Research Article
Volume 5 Issue 1 - 2023
Novel Glycinate N-halamine Siloxane coated Cotton and Cotton/ZnO-NPs Composites and their Antibacterial Activities.
1Department of Chemistry, Al-Azhar University-Gaza, P O Box 1277, Gaza, Palestine.
2Department of Chemistry, College of Science. King Faisal University, AlAhsa, PO Box 380, Hofuf 31982, Saudia Arabia.
2Department of Chemistry, College of Science. King Faisal University, AlAhsa, PO Box 380, Hofuf 31982, Saudia Arabia.
*Corresponding Author: Issa M. El Nahhal, Department of Chemistry, Al-Azhar University-Gaza, P O Box 1277, Gaza, Palestine.
Received: June 11, 2023; Published: June 28, 2023
Abstract
Nanotechnology has great potential to enhance disinfection and decontamination efficiency. It increases antibacterial characteristics and guard against bacterial cross-infection by coating cotton fabrics and medical clothes and other hygiene's with antibacterial agents eg N-halamine glycinate siloxane with or without ZnO-NPs. This was achieved by Pad-dry-cur method through tethering the surface of cotton or cotton/ZnO-NPs fibers with glycinate silane agent of formula (MeO)3Si(CH2)3NHCH2COOMe through covalent ether linkages. Two different glycinate siloxane coated cotton materials: Gly-sil.@cotton or Gly-sil.@cotton/ZnO-NPs materials were obtained (where Gly = glycinate and sil. = siloxane). Their corresponding N-halamine glycinate siloxane coated cotton materials Gly-Cl-sil.@cotton or Gly-Cl-sil.@cotton/ZnO-NPs were then obtained by exposure the parent glycinate siloxane coated materials with dilute sodium hypochlorite solutions. The antibacterial activity of N-halamine glycinate siloxane coated cotton materials have 100% sterilized fecal coliform bacteria, mostly Escherichia coli, and Pseudomonas aeruginosa. It has been concluded that these products have experienced good stability and reproducibility after five times usability. The Gly-Cl-sil.@cotton/ZnO-NPs material which bearing two effective antimicrobial agents (ZnO-NPs along N-halamine glycinate) has very effective antimicrobial activity. In this study, FTIR, TGA, XRD, and SEM techniques were used in this research to elucidate chemical structural properties of the coated materials.
Keywords: Impregnated cotton; N-halamine glycinate; coated cotton/ZnO-NPs; Antibacterial activity; Nanocomposite; Coated cotton; Siloxane; Bacteria
Introduction
Cotton's great absorption ability allows it to easily absorb sweat, metabolites, and sebum from the skin's sweat/sebaceous glands [1,2]. These secretions, on the other hand, could be harmful, provide appropriate environment for microorganisms like bacteria and fungus to proliferate and growth [3]. As a result, under some conditions, these microorganisms can grow quickly and constitute a threat to the public's health. Antibacterial modification of material surfaces is one of the most commonly used strategies for de-contamination control and remove treats. Therefore, the introduction of an effective antimicrobial agent onto the cotton fabrics is of great importance for eradicating bacteria and avoiding major infections [4]. Various antibacterial agents such as chitosan [5], nanometals [6-8] nanometal oxides [9-12], quaternary ammonium salts [13,14], guanidine polymers [15] and N-halamine [16-22] have been used in antibacterial finishing for cotton fabrics. Although, metals or metal oxides nanoparticles coated cotton systems [10,11] are physically coated onto cotton fibers by different means e.g. ultrasound irradiation. Other antimicrobial silane agents functionalized mesoporous silica are chemically introduced onto the surface cotton fibers through Cur-Pad-Dry method [20]. Therefore, polycondensation of biocidal functional silane groups or biocidal functional mesoporous silica hydroxyl groups may involve both the hydroxyl groups of cotton as well as crosslinked with other biocidal silane or silica functional groups on surface to form crosslinked polymeric silicon coating [20,23-26]. To solve the limitation of a single functionality or to combine several different types of functionalities is a method used to lessen the shortcomings of each one separately. Recent research has demonstrated that an N-halamine and cationic salt can work together to maximize their benefits and create synergism/higher biocidal effectiveness [27–32]. Although the cationic salt cannot kill bacteria efficiently, its highly water solubility increase the hydrophilicity and its positive center attracts anionic bacteria to the vicinal N-halamine site. Therefore, the resultant effect of the cationic salt in such a combination is to promote the contact between N-halamine and bacteria, leading to the acceleration of the killing process. Also, QAS kills bacteria without depletion so the combined structure still has certain biocidal ability even after all N-halamine sites are consumed. Since the major antibacterial contributor in this type of combined structures is N-halamine, it is hypothesized that increasing the number of N-halamine can achieve even higher biocidability than the basic combination of one cationic center and one N-halamine. However, the research of this area is still in progress.
