Research Article
Volume 6 Issue 1 - 2024
Determination of Phenols, Flavonoids, Antioxidants, and other Chemical Compounds of Alhagi Maurorum (Camelthorn) plant found in Syria
Faculty of Pharmacy, Damascus University, Syria
*Corresponding Author: Loai Al Allan, Faculty of Pharmacy, Damascus University, Syria.
Received: February 03, 2024; Published: March 18, 2024
Abstract
Plants are valuable and important source of human food as well as natural remedies as they are used to treat many health disorders. They usually contain various biologically active compounds classified as secondary metabolites. Among them phenols and flavonoids, and natural phytochemicals which have been explored as an indispensable asset for their antimicrobial and anti-cancer properties. Alhagi or Maurorum is found in many Syrian areas and can be described as is a thorny herbaceous plant and has leguminous perennial known locally by "camel thorn" and is armed with thorns length 1-2.5 cm. Leaves hang from the base with thorns or branches, smooth and simple, oblong, obtuse, abstract, with a rounded apex. The flowers are panicles 2-3 mm long. It is characterized by small crimson-red flowers emerging from the sides of the spines, the fruit is dark horny, spongy in color, showing interstices between the seed sites, flowering period from October to March. The analysis performed in this study have shown that Alhagi or Maurorum plant contains the following active compounds: Apigenin, chrysoerol chrysin, luteolin, campferol and quercetin and conatins the following phenols such as: Caffeic acid, vanillin acid, naringin, syringic acid, chlorogenic, comaric, ferulic acid, cinnabic, isocryctrin, and rutin.
The study also showed a large exchange capacity of antioxidants in roots, leaves and stems in addition to the identification of many important compounds in the organic extract of roots and leaves with STEM on the technology of GC-MS, compounds were identified from the extract of petroleum ether, methanol and aqueous. The study found that the majority of separated compounds possess many biological activities, including anti-cancer (Including Damascenone, trans-Ionone, Levoglucosenone, Stigmasterol Farnesyl acetone, Farnesol, Hydroquinone and Sinapyl alcohol.
Keywords: Alhagi Maurorum; Flavonoids; Anti-inflammatory; Anti-oxidants; Anti-cancer
Introduction
Alhagi or Maurorum has been used in folk medicine as a treatment for rheumatism and kidney stones, and also has been used in the action of anachronism against migraine, and the extract that results from steaming the decoction of the plant is used as an analgesic or soothing for itching. The secretory substance that comes out of the leaves of the plant has a sex-stimulating effect and gives the body vitality and is a laxative, cholagogue, diuretic and blood purifier.
It is one of the plants endemic to Saudi Arabia, Iraq and Syria and contains glycosides, flavonoids, phenols, antioxidants, saponins, carbohydrates and essential oil and is an important medicinal plant belonging to the legume family.
The aim of this study was to evaluate the total contents of the Syrian Alhagi Maurorum plant (camel thorn) of phenols, flavonoids, antioxidants and active compounds found in the leaves and roots and determine the importance of Alhagi Maurorum plant in folk medicine, and its use in the pharmaceutical industry.
Materials and Methods
Materials and fittings
All of the chemicals used in the extraction and analysis processes are of the class of F and F of Merck
All of the chemicals used in the extraction and analysis processes are of the class of F and F of Merck
- Methanol
- Aceton
- Chloroform
- Hexane
- Petroleum ether
- Dichlorometane
- Bi-distilled water
Chitin chemical used to improve the capacity of antioxidant substances dissolved in water ACW materials dissolved company the ACL with sparkle photochemical company Analytik Jena AG (Jena, Germany)
Hardware
Chromatographic gases with mass spectrometry organic GC-MS is from Agilent with the analytical Chemstation and search Willy - Nist.
Chromatographic gases with mass spectrometry organic GC-MS is from Agilent with the analytical Chemstation and search Willy - Nist.
- Rotary evaporator with vacuum
- HPLC from Agilent with the pump bilateral and injector automatic and detector PDA with analytic software Chemstation
- Photochemical scintigraphy device for determining antioxidants, which is from Analytik Jena
Working Method
Sample Preparation
In this study, a range of different polar solvents (water, methanol, petroleum ether, chloroform and methanol) were used to extract the chemical components.
Sample Preparation
In this study, a range of different polar solvents (water, methanol, petroleum ether, chloroform and methanol) were used to extract the chemical components.
1-Isolation and identification of flavonoids from the Syrian wild plant Alhagi Maurorum
Flavonoids have a vital role in nutrition, physiology and the treatment of diseases. Flavonoids are one of the most important classes of phenolic compounds in plants.
