Research Article
Volume 7 Issue 1 - 2025
Comparative Study of the Antidermatophytic Activity of four Cameroonian Medicinal Plants.
1Department of Biochemistry, Faculty of Science, University of Dschang, Dschang P.O. Box 67, Cameroon
2Department of Pharmaceutical sciences, Faculty of Medicine and pharmaceutical sciences, University of Dschang, Dschang P.O. Box 96, Cameroon
3Aix Marseille Université, Assistance Publique-Hôpitaux de Marseille, Service de Santé des Armées, RITMES, 13005 Marseille, France
2Department of Pharmaceutical sciences, Faculty of Medicine and pharmaceutical sciences, University of Dschang, Dschang P.O. Box 96, Cameroon
3Aix Marseille Université, Assistance Publique-Hôpitaux de Marseille, Service de Santé des Armées, RITMES, 13005 Marseille, France
*Corresponding Author: Associate Prof. Dr Guy Sedar Singor Njateng, Department of Biochemistry, Faculty of Science, University of Dschang, Dschang P.O. Box 67, Cameroon.
Received: January 30, 2025; Published: February 05, 2025
Abstract
Introduction: The absence of a comparative study on the antidermatophytic activity of Ageratum houstonianum, Ageratum conyzoides, Polyscia fulva and Mangifera indica hinders the optimization of their use in treating dermatophyte related infections. This study aimed to evaluate the antifungal activity of the methanolic crude extracts of these plants against three dematophyte strains (T. mentagrophytes f0237, T. mentagrophytes f0268 and T. soudanense) that cause ringworm infections and rank the antidermatophytic activity of these plants so as to identify which is most relevant as an antidermatophytic agent for further development.
Method: Plant extracts of Ageratum houstonianum, Ageratum conyzoides, Polyscia fulva and Mangifera indica were prepared by maceration in methanol. The in vitro antidermatophytic activity was evaluated using the broth microdilution method. The ointment for the in vivo test was formulated according to the protocol of the British pharmacopoeia and the degree of dermal irritation of this ointment was evaluated using the occluded dermal irritation test. Also the in vivo testwas carried out using the guinea pig dermal infection model and phytochemical screening was done using standard methods.
Results: Following this work, Ageratum houstonianum showed the best antidermatophytic activity on all three strains with minimal inhibitory concentrations of 16, 32 and 128 µg/mL, on T. mentagrophytes f0237, T. mentagrophytes f0268 and T. soudanense respectively. Polyscia fulva and Ageratum conyzoides had an activity range of 32 to 256 µg/mL and Mangifera indica showed lowest activity which ranged from 512 to 2048 µg/mL. Furthermore, the dermal irritation test showed no erythema or edema on guinea pigs and the in vivo antidermatophytic activity showed that the 10% ointment of Ageratum houstonianum was more effective than all the other ointments. Finally, phytochemical screening showed that all plant extracts contained terpernoids, flavonoids, anthocyanins and phenols.
Conclusion: The results from in vitro and in vivo show that the ointment formulated from Ageratum houstonianum may serve as alternative in eradicating dermatophyte infections.
Keywords: Dermatophytes; Medicinal plants; Antidermatophytic activity; Guinea pigs; Phytochemical screening
Introduction
The dermatophytes are a group of closely related fungi that have the capacity to invade keratinized tissue (skin, hair, and nails) of humans and other animals to produce this infection, called dermatophytosis, commonly referred to as ringworm or tinea [1]. Dermatophytosis is the most commonly encountered superficial cutaneous fungal infection [2]. According to the World Health Organization 20%-25% of world population suffer from dermatophytosis. In West Africa the prevalence is said to be 20% and 10%-70% in other regions of Africa [1]. While in some regions of Cameroon the prevalence is said to be 10.1% for tinea capitis [3] and 8.6% for onychomycosis [4]. In a year treating dermatophytosis cost about $500 million due to its high infection rate. A concrete diagnosis is very important for patient management and therapy guidance [5]. This infection generate reactions from the host that are highly variable (discrete or severe) depending on the species, the anatomical location of the lesions, the intrinsic factors to the host and the environment [6]. They can also be responsible for allergic manifestations [7], and finally, exceptionally, invade deep tissues [8]. They are not life threatening, but constitute a public health problem because of their prevalence and recidivism. A number of antifungal agents such as griseofulvin, allylamines, morpholine and azoles is available treatment of dermatophyte infections. However they have a narrow spectrum of activity and the limited number of effective antifungals some of which present adverse side effects, without forgetting the emergence of the resistance of certain strains to available drugs [9].To this effect, the search for new drug molecules is very necessary and medicinal plants appear to be a better option to handle dermatophyte infections as they are easy to access, less side effects with a lesser likelihood of resistance [10]. In Cameroon plants like Ageratum houstonianum leaves, Polyscia fulva stem bark, Ageratum conyzoides leaves and Mangifera indica leaves have been studied for their antidermatophytic activity by [11, 12, 13, 14] respectively. These plants are readily available, abundant and have been used in traditional medicine against dermatophyte infections ensuring that this study can be carried out without significant logistical challenges. However, the absence of a comparative study on the antidermatophytic activity of these four medicinal plants hinders the optimization of their use in treating dermatophyte related infections. The aim of this work was to evaluate the antifungal activity of the methanolic crude extracts of the leaves of Ageratum houstonianum, Ageratum conyzoides, Mangifera indica and the stem bark of Polyscia fulva, against three dematophyte strains (T. mentagrophytes f0237, T. mentagrophytes f0268 and T. soudanense) that commonly cause ringworm infections and rank the antidermatophytic activity of these plants so as to identify which is most relevant as an antidermatophytic agent for further development.