For instance, ZnO-NPs as one antimicrobial functionality was coated onto cotton fabrics [10,11]. N-halamines has complementary properties, its combination with ZnO-NPs is hypothesized to have good synergism. Due to few numbers of silane coupling agents bearing N-halamines are known, we have had constructed a new silane coupling agent bearing amino acid functionality group by modification of 3-(2-aminoethylaminopropyl) trimethoxysilane with monochloromethylacetate, then immobilized it onto the surface of cotton or cotton/ZnO substrate. This combined coated system contains two different functionalities, one is glycinate N-halamine and the other functionality is ZnO-NPs and each of them has effective antimicrobial activity. The N-halamine glycinate coated cotton materials were obtained through chlorination of the corresponding materials with diluted sodium hypochlorite solution. The presence of functionalized N-halamine glycinate system onto the surface of cotton coated ZnO-NPs would increase its antimicrobial effectiveness due to presence of two different active centers (N- halamine glycinate and ZnO-NPs) and also would further increasing of hydrophilicity of the system.
We further hypothesized that the combination of ZnO-NPs along with N-halamine glycinate can achieve a higher antibacterial efficacy than one counterpart of ZnO-NPs or N-halamine glycinate.
In our previous reports we have focused our research efforts on employing cotton that has had concentrated onto metal oxides coated cotton and using starched cotton to operate as antibacterial systems [33,34], where effective antimicrobial activities have been noted. In this research, we have used a combined-system of the presence of metal oxides coated cotton along with N-halamine glycinate silane functional group, where the glycinate silane was coated onto the surface of cotton or cotton/ZnO fibers by Cur-Pad-Dry method obtaining an organic–inorganic hybrid precursors. For this purpose, we have prepared a system with glycinate coated cotton and the other system is glycinate coated cotton/ZnO-NPs. This new system was chlorinated using sodium hypochlorite before tested as antimicrobial activities. Variety of several techniques such as Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy X-Ray spectrometer (SEM) were used for elucidation of chemical structures of the materials.
Experimental
Materials and reagents
Ethylmonochloroacetate (ClCH2COOEt), (99%), 3-(aminopropyl) triethoxysilane, (97%), zinc acetate dihydrate Zn(CH3COO)2.2H2O,(95.5%) and sodium hydroxide (NaOH) were purchased from Merck and used without further purifications. Dehydrated nutrient agar powder and nutrient broth powder were purchased from Difco.
Ethylmonochloroacetate (ClCH2COOEt), (99%), 3-(aminopropyl) triethoxysilane, (97%), zinc acetate dihydrate Zn(CH3COO)2.2H2O,(95.5%) and sodium hydroxide (NaOH) were purchased from Merck and used without further purifications. Dehydrated nutrient agar powder and nutrient broth powder were purchased from Difco.
Methods
Infrared spectra for the materials were recorded on Shimadzu FTIR Tracer 100 spectrophotometer using KBr in the range 4000–400 cm-1. Thermogravimetric analysis (TGA) was carried out using Mettler Toledo TGA/SDTA 851e analyzer in the range of 25–600ºC of heat rate of 10º C/min. Series II/CHN-S 2400. Scanning electron microscopy (SEM) were obtained using Carl Zeiss AG - EVO® 60). X-ray diffraction (XRD) using EQ uniox 3000, INEL, France.