Flavonoids have a vital role in nutrition, physiology and the treatment of diseases. Flavonoids are one of the most important classes of phenolic compounds in plants.
Sample preparation and extraction: the aerial part (leaves and stems) was washed with water and air dried. Then drying in oven at 50 m degrees and after 3 days until reached a constant weight. The leaves and stems of Alhagi Maurorum were ground into a very fine powder in a blender and stored in opaque glass containers in preparation for extraction and analysis.
The roots of Alhagi Maurorum were cleaned and dried in the shade and air under natural conditions and a well-ventilated place until reaching a fixed weight, after which they were ground into powder and kept in opaque glass containers for extraction and analysis.
Extraction
The desiccants were extracted by petroleum ether (nonpolar) and methanol (moderate polarity) using the method of DL, and also by soaking in water (high polarity) at room temperature. Extraction was then carried out using an ultrasonic bath and the extracts were filtered and concentrated to 1ml using a rotary evaporator at room temperature.
The desiccants were extracted by petroleum ether (nonpolar) and methanol (moderate polarity) using the method of DL, and also by soaking in water (high polarity) at room temperature. Extraction was then carried out using an ultrasonic bath and the extracts were filtered and concentrated to 1ml using a rotary evaporator at room temperature.
The extracts obtained by sequential extraction were then separated using petroleum ether first and then using diethyl ether second and ethyl acetate third separately. Each method was done three times to completely isolate the compound; the first droplet was discarded because it contained fatty substances, while the second and third droplet were used to identify flavonoids.
The second and third pieces were collected and after drying dissolved in ethanol, separated and identified by thin-layer chromatography using silica gel 400 microns and using a solvent mixture of benzene, acetic acid and water according to the proportions (125: 72: 3)
With standard material loading and in the presence of UV light, both the separated spots and the corresponding macroscopic matter have been observed. The stains were isolated and loaded again against the standard materials to ensure the purity of the separation and insulation and then determined using the HPLC.
The distinction of six patches of flavonoids in the plant parts (roots and leaves with the stem ) of the sample were selected on plates chromatograph thin layer in comparison with the supplier's Standard Terms of the show stains by spraying the colour with FeCl3 5% and observed the appearance of spots with similar materials standard was identified as Chrysoeriol Chrysin Apigenin Luteolin Kaempferol Quercetin, through the use of a solution of the dose benzene: acetic acid: water (125: 72: 3) so I gave the best results with the proper value of the RF to confirm the presence of flavonoids Chrysoeriol Chrysin Apigenin Luteolin Kaempferol Quercetin
Results
The plant samples (roots and leaves with stem) were extracted after being cleaned and dried in the oven at 40 degrees Celsius with the extraction solution prepared from 1.2 molar hydrochloric acid in 50% aqueous methanol
The mixture was carefully mixed and then distilled for two hours. The extract was cooled and filtered using the Buchner filter, then the sample was filtered on the 0.45 filter and the sample became ready for analysis on HPLC technology according to the following conditions:
Column: Reverse phasep-C-18(250 4 4.6, 5 micrometers)
Mobile Phase: Methanol, acetonitrile, water (40:15:45, v/v/v) containing 1.0% acetic acid
Flow rate: 0.9 mL / min
Detector: Ultraviolet DAD at wavelengths-279 2 257 - 368 Nm
The Results are as in Table 1
Column: Reverse phasep-C-18(250 4 4.6, 5 micrometers)
Mobile Phase: Methanol, acetonitrile, water (40:15:45, v/v/v) containing 1.0% acetic acid
Flow rate: 0.9 mL / min
Detector: Ultraviolet DAD at wavelengths-279 2 257 - 368 Nm
The Results are as in Table 1
| Flavonoid | Retention time | Concentration μg/ml (leaf, stem) |
Concentration μg/ml (Roots) |
| Chrysoeriol | 2.3 | 96.5 | 105.9 |
| Chrysin | 4.7 | 115.2 | 225.8 |
| Apigenin | 5.9 | 103.8 | 125.41 |
| Luteolin | 7.4 | 52.6 | 165.93 |
| Kaempferol | 8.3 | 85.1 | 143.2 |
| Quercetin | 9.6 | 112.9 | 173.4 |
Table 1: Detecting flavonoid of Alhag maurorum using HPLC.
