Research Article
Volume 7 Issue 1 - 2025
Studies on Phytochemical and Biological Screenings of Caragana sukiensis of Nepal.
1Department of Chemistry, Patan Multiple Campus, Tribhuvan University, Kathmandu, Nepal
2Nepal Academy of Science and Technology (NAST), Khumaltar, Lalitpur, Nepal
2Nepal Academy of Science and Technology (NAST), Khumaltar, Lalitpur, Nepal
*Corresponding Author: Reeta Mandal, Department of Chemistry, Patan Multiple Campus, Tribhuvan University, Kathmandu, Nepal.
Received: January 15, 2025; Published: January 27, 2025
Abstract
Objective: The main aim of this study was to find out, antibacterial, antioxidant and cytotoxic activities and also screen the phytochemicals in n-hexane, dichloromethane and methanol extracts of Caragana sukiensis for first time.
Methods: Aerial parts of C. sukiensis were extracted out by solvents of increasing polarity. Preliminary Phytochemical screening of extracts was carried out using standard procedures. Furthermore, extracts were also evaluated for (i) antibacterial assay, (ii) brine shrimp lethality bioassay and (iii) antioxidant assay. American Type Culture Collection (ATCC) strains six bacteria, one gram-positive (Staphylococcus aureus ATCC 25525), five gram-negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsella pneumonia ATCC 700603, Serratia marcescens ATCC 13880 and Salmonella Typhimurium ATCC 14028 were used to evaluate its antimicrobial activity. Brine shrimp lethality assay was done on the extracts for cytotoxic. For the antioxidant test, 2, 2- Diphenyl 1, picrylhydrazyl (DPPH) free radicals scavenging assay was done.
Results: The phytochemical analysis indicated the presence of alkaloids, flavonoids, saponins, and terpenoids in aerial parts of C. sukiensis. In the case of the antibacterial, different extracts showed 9-13 mm and 0-15 mm zone of inhibition in diameter against gram positive bacteria S. aureus and gram-negative bacteria (E. coli, P. aeruginosa, K. pneumonia, S. marcescens and S. Typhimurium). This inhibition was found less in comparison to the standard compounds, Chloramphenicol (26±0.5mm to 32±0.5mm) and Gentamicin (26±0.5mm to 30±0.5mm) antibiotics. Similarly, in brine shrimp lethality assay, methanol extracts showed a potent lethal effect against the brine shrimp nauplii with LC50 of 28.18±2.6µg/ml. In the DPPH free radical scavenging assay, the extracts showed strong antioxidant activity in the order: methanol extract> dichloromethane extract> hexane extract, however all the extracts little less active than the standard compound, ascorbic acid. Methanol extract showed the highest DPPH free radical scavenging with IC50 value of 6.47±0.01 µg/ml.
Conclusion: The present study suggests the potentiality of C. sukiensis to become a natural source of antioxidant for the protective as well as prevention of diseases.
Keywords: Caragana sukiensis; Phytochemicals; Antibacterial; Antioxidant and Lethality assay
Introduction
Natural products have been one of the important sources to obtain useful drugs since last two centuries. The realm of drugs obtained from plants is vast wider than any other source of natural products. Plants also form the basis for the treatment in the traditional medicinal systems and practices such as the Ayurveda, Chinese Traditional Medicine, the Unani, Kampo, Yaku and others. Folk medicine also employs a wide variety of the plants as medicines. Therefore, it can be safely said that plants still hold promise for providing useful medicines. The compounds are found in various plant parts such as stems, roots, leaves, bark, flowers or fruits and seeds and include alliin/allicins, isothiocyanates, plant pigments, proteins, essential oils, and phytoalexins or phenolic compounds [1,2].