Materials and Methods
Plant material
The leaves of Ageratum houstonianum (Asteraceae), Mangifera indica (Anacardiaceae)and the sterm bark of Polyscia fulva (Araliaceae)were harvested in December 2023 in Dschang town whereas the leaves of Ageratum conyzoides (Asteraceae) were harvested in the town of Bafoussam (west region of Cameroon). Botanical identification was done at Cameroon National Herbarium in Yaounde where voucher specimens were kept under the identification numbers; No. 3114/39564/CNH, 21155/CNH, 43546/CNH and 57347/CNH respectively.
The leaves of Ageratum houstonianum (Asteraceae), Mangifera indica (Anacardiaceae)and the sterm bark of Polyscia fulva (Araliaceae)were harvested in December 2023 in Dschang town whereas the leaves of Ageratum conyzoides (Asteraceae) were harvested in the town of Bafoussam (west region of Cameroon). Botanical identification was done at Cameroon National Herbarium in Yaounde where voucher specimens were kept under the identification numbers; No. 3114/39564/CNH, 21155/CNH, 43546/CNH and 57347/CNH respectively.
Microorganisms
The test was performed on three dermatophyte strains including T. mentagrophytes (f0237), T. mentagrophytes (f0268) and T. soudanense which all came from the University ofAix-Marseille in France.
The test was performed on three dermatophyte strains including T. mentagrophytes (f0237), T. mentagrophytes (f0268) and T. soudanense which all came from the University ofAix-Marseille in France.
Animals
The dermal irritation test and in vivo antidermatopytic test of the extract were carried out using guinea pig of weight 350 g ± 400 g.These guinea pigs were nurtured in the Animal House of the Department of Biochemistry, Dschang, Cameroon for 2 weeks at room temperature and were feed with standard guinea pig food throughout the experimental period (22 ± 2°C) and were handled according to standard protocols for the use of laboratory animals. The animals were randomly assigned to control and test groups and caged.
The dermal irritation test and in vivo antidermatopytic test of the extract were carried out using guinea pig of weight 350 g ± 400 g.These guinea pigs were nurtured in the Animal House of the Department of Biochemistry, Dschang, Cameroon for 2 weeks at room temperature and were feed with standard guinea pig food throughout the experimental period (22 ± 2°C) and were handled according to standard protocols for the use of laboratory animals. The animals were randomly assigned to control and test groups and caged.
Preparation of plant extract
The plants were dried for 10 days in a plant drying machine, grind into fine powder after which 100 g of each plant was macerated in 300 ml of methanol for 48 hours and this mixture was being stirred 3 times a day to optimize the yield. After filtration with a whatmann No 1 filtration paper, the filtrate was evaporated to dryness under vacuum at 45°C using a rotatory evaporator (Buchi R-200).
The plants were dried for 10 days in a plant drying machine, grind into fine powder after which 100 g of each plant was macerated in 300 ml of methanol for 48 hours and this mixture was being stirred 3 times a day to optimize the yield. After filtration with a whatmann No 1 filtration paper, the filtrate was evaporated to dryness under vacuum at 45°C using a rotatory evaporator (Buchi R-200).
Phytochemical screening
The phytochemical screening of the extracts was carried out using standard method [15]. The plants were screened for the presence of alkaloids, terpennoids, phenols, anthocyanins, saponins and flavonoids.