The results of the analysis in Table 1 showed that the presence of flavonoids in the roots in higher proportions than in the leaves with the stem
Flavonoids are a natural product of phenolic glycosides, found almost naturally in angiosperms. They provide color to flowers and fruits, and play a role in attracting pollinating insects. Flavonoids are important in plants by providing resistance to plants against pests and insects.
Extraction of phenols from the roots, leaves and stems of the Alhagi Maurorum plant.
100g of lyophilized roots, leaves and stems of Alhagi Maurorum plant after homogenization separately so as to maintain high levels of phenols more than drying by exposure to air currents and solvent extraction was carried out from the roots, leaves and stems of Alhagi Maurorum plant as the yield of chemical extraction depends on the type of solvents with different polarity, extraction time, temperature and acid-free? Of hydrochloric acid to increase the yield of extraction where he took 100 g of the roots, leaves and stem of Alhagi Maurorum plant each separately leaves with the stem and roots alone and after grinding was soaked in about 200 ml solution 50/50 ( ethanol /water) and put in a tumble in a water bath at 40 degrees Celsius for half an hour dehydration by the rotary evaporator and then dissolved by the moving phase liquid and the extracted phenols were determined by the technology OFL G ??? according to the following analytical conditions:
Analytical column: reverse phase column - C-18(250 4 4.6, 5 micrometers)
Mobile phase: a mixture of solvent - (2% acetic acid in water) and solvent - (70: 30, acetonitrile / water).
100g of lyophilized roots, leaves and stems of Alhagi Maurorum plant after homogenization separately so as to maintain high levels of phenols more than drying by exposure to air currents and solvent extraction was carried out from the roots, leaves and stems of Alhagi Maurorum plant as the yield of chemical extraction depends on the type of solvents with different polarity, extraction time, temperature and acid-free? Of hydrochloric acid to increase the yield of extraction where he took 100 g of the roots, leaves and stem of Alhagi Maurorum plant each separately leaves with the stem and roots alone and after grinding was soaked in about 200 ml solution 50/50 ( ethanol /water) and put in a tumble in a water bath at 40 degrees Celsius for half an hour dehydration by the rotary evaporator and then dissolved by the moving phase liquid and the extracted phenols were determined by the technology OFL G ??? according to the following analytical conditions:
Analytical column: reverse phase column - C-18(250 4 4.6, 5 micrometers)
Mobile phase: a mixture of solvent - (2% acetic acid in water) and solvent - (70: 30, acetonitrile / water).
Syringe size: 20 microliters and analyzed at a constant temperature of 30 degrees Celsius
| Time | B % |
| 0 | 12 |
| 6 | 18 |
| 9 | 26 |
| 14 | 36 |
| 18 | 25 |
| 19 | 12 |
Table 2: The flow rate is constant at 1 ml per minute using programming for the gradient of the carrier fluid over time as follows.
Reagent: PDA by measuring with three wavelengths that are 280, 315 and 350 Nm, to determine most phenols
| Phenols | Retention time | Concentration μg/ml (leaf, stem) | Concentration μg/ml (Roots) |
| Caffeic acid | 4.11 | 41.3 | 75.2 |
| Vanillic acid | 6.53 | 32.5 | 48.2 |
| vitexin | 7.95 | 115.9 | 228.9 |
| ferulic acid | 9.34 | 129.5 | 180.2 |
| Chlorogenic | 11.41 | 130.7 | 236.4 |
| Coumaric | 12.84 | 114.8 | 195.3 |
| Ferulic acid | 13.58 | 98.4 | 185.24 |
| Sinapic acid | 14.92 | 178.9 | 240.6 |
| Isoqurectrin | 15.57 | 12.9 | 53.21 |
| Rutin | 17.11 | 86.5 | 184.9 |
Table 3: Detecting phenols in extracts of Alhagi maurorum Syrian using HPLC method.
Also from Table (3), it is shown that the percentage of phenols in the roots is higher than the leaves with the stem and this gives great importance to the plant Alhagi Maurorum through the presence of high proportions of phenols variety in it.
3 - Antioxidant effects in the roots and leaves of the Syrian wild Alhagi Maurorum plant
To examine the antioxidant effect of the aqueous extract of the roots and leaves of the Syrian wild Alhagi Maurorum plant: the total antioxidant capacity of the antioxidant activity of acetylsalicylic acid was measured. The results showed a marked decrease in levels of llll and strong antioxidant activity.
To examine the antioxidant effect of the aqueous extract of the roots and leaves of the Syrian wild Alhagi Maurorum plant: the total antioxidant capacity of the antioxidant activity of acetylsalicylic acid was measured. The results showed a marked decrease in levels of llll and strong antioxidant activity.