The genus Caragana belongs to the family Fabaceae and comprises over 80 species distributed in Asia and Europe [3]. Among them 14 species are available in the central and western Nepal [4]. Dense populations of Caragana species have been reported in the lower and upper Mustang, Annapurna Conservation Area of Nepal. Many species of this genus have been used in the Traditional Chinese Medicine for the treatment of hypertension, irregular menstruation and fatigue [5]. The major constituents of Caragana species were reported to be the flavonoids and stilbenoids [5]. Several novel compounds have also been isolated from species of Caragana and they are shown to possess anti-tumor, antivirus, anti-inflammation, sedative, acetyl cholinesterase inhibitory, immunosuppressant activities phytoestrogenic and anti-HIV activities [6-8]. The ethnopharmacological importance and wide traditional therapeutic values of Caragana genus have prompted us to carry out the phytochemical studies on C. sukeinsis found in Nepal. Among the Caragana species, C. sukiensis is found generally in open dry slopes; 2100- 4200 m, abundant in Mustang, Annapurna conservation area, and Manaslu conservation area and also other parts of Central Nepal [9]. Ethanol extract has been reported to be antimicrobial activity against fungus Cryptococcus neoformans [10]. Though the shrub is used for different purposes, few phytochemical as well as bioactivity studies have been reported sukienside A (1), sukienside B (2) [11], sukienside D (3), sukienside E (4), sukienside F (5),20(R), 24(S)-epoxycycloartane-3b,6a,16b,25-tetraol-3b-O-D-(2-O-acetyl)-xylopyranoside (6), 3-O-b-D-xylo cyclo siversigenin (7), cyclosiversigenin (8), 3b-hydroxy-25-methylenecycloartan-24-ol (9), b amyrin (10), soyasapogenol B (11), melilotigenin C (12)[12]and sukienside C(13)[11]. The present study describes the phytochemical screening as well as the antimicrobial, antioxidant and brine shrimp lethality test of various fractions from C. sukeinsis.
Materials and Methodology
Plant material and preparation of extracts
An aerial part of C. sukiensis was collected from an altitude 2249m, Prok VDC in the Manaslu Conservation Area, Nepal in July 2011. The respective herbariums were identified by a plant taxonomist, Rita Chhetri, National Herbarium and Plant Laboratory, Godavari, Lalitpur, Nepal. Voucher number of C. sukiensis, was 201179. The aerial parts were dried under shade for one month. The plant material was grounded into a fine powder. The powder sample was subjected to the successive extraction using different solvents with increasing polarity-hexane, dichloromethane, methanol two times heating each for 12-18 hr respectively. The extracts were then filtered through a cotton plug. The filtrates were dried under vacuum in a rotary evaporator. The hexane, dichloromethane, methanol extracts were collected, stored at 4°C and used for further investigation for the phytochemical as well as bioassay studies.
An aerial part of C. sukiensis was collected from an altitude 2249m, Prok VDC in the Manaslu Conservation Area, Nepal in July 2011. The respective herbariums were identified by a plant taxonomist, Rita Chhetri, National Herbarium and Plant Laboratory, Godavari, Lalitpur, Nepal. Voucher number of C. sukiensis, was 201179. The aerial parts were dried under shade for one month. The plant material was grounded into a fine powder. The powder sample was subjected to the successive extraction using different solvents with increasing polarity-hexane, dichloromethane, methanol two times heating each for 12-18 hr respectively. The extracts were then filtered through a cotton plug. The filtrates were dried under vacuum in a rotary evaporator. The hexane, dichloromethane, methanol extracts were collected, stored at 4°C and used for further investigation for the phytochemical as well as bioassay studies.
Preliminary Phytochemical Screening
The different extracts collected after successive extraction of the aerial part of C. sukiensis were subjected to qualitative chemical analysis for the identification of different phyto-constituents [14, 15]. Alkaloid detection was carried out by addition of 2N HCl to different extracts collected by extraction in 1:1 ratio and then treating the filtrate with Meyer's and Wagner's reagents. The samples were scored positive on the basis of turbidity or precipitation. Flavonoids were tested by treating extracts with 1 ml dilute ammonia. A yellow coloration demonstrated positive test for flavonoids. The presence of tannins was confirmed by boiling extracts with 6 drops of 5% FeCl3 to the filtrate. Development of brownish-green or blue black colorations was taken as positive for the presence of tannins. Detection of saponins: Saponins content was determined by shaking the different extracts vigorously to record froth formation. Cardiac glycosides were identified by treating different extracts with 1 ml glacial acetic acid containing 1 drop of 5% FeCl3 solution. This solution was carefully transferred to surface of 0.5 ml conc. H2SO4. The formation of reddish-brown ring at the junction of two liquids was indicative of cardenolides/cardiac glycosides. Terpenoids was determined by adding 2ml of chloroform with 5 ml (1 mg/ml) of extracts in a test tube then 3 ml of concentrated H2SO4 was added to develop the color. Exhibition of reddish-brown coloration at the interface confirmed the presence of terpenoids.