The phytochemical screening of the extracts was carried out using standard method [15]. The plants were screened for the presence of alkaloids, terpennoids, phenols, anthocyanins, saponins and flavonoids.
In vitro antidermatophytic activity
Preparation of dermatophyte inocula
The spore suspensions used for the inoculation were prepared from 10 days old cultures on sabouraud dextrose agar culture medium. The culture surfaces were scraped and placed in test tubes containing 10 mL of sterile saline. It was then homogenized for 5 minutes and filtered.
Preparation of dermatophyte inocula
The spore suspensions used for the inoculation were prepared from 10 days old cultures on sabouraud dextrose agar culture medium. The culture surfaces were scraped and placed in test tubes containing 10 mL of sterile saline. It was then homogenized for 5 minutes and filtered.
Antidermatophytic activity using broth microdilution method
The broth microdilution method described by CLSI [16] was used to determine the minimal inhibitory concentration and minimal fungicidal concentration of the tested substances using 96 well microplates. The test substances were dissolved in 200 µg/mL of dimethyl sulphoxide. The stock solution of the extracts were prepared at a concentration of 8192 µg/mL and griseofulvin at a concentration of 1024 µg/mL. Two fold serial dilution of the extract (concentration range of 1-2048 µg/mL) and griseofulvin (concentration range of 1-256 µg/mL) were perfomed in sabouraud dextrose broth (SDB) in a total after which 100 µL of dermatophyte suspension was added to the wells. The microplates were covered and incubated at room temperature for seven days. Fungal growth in the wells was determined by observing and comparing the turbidity of the test wells to that of the positive control wells and negative control wells. The MIC was the lowest concentration of the tested substances that prevented the growth of the microorganisms. The minimum fungicidal concentration (MFC) values were determined by sub culturing 50 μL aliquots of the preparations, which did not show any visible growth of the micro-organisms during MIC determination in 150 µL of SDB. These preparations were further incubated at 25°C for seven days. Microbial growth in each well was determined by observing and comparing the turbidity of test wells to that of the positive and negative controls. The MFC was the lowest concentration of the tested substances that prevented visible growth of the microorganisms in the sub-cultures.
The broth microdilution method described by CLSI [16] was used to determine the minimal inhibitory concentration and minimal fungicidal concentration of the tested substances using 96 well microplates. The test substances were dissolved in 200 µg/mL of dimethyl sulphoxide. The stock solution of the extracts were prepared at a concentration of 8192 µg/mL and griseofulvin at a concentration of 1024 µg/mL. Two fold serial dilution of the extract (concentration range of 1-2048 µg/mL) and griseofulvin (concentration range of 1-256 µg/mL) were perfomed in sabouraud dextrose broth (SDB) in a total after which 100 µL of dermatophyte suspension was added to the wells. The microplates were covered and incubated at room temperature for seven days. Fungal growth in the wells was determined by observing and comparing the turbidity of the test wells to that of the positive control wells and negative control wells. The MIC was the lowest concentration of the tested substances that prevented the growth of the microorganisms. The minimum fungicidal concentration (MFC) values were determined by sub culturing 50 μL aliquots of the preparations, which did not show any visible growth of the micro-organisms during MIC determination in 150 µL of SDB. These preparations were further incubated at 25°C for seven days. Microbial growth in each well was determined by observing and comparing the turbidity of test wells to that of the positive and negative controls. The MFC was the lowest concentration of the tested substances that prevented visible growth of the microorganisms in the sub-cultures.
In vivo studies
Ethical statement
All studies involving animals were conducted according to the ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (Registration no. 173/CPCSEA, dated 28 January, 2000), Government of India, on the use of animals for scientific research.
Ethical statement
All studies involving animals were conducted according to the ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (Registration no. 173/CPCSEA, dated 28 January, 2000), Government of India, on the use of animals for scientific research.
Skin irritation test
The degree of dermal irritation of the methanol extract of the leaves of A. houstonianum was determined on guinea pigs using the occluded dermal irritation test method [17]. Four guinea pigs were used and each animal served as its own control. The animals were divided into two groups (group 1 and group 2) of 2 animals each and then treated with water-moistened extract and oil-moistened extract respectively. On day 0, fur was shaved from the back of each animal, about 6 cm2 on both sides. The left side served as a negative control, while the right one served as a test site. The guinea pigs were caged individually for 24 hours. On day 1, 0.5 g of extract was moistened with distilled water and evenly applied to the test sites of group 1 animals and the skin was covered with a gauze patch and a non-irritant adhesive plaster. The control sites were treated with distilled water and covered as above. The animals of group 2 were treated with oil-moistened extract in the same manner as above and palm kernel oil was also used as the negative control for the group 2 animals.
