular cancer but cisplatin is generally more effective. It has also been used to treat triple-negative breast cancer [9]. It was approved by FDA in 1989 [10]. The chelate effect of the six-member ring in carboplatin is thought to reduce the chemical reactivity of the platinum drug; thus, lowering its nephrotoxicity and ototoxicity.
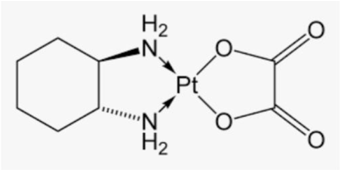
Oxaliplatin
Oxaliplatin ((1R, 2R) -(N, N-1,2-diamminocyclohexane) -(O-O)-ethanedioato) platinum (II)) (Figure 9) is used for treatment of colorectal cancer, typically along with folinic acid and 5-fluorouracil in a combination known as FOLFOX. It was approved by FDA on 2004.
Oxaliplatin ((1R, 2R) -(N, N-1,2-diamminocyclohexane) -(O-O)-ethanedioato) platinum (II)) (Figure 9) is used for treatment of colorectal cancer, typically along with folinic acid and 5-fluorouracil in a combination known as FOLFOX. It was approved by FDA on 2004.
Of the platinum drugs in clinical trials, satraplatin (bis(acetato)-amminedichloro (cyclohexylamine) platinum (IV)) offers the unique advantage of oral administration to the patient, which is not only convenient but also cheap. Satraplatin contains a Pt (IV) centre that after administration is reduced to Pt (II), the active form in the bloodstream by the action of redox proteins.
Ruthenium complexes
Platinum is not the only transition metal used in the treatment of cancer; various other transition metals have been used in anticancer drugs. Many ruthenium complexes were studied which showed anti-proliferative effects in human ovarian cancers. Ruthenium complexes with oxidation state +2 or +3 show antitumor activity against metastasis cancers.
Platinum is not the only transition metal used in the treatment of cancer; various other transition metals have been used in anticancer drugs. Many ruthenium complexes were studied which showed anti-proliferative effects in human ovarian cancers. Ruthenium complexes with oxidation state +2 or +3 show antitumor activity against metastasis cancers.
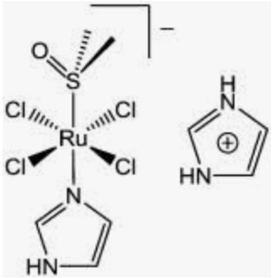
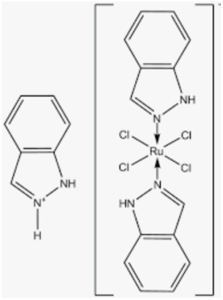
Two structurally related Ru (III) coordination compounds, known as NAMI-A (Figure 10) ((ImH)[trans-RuCl4(dmso-S) (Im)], Im = imidazole) and KP1019/KP1339 (KP1019 = (IndH)[trans-RuCl4(Ind)2], Ind = indazole; KP1339 = Na[trans-RuCl4(Ind)2], i.e., the sodium salt of KP1019) (Figure 11), have eventually reached the stage of clinical evaluation in humans, opening the way to large expectations for a new class of metal-based anticancer drugs [11,12]. Both NAMI and NAMI-A share the same pseudo-octahedral ruthenium (III) center surrounded by four chloride ligands in the equatorial plane. One S-bonded dmso (dmso-S) and one imidazole occupy the two axial positions. The negative charge is neutralized by Na+ in NAMI and by the imidazolium cation in NAMI-A. Ru coordination compounds have a greater lability compared to Pt drugs, and this marks a relevant difference. As a matter of fact, the relatively fast aquation processes and ligand-exchange reactions of Ru compounds produce several species that are capable of reacting with a variety of biological components. This is particularly true for NAMI-A, which is far more labile than KP1019.