The different extracts collected after successive extraction of the aerial part of C. sukiensis were subjected to qualitative chemical analysis for the identification of different phyto-constituents [14, 15]. Alkaloid detection was carried out by addition of 2N HCl to different extracts collected by extraction in 1:1 ratio and then treating the filtrate with Meyer's and Wagner's reagents. The samples were scored positive on the basis of turbidity or precipitation. Flavonoids were tested by treating extracts with 1 ml dilute ammonia. A yellow coloration demonstrated positive test for flavonoids. The presence of tannins was confirmed by boiling extracts with 6 drops of 5% FeCl3 to the filtrate. Development of brownish-green or blue black colorations was taken as positive for the presence of tannins. Detection of saponins: Saponins content was determined by shaking the different extracts vigorously to record froth formation. Cardiac glycosides were identified by treating different extracts with 1 ml glacial acetic acid containing 1 drop of 5% FeCl3 solution. This solution was carefully transferred to surface of 0.5 ml conc. H2SO4. The formation of reddish-brown ring at the junction of two liquids was indicative of cardenolides/cardiac glycosides. Terpenoids was determined by adding 2ml of chloroform with 5 ml (1 mg/ml) of extracts in a test tube then 3 ml of concentrated H2SO4 was added to develop the color. Exhibition of reddish-brown coloration at the interface confirmed the presence of terpenoids.
Antimicrobial Activity
Microbial strains
The collected extracts of C. sukiensis were tested for their antibacterial activities against six bacterial strains namely Staphylococcus aureus ATCC 25923), Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsella pneumonia ATCC 100603, Serratia marcescens ATCC 13880 and Salmonella Typhimurium ATCC 14028. These strains of bacteria were gift from National Public Health Laboratory, Teku, Nepal and Institute of Medicine, Tribhuvan University, Maharajgunj, Nepal.
Microbial strains
The collected extracts of C. sukiensis were tested for their antibacterial activities against six bacterial strains namely Staphylococcus aureus ATCC 25923), Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsella pneumonia ATCC 100603, Serratia marcescens ATCC 13880 and Salmonella Typhimurium ATCC 14028. These strains of bacteria were gift from National Public Health Laboratory, Teku, Nepal and Institute of Medicine, Tribhuvan University, Maharajgunj, Nepal.
Microbial assay
The agar well diffusion assay was used to determine the antimicrobial activity of the extracts of C. sikeinsis [15, 16]. Mueller Hinton agar (Himedia) was prepared as per the manufacturer’s protocol. The sterile Mueller Hinton agar was poured into sterile petri dishes and seeded with test microorganisms. The concentration of microorganism used was equivalent to McFarland solution (standard). Seven holes (6mm diameter) were made by cutting out from agar plates using a sterilized stainless-steel borer of 6mm. They were bore and filled with 20µl (50 mg/ml) of the fraction. The plates inoculated with different bacteria were incubated at 37°C up to 48 h. The diameter of any resultant zone of inhibition was measured. For each combination of extracts and the bacterial strain, the experiment was performed in duplicate and repeated thrice. The antibacterial activity of different plant extracts was compared with two commonly employed antibiotics was (i) Chloramphenicol (30μg/ml) and (ii) Gentamicin (10μg/ml) as positive control and DMSO as negative control.
The agar well diffusion assay was used to determine the antimicrobial activity of the extracts of C. sikeinsis [15, 16]. Mueller Hinton agar (Himedia) was prepared as per the manufacturer’s protocol. The sterile Mueller Hinton agar was poured into sterile petri dishes and seeded with test microorganisms. The concentration of microorganism used was equivalent to McFarland solution (standard). Seven holes (6mm diameter) were made by cutting out from agar plates using a sterilized stainless-steel borer of 6mm. They were bore and filled with 20µl (50 mg/ml) of the fraction. The plates inoculated with different bacteria were incubated at 37°C up to 48 h. The diameter of any resultant zone of inhibition was measured. For each combination of extracts and the bacterial strain, the experiment was performed in duplicate and repeated thrice. The antibacterial activity of different plant extracts was compared with two commonly employed antibiotics was (i) Chloramphenicol (30μg/ml) and (ii) Gentamicin (10μg/ml) as positive control and DMSO as negative control.