The degree of dermal irritation of the methanol extract of the leaves of A. houstonianum was determined on guinea pigs using the occluded dermal irritation test method [17]. Four guinea pigs were used and each animal served as its own control. The animals were divided into two groups (group 1 and group 2) of 2 animals each and then treated with water-moistened extract and oil-moistened extract respectively. On day 0, fur was shaved from the back of each animal, about 6 cm2 on both sides. The left side served as a negative control, while the right one served as a test site. The guinea pigs were caged individually for 24 hours. On day 1, 0.5 g of extract was moistened with distilled water and evenly applied to the test sites of group 1 animals and the skin was covered with a gauze patch and a non-irritant adhesive plaster. The control sites were treated with distilled water and covered as above. The animals of group 2 were treated with oil-moistened extract in the same manner as above and palm kernel oil was also used as the negative control for the group 2 animals.
After 24 hours of exposure, the coverings were removed and the test site rinsed with distilled water and dried. The animals were examined for the presence of erythema and edema according to the Draize dermal irritation scoring system [18] (0, no erythema or no edema; 1, barely perceptible erythema or edema; 2, well defined erythema or slight edema; 3, moderate to severe erythema or moderate edema; 4, severe erythema or edema) at grading intervals of 1, 24, 48 and 72 hours.
In vivo antidermatophytic test
This experiment was carried out using the ointment formulated with the extract of Ageratum houstonianum in the proportions of 1%, 5%, 10% and 1% w/w griseofulvin ointment. The dermatophyte suspendion was prepared same way as for the In vitro test.
This experiment was carried out using the ointment formulated with the extract of Ageratum houstonianum in the proportions of 1%, 5%, 10% and 1% w/w griseofulvin ointment. The dermatophyte suspendion was prepared same way as for the In vitro test.
Dermal infection of animals
The fur from the back of each guinea pig was shaved (approximately 16cm2) and 1 mL of a suspension of T. Mentagrophytes f0237 spores was inoculated to a surface of about 4cm2 area (previously lightly abraded with sandpaper) within the shaved zone [19]. Animals were randomly assigned to different treatment groups. Evidence of infection was revealed by direct observation of the infected area, followed by agar culture of scrapings from the area, and microscopic observation of the resulting fungi from the scrapings.
The fur from the back of each guinea pig was shaved (approximately 16cm2) and 1 mL of a suspension of T. Mentagrophytes f0237 spores was inoculated to a surface of about 4cm2 area (previously lightly abraded with sandpaper) within the shaved zone [19]. Animals were randomly assigned to different treatment groups. Evidence of infection was revealed by direct observation of the infected area, followed by agar culture of scrapings from the area, and microscopic observation of the resulting fungi from the scrapings.
Treatment of infected animals
Treatment started six days after infection and was done on a daily basis. Animals were treated using the test substances. Only group one animals were not infected and treated (table 1).
Treatment started six days after infection and was done on a daily basis. Animals were treated using the test substances. Only group one animals were not infected and treated (table 1).
| Group | Group type | Infection | Treatment |
| 1 | Neutral control | No | No |
| 2 | Negative control | Yes | No |
| 3 | Test | Yes | 1% extract ointment |
| 4 | Test | Yes | 5% extract ointment |
| 5 | Test | Yes | 10% ointment |
| 6 | Positive control | Yes | 1%w/w griseofulvin |
Table 1: Infection and treatment of guinea pigs.
Statistical analysis
Data on dermal irritation test were presented as visual scores based on Draize method of erythema and edema grading system. SPSS was used to analyze the diameter of regression of the infected zone or inhibition by the crude extract which was expressed as the mean ± standard deviation. The Waller Duncan test was used to compare the means of different substances at a significant value of 5%.
Data on dermal irritation test were presented as visual scores based on Draize method of erythema and edema grading system. SPSS was used to analyze the diameter of regression of the infected zone or inhibition by the crude extract which was expressed as the mean ± standard deviation. The Waller Duncan test was used to compare the means of different substances at a significant value of 5%.
Results
Phytochemical screening of extracts
In other to know the secondary metabolites present in these plant extracts, a phytochemical screening was carried out which showed that Ageratum houstonianum contained all tested metabolites except alkaloids. The overall results are summarized in table 2.