Extraction
Two separate extraction processes were carried out for both the dissolved parts in the water and in the lipoid to ensure the availability of kits for the appointment of antioxidants in both cases and to ensure correct results.
Two separate extraction processes were carried out for both the dissolved parts in the water and in the lipoid to ensure the availability of kits for the appointment of antioxidants in both cases and to ensure correct results.
We prepared the plant samples (drying-cleaning-roots and leaves and homogenization procedure for both leaves and roots) and then saved each sample with a number corresponding to the name and type of sample and thus the samples are ready to extract the (basic) compounds from them. 100g of the roots, leaves and stem of the Alhagi Maurorum plant each separately and after grinding it was soaked in about 200 ml solution 50/50 (etanol /water) and put in a tumultuous flask in a water bath at 40 degrees Celsius for half an hour with stirring then stir constantly and put in an ultrasonic bath for 25 minutes after that 30L a 50% aqueous solution of methanol, then put the beaker containing the plant extract in a bath of two minutes, and then evaporate the methanol with the rotary evaporator to a lesser degree than 30-using the vacuum, we get the water extract to be placed in the refrigerator in preparation for analysis
Measurement of antioxidants using photochemical luminescence technology
The method of luminescence was applied in the same way as described by Bulova and Levin [4]. This method is easy and fast and has a number of qualities compared to other methods, it does not require high temperatures to generate roots and is more accurate (field It takes about a few minutes (and less than three minutes) to measure the effectiveness of an antioxidant's ability to annihilate superoxide 2 free radicals-the most reactive radicals encountered in the human body.
The method of luminescence was applied in the same way as described by Bulova and Levin [4]. This method is easy and fast and has a number of qualities compared to other methods, it does not require high temperatures to generate roots and is more accurate (field It takes about a few minutes (and less than three minutes) to measure the effectiveness of an antioxidant's ability to annihilate superoxide 2 free radicals-the most reactive radicals encountered in the human body.
While most other methods such as (TEAC, TRAP, DPPH, ORAC and FRAP) the appointment by the effectiveness of anti-oxidant in the field of Cameron and require minutes, maybe hours. The measurement of antioxidant effectiveness by the previous methods involves generating root types and the presence of antioxidant substances that cause the disappearance of those roots, and the principle of thel method is as follows: the generation of photochemical free radicals is combined with the careful investigation of the use of the photoluminescence method. The V method differs on the photoabsorption of the self - oxidative termination of luminol by antioxidants and is a process mediated by the superoxide anionic free root .2* - which is very suitable for measuring the annihilation potential possessed by individual antioxidants as well as complex antioxidants in the Nano-mole range. Photochemical generation of free radicals thus combines with delicate, sensitive investigation. The following reaction is catalyzed by photohydrogenation (h)of a photosensor the overall process is as follows:
S + hv + O2 → [S* O2] → S*+ + O2*-
S + hv + O2 → [S* O2] → S*+ + O2*-
Thus, free radicals become visible with the luminol fluorescence detector, which acts as a photosensitive and oxygen radical detection agent. Antioxidant efficacy is measured by the phase difference in different concentrations calculated according to ascorbic acid or trolleux calibration curve and expressed in milli-Mol equivalent antioxidant efficacy compared to a reference compound (i.e. trolleux for dissolved substances in lipids, ascorbic acid for dissolved substances in water.)
Antioxidant Measurement
The method developed by Popov and Lewin [5] applied the total antioxidant capacity protocol for substances dissolved in water. In this work , where the antioxidant effectiveness of all components found in the roots and leaves of the Syrian wild plant Alhagi Maurorum was measured, we purchased the necessary reagents kits from the company analytic Jena – Germany. The ACW protocol is explained below. The first three reagents are: reagent 1 (solvent), reagent 2 (aqueous solution10.5), and reagent 3(photosensitive). The working solution of reagent 3 (3-WS) is prepared by taking the solution of reagent 3 and extending it by 750l L of reagent 2. The working solution of reagent 4 (4-WS) is prepared by taking reagent 4, adding μ 490 of reagent 1 and mixing it with 10 of sulfuric acid at a concentration of 95-97% from Merck. Stir for several seconds, take from the previous mixture 10 µl and extend by 990 µl from reagent 1 to obtain a working solution 4 (4-WS). Table 4 shows all procedures and volumes used in the analysis.