Brine shrimp lethality assay
About 1gm of brine shrimps (Artemia salina) eggs (Ocean Star International, Inc., USA) was allowed to hatch in one liter of artificial sea water. The temperature was maintained at 22-28°C in presence of fluorescent lamp with continuous aeration for 48 hrs. This assay was performed by using the method described by Meyer et al. [17-18]. Stock solutions were prepared by dissolving 2mg of extract in 2ml of DMSO. Different concentrations of extracts: 10µg/ml, 100µg/ml and 1000µg/ml in DMSO were used against brine shrimp larvae. The experiment was in triplet. These vials were incubated for 24 hr. The survival rate of these larvae was observed against all concentration of test samples. For this purpose, 5 ml of brine and 10 shrimp in each vials containing of three replicates solutions with 0.5ml of each fraction was applied. These vials were incubated for 24 hr.
About 1gm of brine shrimps (Artemia salina) eggs (Ocean Star International, Inc., USA) was allowed to hatch in one liter of artificial sea water. The temperature was maintained at 22-28°C in presence of fluorescent lamp with continuous aeration for 48 hrs. This assay was performed by using the method described by Meyer et al. [17-18]. Stock solutions were prepared by dissolving 2mg of extract in 2ml of DMSO. Different concentrations of extracts: 10µg/ml, 100µg/ml and 1000µg/ml in DMSO were used against brine shrimp larvae. The experiment was in triplet. These vials were incubated for 24 hr. The survival rate of these larvae was observed against all concentration of test samples. For this purpose, 5 ml of brine and 10 shrimp in each vials containing of three replicates solutions with 0.5ml of each fraction was applied. These vials were incubated for 24 hr.
After 24 hr, the survivors were counted with the help of magnifying glass. Percentage mortality was determined for three replications. From this data, the % lethality of fraction was calculated for each concentration. The LC50 of the extracts were obtained by plotting percentage of shrimp killed against the logarithm of sample concentration.
Antioxidant activity evaluation using DPPH free radical scavenging activity
The DPPH radical-scavenging assay was performed as described by Blois et al. [19] with some modification. DPPH solutions (50 μL, 100μg/ml) were added to the methanol solution of sample (200 μL, 12.5-100 μg/ml). After storage at the room temperature for 30 min in dark, the absorption of the reaction mixture was recorded at 517 nm in the spectrophotometer against the solvent. Inhibition of DPPH radical scavenging activity in percent (I%) was calculated by using the following equation [20].
The DPPH radical-scavenging assay was performed as described by Blois et al. [19] with some modification. DPPH solutions (50 μL, 100μg/ml) were added to the methanol solution of sample (200 μL, 12.5-100 μg/ml). After storage at the room temperature for 30 min in dark, the absorption of the reaction mixture was recorded at 517 nm in the spectrophotometer against the solvent. Inhibition of DPPH radical scavenging activity in percent (I%) was calculated by using the following equation [20].
The inhibition curve was plotted for triplicate experiments and represented as % of mean inhibition with standard deviation. IC50 value was determined by interpolation from linear regression of plot of percentage of inhibition against the logarithm concentration of extracts, as the amount of extract needed to scavenge 50% of DPPH radicals.
Statistical Analysis
All assays were carried out in triplicate. Results are expressed as means ± SD. The LC50 and IC50 values were calculated by linear interpolation between values above and below 50% activity by Microsoft excel analysis.
All assays were carried out in triplicate. Results are expressed as means ± SD. The LC50 and IC50 values were calculated by linear interpolation between values above and below 50% activity by Microsoft excel analysis.
Result and Discussion
Qualitative phytochemical tests of C. sukiensis were performed for collected extracts. The results of various chemical tests for the detection of group of secondary metabolites were summarized in the Table 1.
| Test | n-Hexane soluble extract | Dichloromethane Soluble extract | Methanol Soluble extract | Aqueous extract |
| Alkaloids | - | + | + | - |
| Terpenoids | + | + | + | + |
| Saponins | - | + | + | + |
| Tannins | - | + | + | + |
| Cardiac glycoside | - | - | + | + |
| Phenolics | - | - | + | - |
| Flavanoids | - | + | + | - |
Note: “+” present; “-” absent
Table 1: Phytochemical constituents of the different extracts in aerial part of C. sukiensis.