In other to know the secondary metabolites present in these plant extracts, a phytochemical screening was carried out which showed that Ageratum houstonianum contained all tested metabolites except alkaloids. The overall results are summarized in table 2.
| Plant extracts | Phytoconstituents | |||||
| Alkaloids | Terpenoids | Flavonoids | Phenols | Anthocyanins | Saponins | |
| Ageratum conyzoides | - | + | + | + | + | + |
| Ageratum houstounianum | - | + | + | + | + | + |
| Mangifera indica | - | + | + | + | + | + |
| Polyscia fulva | + | + | + | + | + | - |
(+) stands for presence and (-) for absence, AH; Ageratum houstonianum, AC; Ageratum conyzoides, MI; Mangifera indica, PF; Polyscia fulva.
Table 1: Secondary metabolites found in extracts.
Table 1: Secondary metabolites found in extracts.
In vitro antidermatophytic activity
The evaluation of anti-dermatophytic activity of various crude extracts was carried out by determining the MIC and the MFC of each extract. Table 3 summarizes the results and from this table, it appears that all tested plant extracts have anti-dermatophytic activity which varying from one fungal isolate to another. The anti-dermatophytic activities (MIC) of the various extracts are generally between 16 and > 2048 μg/mL. The plant with the best anitdermatophytic activity was Ageratum houstonianum with MIC between 16 to 128 μg/mL and the most sensitive strain was T. mentagrophytes f0237 which was susceptible to all the tested extracts.
The evaluation of anti-dermatophytic activity of various crude extracts was carried out by determining the MIC and the MFC of each extract. Table 3 summarizes the results and from this table, it appears that all tested plant extracts have anti-dermatophytic activity which varying from one fungal isolate to another. The anti-dermatophytic activities (MIC) of the various extracts are generally between 16 and > 2048 μg/mL. The plant with the best anitdermatophytic activity was Ageratum houstonianum with MIC between 16 to 128 μg/mL and the most sensitive strain was T. mentagrophytes f0237 which was susceptible to all the tested extracts.
| Extracts | Strains | |||||
| T. mentagrophytes f0237 | T. mentagrophytes f0268 | T. soudanense | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | |
| Mangifera indica | 512 | 1024 | >2048 | >2048 | >2048 | >2048 |
| Ageratum conyzoides | 32 | 64 | 128 | 512 | 256 | 512 |
| Ageratum houstounianum | 16 | 1024 | 32 | 512 | 128 | 1024 |
| Polyscia fulva | 32 | 256 | 32 | 256 | 256 | 1024 |
| Griseofulvin | 2 | 4 | 8 | 16 | 2 | 2 |
Table 3: Minimal inhibitory concentration and the minimal fungicidal concentration of the different plant extracts.
Skin irritation test
After 24 hours of exposure to 0.5 g of the leaves of Ageratum houstonianum, rinsing and observation for 72 hours, the test and control sites showed no edema and erythema.
After 24 hours of exposure to 0.5 g of the leaves of Ageratum houstonianum, rinsing and observation for 72 hours, the test and control sites showed no edema and erythema.
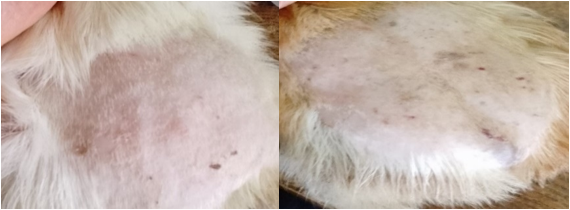
Evolution of treatment after infection with T. mentagrophytes f0237 The results from this test showed that, up to day 3 of the treatment, there were no visible changes observed in the lesions for the test groups, positive control group and negative control group until day four where regression became visible in the test groups. For the test groups and positive control group it was seen that, treatment efficacy was in a dose dependent manner as the 10% treatment group was healed on day 13, positive control group (1% griseofulvin cream) healed on day 15, the 5% treatment group was healed on day 17 and the 1% treatment group on day 18 meanwhile the negative control group showed a slightly progressive increase in lesions throughout the test. This is illustrated in table 4.