The method developed by Popov and Lewin [5] applied the total antioxidant capacity protocol for substances dissolved in water. In this work , where the antioxidant effectiveness of all components found in the roots and leaves of the Syrian wild plant Alhagi Maurorum was measured, we purchased the necessary reagents kits from the company analytic Jena – Germany. The ACW protocol is explained below. The first three reagents are: reagent 1 (solvent), reagent 2 (aqueous solution10.5), and reagent 3(photosensitive). The working solution of reagent 3 (3-WS) is prepared by taking the solution of reagent 3 and extending it by 750l L of reagent 2. The working solution of reagent 4 (4-WS) is prepared by taking reagent 4, adding μ 490 of reagent 1 and mixing it with 10 of sulfuric acid at a concentration of 95-97% from Merck. Stir for several seconds, take from the previous mixture 10 µl and extend by 990 µl from reagent 1 to obtain a working solution 4 (4-WS). Table 4 shows all procedures and volumes used in the analysis.
| Reagent | 1 | 2 | 3-WS | 4-WS | Sample |
| Control | 1500l | 1000l | 25l | 0 | 0 |
| Calibration | 1500l-x | 1000l | 25l | x | 0 |
| Measurement | 1500l-y | 1000l | 25l | 0 | y |
x= 10, 15, 20l, y= 10l, WS (working solution)
Table 4: Sizes used in different measurements.
Table 4: Sizes used in different measurements.
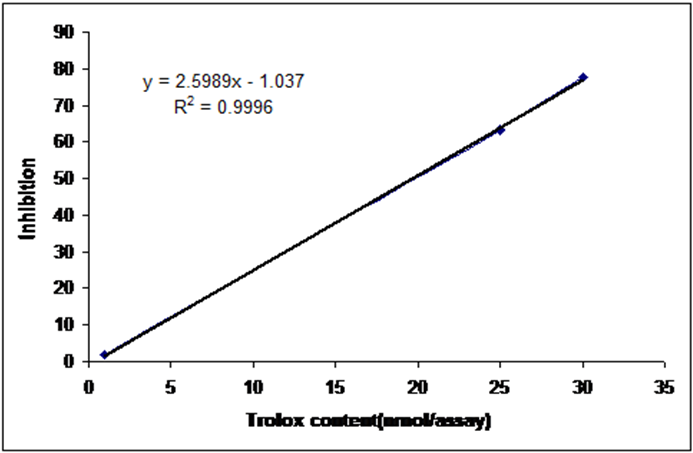
The measurements were calibrated according to the standard kit protocol as shown in the table, and the measurements were made with a photochem (Nm) device from analytic Jena - Germany. The used sizes were prepared in microliters and the measurements were repeated twice. A light emission curve was recorded in 240 seconds using an inhibitor as a parameter to estimate the antioxidant capacity, and the antioxidant capacity is determined by taking the integration given by the previous curve and expressed in mmol/L ascorbic acid used as the criterion for obtaining the calibration curve.
Table (5) shows the water-soluble antioxidant capacity equivalent to the efficiency expressed in nanomol equivalent of ascorbic acid per gram of the studied product.
| Measurements | (leaf, stem) Total antioxidant with ascorbic acid equivalents (nmol/g)** | (Roots) Total antioxidant with ascorbic acid equivalents (nmol/g)** |
| 1st repetition | 156.31 | 493.12 |
| 2nd repetition | 152.11 | 489.45 |
| 3rd repetition | 149.87 | 495.24 |
| Average | 152.76 | 492.60 |
Table 5: Total antioxidant rated with ascorbic acid equivalents using photochemical luminescence technology.
*: One gram of dry matter for fruits or flowers
**: Average score for three measurements
**: Average score for three measurements
It is clear from Table (5) that the highest antioxidant capacity values of Alhagi Maurorum plant roots expressed in ascorbic acid are at values of 495.24 (nmol/G) present in the root extract. Refer to Table (1) and (2) shows that the roots have the highest percentage of antioxidant components due to the presence of high levels of flavonoids and phenols.
From Table (5), It turns out that the results obtained support the view that the roots and leaves of the plant Alhagi Maurorum promising source of natural antioxidants for their ability to stop oxidation, especially as they contain large amounts of flavones and phenols, they are a promising source in the pharmaceutical industry
4-methanol extraction from the leaves and stems of the Syrian wild Alhagi Maurorum plant:
After that, the methanol extract was taken to the rotary evaporator and dried until dry with a vacuum and a temperature of 30 degrees Celsius, then dissolved in one milliliter of methanol, dried with anhydrous sodium sulfate, stored in a clean glass tube and kept in the dark at 4 degrees Celsius for analysis.