Table 1: Phytochemical constituents of the different extracts in aerial part of C. sukiensis.
Plants were selected on the basis of a potentially useful phytochemical composition by consulting ethnopharmacological and ecological information [21-22]. Therefore C. sukiensis was selected as well. Acceptance of medicines from such plant origin as an alternative form of healthcare is increasing because they are serving as promising sources of novel antibiotic prototypes [23]. Tannins are known to be useful in the treatment of inflamed or ulcerated tissues and they have remarkable activity in cancer prevention and anticancer [24]. Flavonoids have been shown to exhibit their actions through effects on membrane permeability, and by inhibition of membrane-bound enzymes such as the ATPase and phospholipase. The presence of these phenolic compounds in this plant contributed to their antioxidative properties [25]. Flavonoids serve as health promoting compound as a result of its anion radicals [26] and this property may explain the mechanisms of antioxidative action of C. sukiensis. These observations support the usefulness of this plant in folklore remedies in the treatment of stress related ailments and as dressings for wounds normally encountered in circumcision rites, bruises, cuts and sores. Also, the plant extract was revealed to contain saponins, known to produce inhibitory effect on inflammation and are major ingredients in traditional Chinese medicine [27]. This may be responsible for most of the observed biological effects, and these tend to the use of C. sukiensis in traditional medicine. Strong antifungal activity of C. sukiensis reported against Crytococcus neoformans. The result of phytochemical screening of plants indicated the presence of flavonoids, terpenoids, tannins, alkaloids and saponins are response for the activity.
Aerial part of C. sukiensis showed antibacterial activity against different ATCC bacteria (Table: 2) as measured by zone of inhibition. In antibacterial screening,the extracts showed less zone of inhibition (9-11 mm in diameter) were observed against gram positive S. aureus and promising zone of inhibition (0-13 mm in diameter) against gram-negative S. Typhimurium, K. pneumonia, S. marcescens, P. aeruginosa. But extracts had not shown any sensitivity against E. coli in comparison to standard Gentamicin and Chloramphenicol showed zone of inhibition 26-30mm. Methanol extract showed more active against tested bacteria than other extracts.
| S.N | Samples / Standards | Bacterial Inhibition zone | |||||
| E. coli 25922 | K. pneumoniae 100603 |
S. Typhimurium 14028 | S. marcescens 13880 | P. aeruginosa 27853 | S. aureus 25923 | ||
| 1 | Hexane soluble extract | - | 7.0 ± 0.5 | 10.7±0.6 | 11.7 ± 0.6 | 10.0 ± 1.0 | 10.3 ± 0.6 |
| 2 | Dichloromethane soluble extract | - | 9.3 ± 1.2 | - | 12.0 ± 1.5 | 10.0 ± 1.0 | 9.0 ± 1.0 |
| 3 | Methanol soluble extract | - | 13.0 ± 1.5 | 11.3 ± 1.5 | 10.3±1.5 | 12.7 ± 1.5 | 10.7 ± 1.0 |
| 4 | Chloramphenicol | 30.0 ± 0.5 | 26.0 ± 0.5 | 30.0 ± 0.5 | 30.0 ± 1.2 | 26.0 ± 0.5 | 32.0 ± 1.2 |
| 5 | Gentamicin | 26.0 ± 0.5 | 30.0 ± 1.2 | 30.0 ± 0.5 | 26.0 ± 0.5 | 30.0 ± 0.5 | 26.0 ± 0.5 |
Table 2: Antibacterial activities of different extracts of C. sukiensis evaluated using Agar well (6mm) diffusion Assay at 20 µl per well.
All results are presented as mean ± standard mean error of three assays.