NAMI-A was introduced into a phase 1 study in 1999; it was the first ruthenium drug candidate to be tested in humans. In 2006, a preliminary phase 1 study was performed with KP1019 on a few patients with advanced solid tumors. Ruthenium (III) compounds are activated by reduction from Ru (III) to Ru (II). Cancer cells generally contain a lower oxygen concentration as well as higher levels of glutathione and a lower pH than normal tissues creating a reducing environment. Ruthenium mimics iron metabolism and, as a consequence Transferrin is a selective carrier for ruthenium-based drugs. DNA is the main target for ruthenium drugs [13]. For ruthenium compounds only mild toxicity was observed.
Anti-Arthritic Drugs
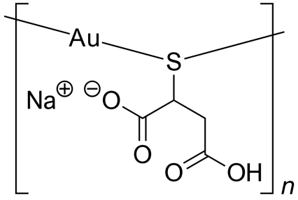
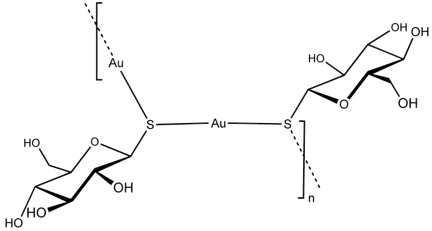
Arthritis is an inflammation of joints of the body causing pain due to erosion of bone. The early gold compounds used for the treatment of rheumatoid arthritis were gold(I) thiolates (AuSR) such as sodium aurothiomalate (Myocrisin) (Figure 12) and aurothioglucose (Solganol) (Figure 13), SR being thiomalate and thioglucose respectively [14]. These are charged polymeric species which are administered weekly or monthly by intramuscular injection.
Arthritis is an inflammation of joints of the body causing pain due to erosion of bone. The early gold compounds used for the treatment of rheumatoid arthritis were gold(I) thiolates (AuSR) such as sodium aurothiomalate (Myocrisin) (Figure 12) and aurothioglucose (Solganol) (Figure 13), SR being thiomalate and thioglucose respectively [14]. These are charged polymeric species which are administered weekly or monthly by intramuscular injection.
After rapid absorption the gold is rapidly cleared from the bloodstream and distributed to various tissues including the kidneys where it accumulates and gives rise to nephrotoxicity, a major side effect. Other adverse reactions include mouth ulcers, skin reactions, blood disorders and occasional liver toxicity.
This led to the search for a drug with an improved pharmacokinetic profile and reduced toxicity. In 1985, Auranofin (Figure 14), tetraacetyl-β-D-thioglucose-gold(I)-triethylphosphine was introduced as an orally bioavailable gold drug for arthritis [15]. Auranofin is a monomeric thiolate with the general formula R3PAuSR, the phosphine ligand making the compound lipophilic. It is worth to mention that Auranofin may inhibit replication of SARS-CoV-2, the virus responsible for causing COVID-19 in cell culture. Inflammation may also be reduced.
The mechanism of action of the antiarthritic gold compounds is still unclear and needs further understanding of the chemical interaction of gold complexes with naturally occurring ligands in a biological environment.
Diagnostic Agents
Metal complexes those who have radioactive nuclei can be used as diagnostic agents for tumor, organ and tissue imaging. The radioisotopes which are used in treatment should be short lived, emit low energy photons and no emotion of alpha and beta particles.
Technetium. 99mTc is the most commonly used radioisotope agent for imaging purposes. It has a short half-life, emits only gamma ray photons, and does not emit beta or alpha particles (which are more damaging to surrounding cells), and thus is particularly suitable as an imaging radioisotope [16].
Nuclear magnetic resonance (NMR) spectroscopy can be used to image specific tissues of biological specimens because of differences in the relaxation times of water proton resonances, usually brought about by paramagnetic metal ions. Of the various metal ions surveyed in attempts to provide clinically useful NMR images in humans, Gd (III), Fe (III), and Mn(lI) were found to give the best proton-relaxation enhancements. These paramagnetic metals are able to alter the tissue relaxation times and produce a contrast image. The gadolinium complex [Gd (DTPA) (H2O)], an agent currently used in the clinic, has been successfully employed to image brain tumors. Ferric chloride improves gastrointestinal tract images in humans and manganous salts can be used for heart imaging.