Infrared spectra for the materials were recorded on Shimadzu FTIR Tracer 100 spectrophotometer using KBr in the range 4000–400 cm-1. Thermogravimetric analysis (TGA) was carried out using Mettler Toledo TGA/SDTA 851e analyzer in the range of 25–600ºC of heat rate of 10º C/min. Series II/CHN-S 2400. Scanning electron microscopy (SEM) were obtained using Carl Zeiss AG - EVO® 60). X-ray diffraction (XRD) using EQ uniox 3000, INEL, France.
Preparation of 3-glycinatepropyltriethoxysilane (Gly-S)
The functionalized 3-glycinatepropyltrimethoxysilane (Gly-S) was prepared in a similar way as previously reported [35-37] by refluxing 0.035 mol of (3-aminopropyl) triethoxysilane with 0.035 mol of ethylchloroacetate in 50 mL ethanol at 100ºC for 12h. The functionalized 3-glycinatepropyltrimethoxysilane (Gly-S) was obtained under vacuum by evaporation of ethanol using rotary evaporator at 60ºC. The new silane agent (Gly-S) was identified using FTIR spectroscopy.
The functionalized 3-glycinatepropyltrimethoxysilane (Gly-S) was prepared in a similar way as previously reported [35-37] by refluxing 0.035 mol of (3-aminopropyl) triethoxysilane with 0.035 mol of ethylchloroacetate in 50 mL ethanol at 100ºC for 12h. The functionalized 3-glycinatepropyltrimethoxysilane (Gly-S) was obtained under vacuum by evaporation of ethanol using rotary evaporator at 60ºC. The new silane agent (Gly-S) was identified using FTIR spectroscopy.
Preparation of coated-cotton/ZnO-NPs.
As previously described [10, 11, 33, 34], ZnO-NPs coated cotton was made by treating 1.00g of cotton with zinc (II) acetate dihydrate (0.01 mol) in the presence of NaOH (0.02 mol) as a base in 100 mL deionized water. To make sure that cotton fibers were uniformly coated with ZnO-NPs, the combination was irradiated using an Ultrasnicator (Model US-150 Ti-horn, 20 kHz, output 10 Turning 7 for 60 minutes). At the same time, the cotton sample was rotated at a speed of 250 rps. Following three separate washes with 20 mL of distilled water to remove the unbound ZnO-NPs, the cotton material coated with ZnO-NPs was dried at 80°C overnight. Cotton/ZnO-NPs is the label on the coated material.
As previously described [10, 11, 33, 34], ZnO-NPs coated cotton was made by treating 1.00g of cotton with zinc (II) acetate dihydrate (0.01 mol) in the presence of NaOH (0.02 mol) as a base in 100 mL deionized water. To make sure that cotton fibers were uniformly coated with ZnO-NPs, the combination was irradiated using an Ultrasnicator (Model US-150 Ti-horn, 20 kHz, output 10 Turning 7 for 60 minutes). At the same time, the cotton sample was rotated at a speed of 250 rps. Following three separate washes with 20 mL of distilled water to remove the unbound ZnO-NPs, the cotton material coated with ZnO-NPs was dried at 80°C overnight. Cotton/ZnO-NPs is the label on the coated material.
(a) Preparation of glycinate siloxane coated-cotton (Gly-sil.@cotton) or glycinate siloxane coated-cotton/ZnO (Gly-sil.@cotton/ZnO-NPs).