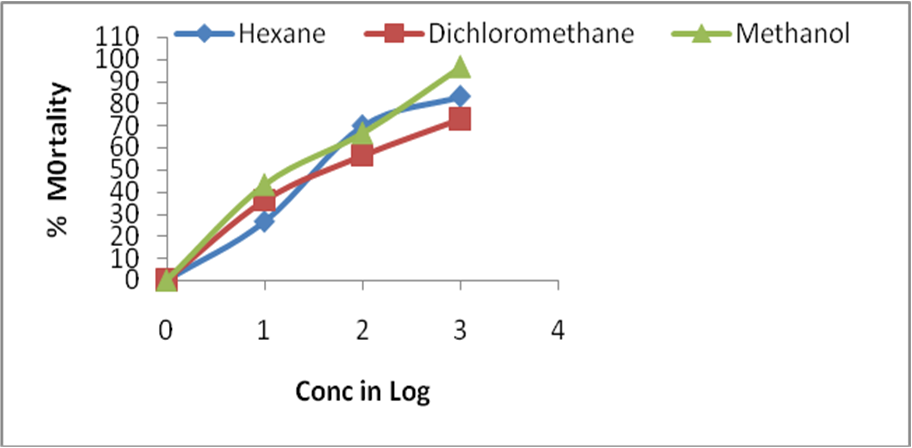
Inhibition zone produced around the well by adding 20 µl of extracts. Brine shrimp lethality is a general preliminary bioassay which is indicative of some form of pharmacologic activities (Table: 3).
Inhibition zone produced around the well by adding 20 µl of extracts. Brine shrimp lethality is a general preliminary bioassay which is indicative of some form of pharmacologic activities (Table: 3).
The LC50 value (28.18 ± 2.6 µg/ml) of methanol extract showed maximum activity for the experimental shrimps in comparison to its other extracts for C. sukiensis. Similarly, hexane extracts of C. sukiensis showed least cytotoxic effect with LC50 70.79 ± 5.5 µg/ml and dichloromethane showed LC50 46.77 ± 5.4µg/ml) respectively. The LC50 value of methanol extracts of C. sukiensis showed more lethality effect in comparison to other extracts.
| S.N | Samples | LC50 (µg/mL) |
| 1 | Hexane soluble extract | 57.00 ±5.4 |
| 2 | Dichloromethane soluble extract | 70.22 ± 5.5 |
| 3 | Methanol soluble extract | 29.05 ± 2.6 |
All results are presented as mean ± standard mean error of three assays
The antioxidant activity of the extract was assessed by the DPPH free radical scavenging assay and their scavenging activity was compared with the standard antioxidant ascorbic acid (Figure: 3). This activity was increase by increase the concentration of sample extract.
The antioxidant activity of the extract was assessed by the DPPH free radical scavenging assay and their scavenging activity was compared with the standard antioxidant ascorbic acid (Figure: 3). This activity was increase by increase the concentration of sample extract.
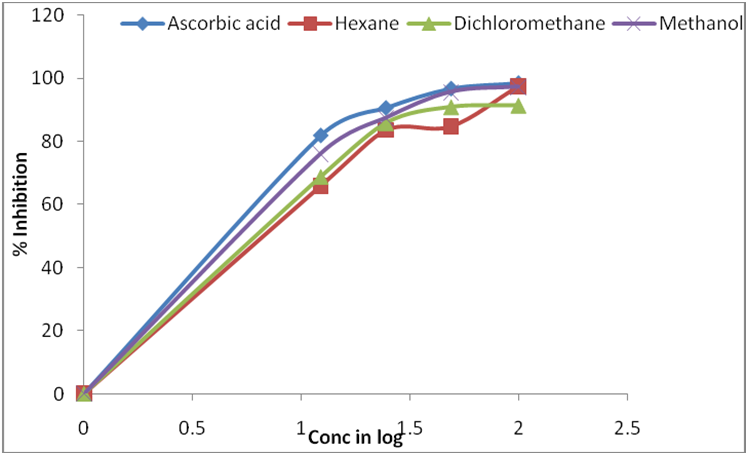
Figure 3: DPPH radical-scavenging activities of different extracts of C. sukiensis in comparision to Ascorbic Acid. Values were means ± SD (n = 3).