| Days | Group 1% ointment | 5% ointment | 10% ointment | Griseofulvin 1% | Negative control |
| 1 | 2,90 ± 0,10ab | 3,00 ± 0,00bc | 2,80 ± 0,00a | 3,00 ± 0,00bc | 3,06 ± 0,05c |
| 2 | 2,90 ± 0,10ab | 3,00 ± 0,00bc | 2,80 ± 0,00a | 3,00 ± 0,00bc | 3,06 ± 0,05c |
| 3 | 2,90 ± 0,10ab | 3,00 ± 0,00bc | 2.80 ± 0,00a | 3.00 ± 0,00bc | 3,06 ± 0,05c |
| 4 | 2.90 ± 0,10b | 2,90 ± 0,10b | 2.60 ± 0,10a | 2,76 ± 0,05b | 3,10 ± 0,00c |
| 5 | 2,76 ± 0,05b | 2,70 ± 0,00b | 2,36 ± 0,05a | 2.66 ± 0,05b | 3,26 ± 0,05c |
| 6 | 2,60 ± 0,10b | 2,65 ± 0,05b | 2,16 ± 0,15a | 2,50 ± 0,00b | 3,26 ± 0,00c |
| 7 | 2,46 ± 0,15b | 2,50 ± 0,10b | 1,86 ± 0,15a | 2,40 ± 0,10b | 3,30 ± 0,00c |
| 8 | 2.36 ± 0,15c | 2,40 ± 0,00c | 1,56 ± 0,15a | 2,10 ± 0,10b | 3,40 ± 0,00c |
| 9 | 2,10 ± 0,10b | 2,30 ± 0,00c | 1,56 ± 0,15a | 1,93 ± 0,11b | 3,40 ± 0,00d |
| 10 | 1,96 ± 0,05c | 2,15 ± 0,05c | 1,26 ± 0,15a | 1,70 ± 0,10b | 3,46 ± 0,05d |
| 11 | 1,86 ± 0,15c | 2,00 ± 0,10c | 1,16 ± 0,05a | 1,46 ± 0,05b | 3,46 ± 0,05d |
| 12 | 1,76 ± 0,05b | 1,80 ± 0,10b | 0,50 ± 0,50a | 1,36 ± 0,05b | 3,46 ± 0,05c |
| 13 | 1,56 ± 0,05c | 1,60 ± 0,20c | 0,00 ± 0,00a | 1,10 ± 0,10b | 3,50 ± 0,00d |
| 14 | 1,36 ± 0,15c | 1,36 ± 0,15c | 0,00 ± 0,00a | 0,50 ± 0,50b | 3,50 ± 0,00d |
| 15 | 1,20 ± 0,10b | 1,20 ± 0,10b | 0,00 ± 0,00a | 0,00 ± 0,00a | 3,50 ± 0,00c |
| 16 | 1,10 ± 0,10c | 0,53 ± 0,55b | 0,00 ± 0,00a | 0,00 ± 0,00a | 3.50 ± 0,00d |
| 17 | 0,50 ± 0,50b | 0.00 ± 0,00a | 0,00 ± 0.00a | 0,00 ± 0.00a | 3,50 ± 0.00c |
| 18 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0,00 ± 0.00a | 3,50 ± 0.00b |
Table 4: Mean values of animal infection diameter expressed in function of time (days).
The table values are presented as mean ± standard deviation of the diameter of infected zones of 3 animals per group. In the same column, the value bearing the different letters are significantly different (p<0,05).
Discussion
Medicinal plants produce a diverse range of bioactive molecules that provide reliable therapy in the treatment of various skin infections in humans [20]. Phytochemical analysis of extracts which served in detecting the composition of secondary metabolites indicated the presence of terpenoids, phenols, and flavonoids in all plant extracts. Individual activity of these components have been proven which explains their antidermatophytic activity. The results of the studies in the context of their constituent composition and antifungal activity are greatly influenced by geo-ecological variations, the part of the plant that is used, the time of collecting sample or postharvest, extract type or solvent taken (essential oil, semisolid, dried/ethanol, aqueous, acetone, ether, or dichloromethane fractions and the fungal strain chosen for the study [21]. These findings were in agreement with those of previous studies, reporting that these plants had antimicrobial (antidermatophytic) properties and in some cases can be used in traditional medicine [11, 12, 13, 14]. The significant inhibition of T. mentagrophytes f0237 and T. mentagrophytes f0268 by three (75%) and two (50%) extracts respectively is in consistence with the study of Ali and Suheil [22] which showed that among 22 extracts studied 5 (28%) completely inhibited T. mentagrophytes. However the plants used were not identical to those in this study. The high sensitivity of T. mentagrophytes was in line with the study of Nasr et al [23] where the azealic acid transethosomal gel significantly inhibited T. mentagrophytes. The fact that there was a variation in MIC values did not only show that this plants have different antifungal potentials but also revealed that the sensitivity of a fungal germ varies depending on the plant species [24]. However, none of the substances tested was as active as the reference drug griseofulvin which can be explained by the fact that, potency and concentration of bioactive compounds may be lower or less effective compared to griseofulvin and the mechanism of action of the extracts may be different from that of griseofulvin. This result was in concurrence with the results of Tamokou et al., [25].