After that, the methanol extract was taken to the rotary evaporator and dried until dry with a vacuum and a temperature of 30 degrees Celsius, then dissolved in one milliliter of methanol, dried with anhydrous sodium sulfate, stored in a clean glass tube and kept in the dark at 4 degrees Celsius for analysis.
The plant extract and some of the calibrated materials were analyzed by gas chromatography with a mass spectrometer detector: MS
Device from the company 7 7890/5975. The capillary column (HP-5., 25 m. 0.25 mm) was used helium carrier gas (.) with a hash ratio of 1: 10. The furnace temperature was 75 degrees Celsius constant for 3 minutes and then to 280 degrees Celsius 3 degrees Celsius/min and the detector temperature was 250 degrees Celsius; the carrier gas flow is (0.9 mL/min) and the ionization energy is 70 electron volts. Retention times have been determined for separated materials. The components of the root plant extract were determined by comparing their mass spectra with the mass spectra of the research library.
| Compound | Peak Area % |
| Damascenone | 2.11 |
| E-Geranyl acetone | 8.24 |
| trans-Ionone | 0.92 |
| Actinidiolide | 1.84 |
| 2-(1,3-Butadienyl)-1,3,5-trimethylbenzene | 2.14 |
| Eugenol epoxide | 6.82 |
| E-Nuciferol | 2.47 |
| Eugenol | 7.28 |
| Octadecane | 4.21 |
| 6,10,14-Trimethyl-2-pentadecanone | 2.39 |
| Lupeol | 3.47 |
| Nonadecane | 5.21 |
| Farnesyl acetate | 9.68 |
| Hexadecanoic acid methyl ester | 5.45 |
| Isopropyl palmitate | 6.23 |
| E-15-Heptadecenal | 5.74 |
| Eicosane | 6.25 |
| Docosane | 3.84 |
| Neophytadiene | 4.36 |
| Tricosane | 3.65 |
| Total | 92.3 |
Table 6: Chemical composition of methanol extract of leaves and stems of wild Alhagi Maurorum.
The percent proportional composition of active compounds separated from peak regions in GC was calculated; the compounds were identified by matching with the search Libraries HMA present in the analytical program.
Through the results indicate the presence of Eugenol increased by 7.28 and Farnesyl acetate increased by 9.68 and Eugenol epoxide increased by 6.82. They are important medicinal compounds with anti-cancer and anti-bacterial applications
Also, 100 grams of root powder were extracted and 200 ml of chloroform with 90% ethanol was added to the soxlet. The heating temperature is set for about 6 hours. The extracts were filtered and concentrated to 2 ml using a rotary evaporator
The plant extract and some of the calibrated materials were analyzed by gas chromatography with a mass spectrometer detector: - - MS
Device from the companyl th 7 7890/5975. Helium carrier gas (He) with a hash ratio of 1: 5 the furnace temperature was 75 degrees Celsius constant for 3minutes and then to 280 degrees Celsius at 3C/min and the detector temperature is 250 degrees Celsius; the carrier gas flow is (0.9mL/min) analytical column HP -5 .and the ionization energy is 70 electron volts. Retention times have been determined for separated materials. The components of the root plant extract were determined by comparing their mass spectra and retention times with the mass spectra of the analysis was carried out according to the following analytical conditions:
Syringe size 1
Flow 0.9 mL / min
Analysis Time 35 minutes
Scanning from 50-500 blocks
Syringe size 1
Flow 0.9 mL / min
Analysis Time 35 minutes
Scanning from 50-500 blocks
| RT | Name of compound | Peak Area % |
| 4.11 | Phenol,2,4-bis (1,1- dimethylethyl)- | 1.24 |
| 4.76 | Levoglucosenone | 4.36 |
| 5.11 | m-Coumaric acid | 3.94 |
| 5.87 | Butanedioc acid, hydroxyl, dimethyl ester | 5.95 |
| 6.21 | 3,7,11,15-Tetramethyl-2- hexadecen-1-ol (Phytol) | 2.25 |
| 6.98 | 2-Propoxy-succinic acid, dimethyl ester | 3.71 |
| 8.76 | 1-Tetradecene | 3.94 |
| 10.27 | Sinapyl alcohol | 2.87 |
| 10.58 | Ledol | 1.92 |
| 11.24 | Phenol, 2,5-bis(1-methylpropyl) | 2.54 |
| 11.79 | Phytol,2-hexadecen-1-ol, 3,7,11,15-tetramethy | 4.85 |
| 12.47 | Hydroquinone | 3.12 |
| 13.95 | 3-O-Methyl-d-glucose | 3.51 |
| 14.77 | Phenol, 4-(methoxymethyl) | 2.32 |