DPPH antioxidant assay is based on the ability of DPPH, a stable free radical, to decolorize in the presence of antioxidants. Both ascorbic acid and extracts showed a dose dependent activity expressed in LC50 value. The LC50 value is defined as the concentration of a substrate that causes 50 % loss of the DPPH activity and was calculated by linear regression of plots of the percentage antiradical activity against the concentration of the tested sample. The LC50 values of all the fractions were calculated and the results are given in Table 4. The lower the LC50 value, the higher is the scavenging potential. Where, LC50 value of ascorbic acid was 5.16 ± 0.27 μg/ml. Methanol extract of C. sukiensis showed maximum activity and LC50 value of 6.47 ± 0.01 μg/ml. Similarly, dichloromethane and hexane extract showed LC50 value of 7.5 ± 0.22 μg/ml and LC50 value of 7.9 ± 0.95 μg/ml The DPPH radical has been used widely as a model system to investigate the scavenging activities of several natural compounds including phenolic compounds, flavonoids or crude mixtures of plants. The effect of antioxidants on DPPH was thought to be due to their hydrogen donating ability. Free radicals from oxidative stress are involved in many disorders like atherosclerosis, angina pectoris, neurodegenerative diseases and cancer. Antioxidants due to their scavenging activity are useful for the management of those diseases. The LC50 values showed that high quantity of scavenging substances are found to be in methanol extract of C. sukiensis in comparison to other extract which plays the key role in showing free radical scavenging activity of this plant.
| S.N | Samples | IC50 of DPPH (µg/ml) |
| 1 | Ascorbic acid | 5.16 ± 0.27 |
| 2 | Hexane soluble extract | 7.9 ± 0.01 |
| 3 | Dichloromethane soluble extract | 7.5 ± 0.22 |
| 4 | Methanol soluble extract | 6.47 ± 0.95 |
Table 4: DPPH free radical scavenging activity of different extracts of C. sukiensis.
>All results are presented as mean ± standard mean error of three assays
All assays were carried out in triplicate. Results are expressed as means ± SD. The LC50 and IC50 values were calculated by linear interpolation between values above and below 50% activity by Microsoft excel analysis.
All assays were carried out in triplicate. Results are expressed as means ± SD. The LC50 and IC50 values were calculated by linear interpolation between values above and below 50% activity by Microsoft excel analysis.
Conclusion
Phytochemical screening and biological activities of aerial parts of C. sukeinsis’s different extracts have been studied here for the first time. Furthermore, the activity exhibited by the extracts may offer scientific justification for the ethno medicinal uses of these plants. More isolation of biologically active components and testing them in advanced enzymatic bioassays are required.
Experimental Section
We have declared no competing interest.
We have declared no competing interest.
Author’s Contributions
Reeta Mandal has been participated in collection of plant materials, performed all the experimental section, data analysis and prepared manuscript and Dr. Kanti Shrestha have been helped selecting the plant, designing experiments, interpreting the data and finalizing manuscript.
Reeta Mandal has been participated in collection of plant materials, performed all the experimental section, data analysis and prepared manuscript and Dr. Kanti Shrestha have been helped selecting the plant, designing experiments, interpreting the data and finalizing manuscript.
Acknowledgements
Reeta Mandal acknowledges Nepal Academy of Science and Technology (NAST), Nepal for the PhD fellowship under NAST- Tribhuvan University Collaborative PhD program and Late Prof Dr. Mohan Bikram Gewali for selecting the plant, designing experiments, interpreting the data as my supervisor for whole research work. Reeta Mandal also thanks Roshan Shrestha for his help in plant collection and Rosa Ranjit for her support in the laboratory.
Reeta Mandal acknowledges Nepal Academy of Science and Technology (NAST), Nepal for the PhD fellowship under NAST- Tribhuvan University Collaborative PhD program and Late Prof Dr. Mohan Bikram Gewali for selecting the plant, designing experiments, interpreting the data as my supervisor for whole research work. Reeta Mandal also thanks Roshan Shrestha for his help in plant collection and Rosa Ranjit for her support in the laboratory.
References
- Cragg GM, Newman DJ (2005). Plants as a source of anti-cancer agents. J Ethnopharmacol 100(1-2): 72- 79.
- Hartwell JL (1976). Types of anticancer agents isolated from plants. Cancer Treat Rep 60(8): 1031- 1067.
- Sanderson MJ, Wojciechowski MF (1996). Diversification Rates in a Temperate Legume Clade: Are there “So Many Species” of Astragalus. Amer J Bot 83(11): 1488-1502.
- Press JR, Shrestha KK, Sutton DA (2000). Annotated checklist of the flowering plants of Nepal. Natural History Museum, London.
- Meng Q, Niu Y, Niu X, Roubin RH, Hanrahan JR (2009). Ethanobotany, Phytochemistry and Pharmacology of the genus Caragana used in traditional Chinese medicine. J Ethnopharmacol 124(3): 350-368.