Following this study, the dermal irritation test which was aimed at evaluating how harmless the extract of Ageratum houstonianum can be showed that, the extract at a dose of 0.5 g did not present any erythema or edema on the guinea pigs of both groups. This results suppose that, although palm kernel oil is a good vehicle and can be used as excipient in drugs used for the treatment of dermatophytosis, it is not essential for dermal antidermatophytic treatment using < 0.5 g of leaf extracts of Ageratum houstonianum. This results are in line with the study of Dongmo et al., [13] on the methanolic extracts of Ageratum conyzoides leaves. This similarity may be as a result of the same solvent used in extraction.
Since Ageratum houstonianum exhibited the best activity, its therapeutic effect was investigated in vivo against trichophytosis on guinea pigs with the aim of evaluating how effective it can be on these animals. Guinea pigs are used because they are a very susceptible model for testing topical antifungals [26]. The efficacy of the treatment was confirmed by regression of the infected zone and growth of hairs. The 10% ointment treated the infection before griseofulvin showing that it can be used as a substitute of griseofulvin. This result may be due to the fact that, plants contained many different phytochemicals which act in a synergistic manner. In line with the present study, Seyyed and Abdolhasan, [27] reported a similar result where clotrimazole cured T. mentagrophytes infection on day 21 whereas Myrtus communise and Cinnamonium zeylenicum extracts cured it on day 9 and 13 respectively.
Conclusion
The results obtained show that the crude extract of Ageratum houstonianum is a promising alternative to be used against dermatophyte infections by this strain. This may justify the use of Ageratum houstonianum in traditional medicine against dermatophytosis.
Acknowledgement
We are grateful to Prof Stéphane Ranque the IHU Méditerranée infection, 13385 Marseille, France for providing the strains used in the study.
We are grateful to Prof Stéphane Ranque the IHU Méditerranée infection, 13385 Marseille, France for providing the strains used in the study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
The authors have no relevant financial or non-financial interests to disclose.
Author’s contribution
All authors contributed to the conception of the study design. Material preparation and data analysis were done by Faith Njang Kah, Patricia Assoua, Dongmo Armel-joseph Agokeng, Larissa Chimi and Guy Sedar Singor Njateng. The first draft of the manuscript was written by Faith Njang Kah and approved by all authors.
All authors contributed to the conception of the study design. Material preparation and data analysis were done by Faith Njang Kah, Patricia Assoua, Dongmo Armel-joseph Agokeng, Larissa Chimi and Guy Sedar Singor Njateng. The first draft of the manuscript was written by Faith Njang Kah and approved by all authors.
References
- Coulibaly, O., L'Ollivier, C., Piarroux, R & Ranque, S. (2018). Epidemiology of human dermatophytoses in Africa. Medical mycology. 56(2), 145–161.
- Shanthini. (2020). A clinicomycological study of dermatophytosis in a tertiary care hospital. Master degree, microbiology- branch IV. 183p.
- Dongmo, J.K.A., Dabou s., Jihane K., Kemnang B. D. A., Khaddim D., Njaten G.S.S & Ranque S. (2024). Epidermiology of tinea capitis among school age children in Dschang west region. Mycopathologia., 189-51.
- Manuela P., Nkondjo S.M &Valentina F. (2012). Onychomycosis in Cameroon: a clinical and epidermiological study among dermatological patients. International journal of mycology. 51: 1474-1477.
- Monise, F., Mariana H., Bruna, A., Gabriel, G., Felipa, G., Tamires, A., Mozart M & Ana L. (2020). Epidemiology and diagnostic perspectives of dermatophytosis. Journal of fungi. 6: 310, 1-15.
- Chabasse D, Guiguen C, Contet-Audonneau N. (1999). Mycologie médicale. Editions Masson, collection Abrégés, Paris.
- Koenig H. (1995). Guide de mycologie médicale. Editons Ellipses, Paris,
- Camille C. (2011). Les dermatophyties d'origine zoonotique : aspects actuels et prise en charge à l'officine. Sciences pharmaceutiques.
- Lorougnon, F., Heron, M., Therizol, J., Kanga, D., Djeha, P., Yoboue & Boussou D. (1991). Efficacité Clinique de la naftifine dans le traitement des dermatomycoses. Médicine d’Afrique Noire., 38: 707-714.
- Tan, L.F., Yap, Y.L., Rajagopal,M. , Wiart, C.,Selvarja, M., Leong, M.Y. & Tan, P.L. (2022). Plants as an alternative source of antifungals against Aspergillus infections: a review. Plants. 11(22): 1-37.