| 18.47 | 6-Hydroxyflavone | 3.65 |
| 23.14 | Stigmasterol | 3.14 |
| 23.87 | Benzene, (1-butylhebtyl)- undecane, 5-phenyl | 3.27 |
| 24.01 | 4?tert?Octyl-o-Cresol | 3.29 |
| 24.93 | Phenol, 2,5-di?tert?butyl | 2.14 |
| 25.24 | Levorphanol | 2.98 |
| 25.89 | Scytalone | 3.18 |
| 26.55 | n-Hexadecanoic acid | 4.65 |
| 27.46 | m-Salicylic acid | 2.52 |
| 28.33 | Apigenin 7-glucoside | 1.16 |
| 29.87 | Tetrahydroxyflavonone -7,3?,4? | 1.21 |
| 32.14 | Farnesol | 2.23 |
| 34.15 | Vanillic acid | 9.53 |
| 36.68 | Hippuric acid, o-hydroxy | 0.43 |
| Total | 99.56 |
Table 7: Shows the chemical composition of the ethanol extract of the roots of the Syrian wild Alhagi Maurorum plant.
Results in Table (7) showed the presence of Levoglucosenone increased by 4.36 and Butanedioc acid 5.95 and Phytol,2-hexadecen increased by 4.85 and Farnesol 2.23 and Vanillic acid increased by 9.53 compounds effective anti-inflammatory, anti-cancer and no medical uses wide .It was also demonstrated the presence of many phenolic compounds by high has the appearance of phenols such as Hydroquinone and Sinapyl alcohol was considered compounds inhibitory to the growth of plant is harmful and it had the effect of clear and effective on many of the herbs are alternate, so you can use this natural product as a vital and "environmentally friendly" to combat weeds.
Alcoholic extract of Alhagi Maurorum yte is used in the treatment of rheumatism [18]. In traditional therapy, the aqueous extract of Alhagi Maurorum yte is used in case of enterocolitis, dyspepsia, gastritis, peptic ulcer and duodenal ulcer, as well as as a diuretic, for respiratory, migraine, and for the treatment of rheumatism [19].
Discussion
The current study aimed to investigate the chemicals found in the leaves, stems and roots of the Alhagi Maurorum plant to determine the medicinal importance of this plant and identified flavonoids, phenols and the exchange capacity of antioxidants. Multiple tests of antioxidant activity have shown that Alhagi Maurorum is a promising source of antioxidants, flavonoids and phenols as well as containing anti-cancer substances such as Phenol, 2,5-di?tert?butyl Stigmasterol Phytol, Farnesol.
Also shown the results of the analysis to the presence of an important group of flavonoids in the roots and in the leaves with the leg in different proportions such as Apigenin: Chrysoeriol Chrysin Luteolin Kaempferol Quercetin and also the existence of a group of phenols is: Caffeic acid, Vanillic acid, Naringin, Syringic Chlorogenic, Coumaric, Ferulic acid, Sinapic acid, Isoqurectrin – Rutin.
The results we obtained confirm that Al-Alhagi Maurorum is a promising plant that must be taken care of and expanded in the separation and isolation of some active compounds found in high proportions, and reliable in medical and therapeutic pharmaceutical uses Since natural products have useful and interesting biological activities in conventional therapy, researchers are gradually turning their attention towards natural products to develop better drugs against diseases, such as cancer or viral and microbial infections [15]. It is considered one of the most important medicinal plants that are used to treat many disorders and spread in several regions around the world, and there are many extraction methods including ethanolic and aqueous methods. These various methods of extraction are useful in identifying more the contents of Alhagi Maurorum than active compounds, but the alcoholic method is more important than the aqueous method because there are more extracted compounds. The extract contains many compounds, the most important of which are flavonoids and phenols because of their many therapeutic properties such as antioxidants and anti-inflammation.
References
- Groombridge, B., and Jenkins. (2000). Global Biodiversity, World Conservation Press. Cambridge, UK.
- Guo, T., Jiao, P. (1995). Hawthorn (Crataegus) resources in China. Hortscience 30: 1131-1134.
- Hobbs, C., Foster, S. (1996). Hawthorn: a literature review. Herbal Gram. 22. pp 29-33.