- Khan AN, Perveen S, Malik A, Afza N, Iqbal L, Latif M, Saleem M (2010). Conferin, potent antioxidant and anti-inflammatory isoflavone from Caragana conferta Benth. J Enz Inh Med Chem 25(3): 440-444.
- Khan R, Malik A, Adhikari A, Qadir MI, Choudhary MI (2009). Conferols A and B, New anti-inflammatory 4- hydroxyisoflavones from Caragana conferta. Chem Pharm Bull 57(4): 415-417.
- Yang GX, Zhou JT, Li YZ, Hu CQ (2005). Anti- HIV bioactive Stilbene Dimers of Caragana rosea. Planta Med 71(6): 562-571.
- Bhattarai S, Chaudhary RP, Quave CL, Taylor RS (2010). The use of medicinal plants in the transHimalayan arid zone of Mustang district, Nepal. J Ethnobiol Ethnomed 6: 14.
- Geol AK,. Kulshrestha DK, Dubey MP, Rajendran SM (2002). Screening of Indian plants for biological activity:Part XVI. Ind J Expt Biology. 40 821-827.
- Mandal R, Shrestha K, Gewali MB (2016). Studies on Phytochemical Screening, Antimicrobial, Antioxidant and Cytotoxic Activities of Caragana Jubata of Nepal. Adv Pharmacol Clin Trials 1, 1.
- Mandal R, Siva B, Babu VSP, Babu KS, Jagadeesh B, Rosa Ranjit, Shrestha K , Gewali MB (2015). Novel cycloartane triterpenoids from the Nepal native plant Caragana sukiensis. Bioorg & Med Chem Letters 25: 5168–5171.
- Harborne JB (1998). Phytochemical Methods: A guide to modern technique of plant analysis 3rd eds: Chapman and Hall press: London, Weinheim, New York, Tokyo, Melbourne and Madras.
- Evan WC (2002). Pharmacogonosy, (15th eds) Saunder- Elsevier press, Edinburg, London, New York, Philadelphia, St. Louis, Sydney.
- Perez C, Pauli M, Bazevque P (1990). An antibiotic assay by the agar well diffusion method. Acta Biol Med Exper 15: 113-115.
- Rojas JJ, Ochoa VJ, Ocampo SA, Munoz JF (2006). Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Com Altern Med 17; 6:2.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, (1982). Brine shrimp: a convenient general bioassay for active plants constituents. Planta Med 45(5): 31-44.
- Mclaughlin JL, Rogers LL (1998). The use of Biological Assays to Evaluate Botanicals. Drug Inform Journal 32: 512-524.
- Blois MS (1958). Antioxidant determination by the use of a stable free radical. Nature 181: 1199-1200.
- Li WJ, Cheng XL, Liu J, Wang GL, Du SS, et al. (2012). Phenolic compounds and antioxidant activities of Liriope muscari. Molecules 17(2): 1797-1808.
- Joshi AR, Edington JM (1990). The use of medicinal plants by two village communities in the central development region of Nepal. Economic Botany 44: 71-83.
- Manandhar NP (1887). Traditional medicinal plants used by tribals of Lamjung District, Nepal. Int J Crude Drug Res 25(4): 236-240.
- Lai HY, Lim YY, Kim KH (2010). Blenchnum orientale Linn - fern with potential as antioxidant, anticancer and antibacterial agent. BMC Com Altern Med 10: 15.
- Li H, Wang Z, Liu Y (2003). Review in the studies on tannins activity of cancer prevention and anticancer. Zhong-Yao-Cai 2003 26(6): 444-448.
- Ferguson LR (2001). Role of plant polyphenols in genomic stability. Mutat Res 475(1-2): 89-111.
- Hausteen B (1983). Flavonoids, A class of natural products of high pharmacological potency. Biochem Pharm 32(7): 1141-1148.
- Hassan HS, Sule MI, Musa AM, Musa KY, Abubakar MS, et al. (2012). Anti- Inflammatory activity of crude saponin extracts from five Nigerian medicinal plants. Afr J Tradit Complement Altern Med 9(2): 250-255.
Citation: Reeta Mandal and Kanti Shrestha. (2025). Studies on Phytochemical and Biological Screenings of Caragana sukiensis of Nepal. Journal of Pharmacy and Drug Development 7(1).
Copyright: © 2025 Reeta Mandal. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.