Gallium-68 is useful as a positron source for Positron emission tomography. 57Cobalt (III) is used with the compound bleomycin (BLM), which is an antibiotic, to selectively be taken up by tumor cells. The use of cobalt results in the best blood-to-tumor distribution ratio, but its half-life is too long to be conducive for imaging purposes. A solution has been proposed to attach an EDTA moiety to the terminal thiazole ring of bleomycin (Figure 15), radiolabeled so that the entire complex can then be traceable. This system could provide tumor locations accurately, leading to earlier detection and more non-invasive procedures in the future.
Conclusion
Metals are endowed with unique properties that are absent in conventional carbon-based drugs. The exploration of transition metal complexes should lead to future generations of drugs which can overcome some of the disadvantages associated with present drug therapies, including the reduction of side-effects, widening the spectrum of activity, and resistance. The field of medicinal inorganic chemistry and interdisciplinary researches related with metallodrug should, therefore, be exploited to solve the molecular activity mechanisms of metallodrugs in the complex biological systems. In future, metallodrugs will certainly hold a primary role in drug development to improve the quality of life of patients.
References
- Coudray C, Rambeau M, Feillet-Coudray C, Gueux E, Tressol JC, Mazur A, Rayssiguier Y (2005). “Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach”. Magnes Res. 18 (4): 215.
- Caruso JB, Patel RM, Julka K, Parish DC (2007). “Health-behavior induced disease: return of the milk-alkali syndrome”. J Gen Intern Med. 22 (7): 1053.
- DrugBank DB11248
- Doi H, Kuribayashi K, Kijima T (2018). “Utility of polaprezinc in reducing toxicities during radiotherapy: a literature review”. Future Oncol. 14(17) 1977.
- Maton A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD (1993). Human Biology and Health. Englewood Cliffs, New Jersey, US: Prentice Hall.
- Kelland L (2007). The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 7, 573.
- Wang D, Lippard SJ (2005). “Cellular processing of platinum anticancer drugs”. Nature Reviews. Drug Discovery. 4 (4), 307.
- Sarafraz Z, Ahmadi A, Daneshi A. (2018). “Transtympanic Injections of N-acetylcysteine and Dexamethasone for Prevention of Cisplatin-Induced Ototoxicity: Double Blind Randomized Clinical Trial”. The International Tinnitus Journal. 22 (1), 40.
- Dasari S, Tchounwou PB (2014). Eur J Pharmacol. 740, 364.
- Fischer J, Robin GC (2006). Analogue-based Drug Discovery. John Wiley & Sons. P. 513.
- Burris HA, Bakewell S, Bendell JC, Infante J, Jones SF, Spigel DR, Weiss GJ, Ramanathan RK, Ogden A, Von Hoff D. (2016). Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open. 1, e000154.
- Pillozzi S, Gasparoli L, Stefanini M, Ristori M, D’Amico M, Alessio E, Scaletti F, Becchetti A, Arcangeli A, Messori L (2004). NAMI-A is highly cytotoxic toward leukaemia cell lines: Evidence of inhibition of KCa 3.1 channels. Dalton Trans. 43, 12150.
- Bergamo A, Sava G (2007). Dalton Trans., 13, 1267.
- Champion G.D., Graham G.G., Ziegler J.B., (1990). Baillieres Clin. Rbeumatol., 4, 491.
- T.G. Davis (ed), Scand. J Rheumatol., Supplement 63, 1986
- Lippard, Stephen J. (1994). “Metals in Medicine.” Bioinorganic Chemistry. Mill City: University Science Books. 505-583.
Citation: Dr. Santarupa Thakurta and Soumya Khatua. (2021). A Drug Design Perspective of Metal Ions in Medicine. Archives of Chemistry and Chemical Engineering 3(1).
Copyright: © 2021 Santarupa Thakurta. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.