- Joseph, G., Zhao, Y., Klaus, W. (1995). PharmAlhagi Maurorum ogic action profile of crataegus extract in comparison to epinephrine, amirinone, milrinone and digoxin in the isolated perfused guinea pig heart [in German; English abstract]. Arzneimittelforschung 45: 1261-1265
- I. Popov, and G. Lewin, Oxidants and antioxidants Part B, 300, (1999). 437-456, ed. Lester Packer: Acdemic Press; M. Buhrin, I. Popov, and G. Lewin, Phys.
- Amir Parviz Tavassoli, Majid Anushiravani. (2020). Phytochemistry and therapeutic effects of Alhagi spp and Tarangabin in traditional and modern medicine: a review. Journal of Herbmed PharmAlhagi Maurorum ogy.
- Nabeela Ahmad, Yamin Bibi, Saboon, Iqra Raza. (2015). Traditional uses and pharmAlhagi Maurorum ogical properties of Alhagi maurorum: A review. Asian Pacific Journal of Tropical Disease.
- Mcgill, HC, Jr., Mcmahan, CA, Zieske, AW et al. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological
- Gulzar Muhammad, Muhammad Ajaz Hussain, Alhagi: A Plant Genus Rich in Bioactives for Pharmaceuticals. 2214
- Stanley Davidson. Davidson's Principles and Practice of Medicine
- Journal of Babylon University/Pure and Applied Sciences/ No.(3)/ Vol.(22): (2014).
- D. J. Charles. (2013). Antioxidant Properties of Spices, Herbs and Other Sources, chapter 8 (Anise) 159-164, Springer, New York.
- D. J. Charles, (2013). Antioxidant Properties of Spices, Herbs and Other Sources, chapter 25 (Fennel) 287-293, Springer, New York.
- Y. HYPERLINK "http://www.sciencedirect.com/science/article/pii/S0031942205001755"Gebhardt, S. Witte, G. HYPERLINK "http://www.sciencedirect.com/science/article/pii/S0031942205001755"Forkmann, R.Luka?in, U. HYPERLINK "http://www.sciencedirect.com/science/article/pii/S0031942205001755"Matern, S. Martens, Phytochemistry, 66, 1273–1284, 2005
- E. Besco, E. Braccioli, S. Vertuani, P. Ziosi, F. Brazzo, R. Bruni, G. Sacchetti and S. Manfredini. (2007). Food Chemistry, 102: 1352-1356.
- C. Sanchez-Moreno, Food Sci Tech. Inter., 8, 121-137. (2002).
- N. J. Miller, A.T. Diplock, C. Rice-Evans, M. J. Davis, V. Gopinathan and A. Milner. (1993). Clinical Science, 84: 407-412.
- Weber DJ, Ansari R, Gul B K M (2007). Potential of halo-phytes as source of edible oil. J Arid Env. 68(2): 315–321.
- Nishanbaev S Z, Bobakulov K M, Nigmatullaev A M, Sham I D, Okhundedaev B S and Abdullaev N D. (2016). Volatile compounds from the aerial parts of four Alhagi species growing in Uzbekistan Chem. Nat. Compd. 52: 167–70
- Muhammad G, Hussain M A, Anwar F, Ashraf M and Gilani A-H. (2015). Alhagi: a plant genus rich in bioactives for pharmaceuticals. Phytother. Res. 29: 1–13.
- Al-Snafi AE. (2015). Alhagi maurorum as a potential medicinal herb. an overview. Inte J Pharm Rev Res. 5(2).
- Arunkumar S, Muthuselvam M. (2009). Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci 5(5): 572-576.
- Asmeda AA. (2013). Ecology and phytochemistry of some taxa of family labiatae (lamiaceae). M SC thesis, Fac Sci Mans univ Egy P 145.
- Awaad AS, El-Meligy RM, Qenawy SA, Atta AH and Soliman GA. (2011). Antiinflammatory, antinociceptive and antipyretic effects of some desert plants. J Saudi Chem Soc. 15: 367-73.
- Rochette L., Zeller M., Cottin Y., Vergely C. (2014). Diabetes, oxidative stress and therapeutic strategies. Biochimica et Biophysica Acta—General Subjects. 1840(9): 2709–2729.
Citation: Loai Al Allan. (2024). Determination of Phenols, Flavonoids, Antioxidants, and other Chemical Compounds of Alhagi Maurorum (Camelthorn) plant found in Syria. Journal of Pharmacy and Drug Development 6(1).
Copyright: © 2024 Loai Al Allan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.