- Njateng G.S.S, Kuiate, J.R., Gatsing, D., Tamokou, J.D.D., Mouokeu R. & Kuete, V. (2010). Antidermatophytic activity and dermal toxicity of essential oil from the leaves of Ageratum houstonianum (Asteraceae). Journal of biological sciences. 10(5): 448-454.
- Njateng GSS, Gatsing D, Mouokeu RS, Lunga PK, & Kuiate JR. (2013). In vitro and in vivo antidermatophytic activity of the dichloromethane-methanol (1:1 v/v) extract from the stem bark of Polyscias fulva Hiern (Araliaceae). BMC Complementary and Alternative Medicine. 13 (95) 26.
- Dongmo, J.K.A., Yetende, C.L., Njateng, G.S.S., Gatsing D., Kuiate, J.R. (2018). Antidermatophytic activity and adverse side effects of the methanolic extract from leaves of Ageratum conyzoides (Asteraceae). Journal of investigational, medicinal chemistry and pharmacology. 1(2); 19.
- Assoua, (2023). Etude ethnopharmacologique et avtivite antidermatophytique de quelque plantes medicinales Cameroonaise. Master’s thesis. University of Dschang. Pages 1-59.
- Harbone SB: Baxter H. (1996). Phytochemical dictionary. London: A handbook of bioactive compounds from plants. Taylor Francis.
- CLSI, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed., CLSI document M07-A9. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Auletta CS, CRC: (1995). From Acute, subchronic toxicology. In Handbook of toxicology. London: Derelanko and Hollinger; 51–162.
- Draize, J. H. The appraisal of chemicals in foods, drugs and cosmetics, Association of food and drug officials of the United States. Austin, Tex.1959, 49-52.
- Ahn, J. C., Mo, S. J., Choi, M., Kim, B., & Cho, S. B. (2023). In vivo Guinea Pig Model Study for Evaluating Antifungal Effect of a Dual-Diode Laser with Wavelengths of 405 Nm and 635 Nm on Dermatophytosis. Clinical, cosmetic and investigational dermatology., 16, 1559–1567.
- Savarirajan D, Ramesh V.M., Muthalyan. (2021). In vitro antidermatophytic activity of bioactive compounds from selected medicinal plants. Journal of analytical sciences and technology. 12 (53): 1-13.
- Chahal, R., Nanda, A. Akkol, E.K.; Sobarzo-Sánchez, E.; Arya, A.; Kaushik, D.; Dutt, R.; Bhardwaj, R.; Rahman, M..H & Mittal,V. (2021). Ageratum conyzoides L. and Its Secondary Metabolites in the Management of Different Fungal Pathogens. Molecules. 26, 2933.
- Ali-shtayeh M.S & Suheil A.G. (1999). Antifungal activity of plant extracts against dermatophytes. Mycoses. 42: 665-672.
- Nasr. A.M., Noha, M.B, Yasmin, H.T., Nader, M.S & Shady, AS. (2023). Development, optimization and in vitro, invivo evaluation of Azealic acid transethomal gel for antidermatophytic activity. Antibiotics. 12, 707.
- Yapi, A.B., Camara., Coulibaly K & Zirihi, G.N. (2018). Ethnobotanical study and comparison of antitrichophytic activity of leaves of Aspilia Africana, Adams var Africana Agerartum conyzoides and Acanthospermum DC on in vitro growth of T. mentagrophytes. Indo American journal of pharmaceutical sciences. 5(5): 4766-4775.
- Tamokou J.D.D., Kuaite, J.R., Gatsing, D., Alango, P., Nkeng & Njouedou. (2011). Antidermatophytic activity and toxicological evaluation of dichloromrthane methanol extract, fractions and compounds isolated form Coula edulis. Irand journal of med sci. 36 (2): 111-121.
- Trieber A., Pitterman W., Schuppe H.C. (2001). Efficacy testing of antimycotic prophylactics in an animal model. Int. j. hyg. Environ health. 204: 239-243.
- Seyyed A.A.M & Abdolhasan. (2015). In vitro and in vivo antidermatophytic activity of some Iranian medicinal plants. Journal of medical mycology. 53: 852-859.
Citation: Faith Njang Kah, Dongmo Armel-Joseph Agokeng, Larissa Chimi, Patricia Assoua and Guy Sedar Singor Njateng. (2025). Comparative Study of the Antidermatophytic Activity of four Cameroonian Medicinal Plants. Journal of Pharmacy and Drug Development 7(1). DOI: 10.5281/zenodo.14829299
Copyright: © 2025 Guy Sedar Singor Njateng. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.