3-glycinatepropyltrimethoxysilane (Gly-S) was firstly hydrolyzed in ethanol/water mixture (10/1). The glycinate siloxane coated-cotton (Gly-sil.@cotton) or glycinate siloxane coated-cotton/ZnO (Gly-sil.@cotton/ZnO-NPs) was them obtained by immersed 0.20 g of cotton or coated cotton/ZnO-NPs samples into hydrolyzed glycinate silane solution. The glycinate silane was coated onto the surface of pristine cotton and cotton/ZnO-NPs by Cur-Pad-Dry method [18,20]. Two glycinate siloxane coated-cotton (Gly-sil.@cotton) and glycinate siloxane coated-cotton/ZnO (Gly-sil.@cotton/ZnO-NPs) were obtained.
3-glycinatepropyltrimethoxysilane (Gly-S) was firstly hydrolyzed in ethanol/water mixture (10/1). The glycinate siloxane coated-cotton (Gly-sil.@cotton) or glycinate siloxane coated-cotton/ZnO (Gly-sil.@cotton/ZnO-NPs) was them obtained by immersed 0.20 g of cotton or coated cotton/ZnO-NPs samples into hydrolyzed glycinate silane solution. The glycinate silane was coated onto the surface of pristine cotton and cotton/ZnO-NPs by Cur-Pad-Dry method [18,20]. Two glycinate siloxane coated-cotton (Gly-sil.@cotton) and glycinate siloxane coated-cotton/ZnO (Gly-sil.@cotton/ZnO-NPs) were obtained.
(b) Preparation of glycinate N-halamine coated cotton (Gly-Cl-sil.@cotton) and coated cotton/ZnO-NPs (Gly-Cl-sil.@cotton/ZnO-NPs)
The glycinate N-halamines coated cotton (G-Cl-sil.@cotton) and the glycinate coated cotton-ZnO (G-Cl-sil.@cotton/ZnO-NPs) materials were prepared by chlorination of glycinate coated cotton(G-sil.@cotton) or glycinate coated cotton/ZnO-NPs materials by washing them with dilute (10% NaClO, pH = 7) at room temperature for 1h, while being constantly shaken the mixture in order to produce the chlorinated (G-Cl-sil.@cotton) and (G-Cl-sil.@cotton/ZnO-NPs) fabrics. The chlorinated materials were then separated, repeatedly washed with deionized water to eliminate free chlorine from the surface of cotton materials and dried at 50ºC for 5h. The loaded chlorine content (mmol Cl/g) was calculated by iodometric/thiosulfate titration [18,20].
The glycinate N-halamines coated cotton (G-Cl-sil.@cotton) and the glycinate coated cotton-ZnO (G-Cl-sil.@cotton/ZnO-NPs) materials were prepared by chlorination of glycinate coated cotton(G-sil.@cotton) or glycinate coated cotton/ZnO-NPs materials by washing them with dilute (10% NaClO, pH = 7) at room temperature for 1h, while being constantly shaken the mixture in order to produce the chlorinated (G-Cl-sil.@cotton) and (G-Cl-sil.@cotton/ZnO-NPs) fabrics. The chlorinated materials were then separated, repeatedly washed with deionized water to eliminate free chlorine from the surface of cotton materials and dried at 50ºC for 5h. The loaded chlorine content (mmol Cl/g) was calculated by iodometric/thiosulfate titration [18,20].
Antibacterial Analysis.
The antibacterial analysis of fecal coliforms, where the major species is Escherichia coli, in addition to Pseudomonas aeruginosa were selected as indicator organisms for the effectiveness of the materials in this study to eliminate these types of bacteria from waste water samples.
The antibacterial analysis of fecal coliforms, where the major species is Escherichia coli, in addition to Pseudomonas aeruginosa were selected as indicator organisms for the effectiveness of the materials in this study to eliminate these types of bacteria from waste water samples.
To ensure the quality of the results all materials had sterilized in an autoclave and all benches had sterilized by ethyl alcohol 73% before the experiments were carried out. The contaminated water sample with the certain bacteria were prepared by mixing 2.5ml of effluent wastewater with 500 ml sterilized distilled water. m-faekal Coliform (mFC) agar and MacConkey agar were used to grow fecal coliforms, including E. coli, and Pseudomonas aeruginosa, respectively. A membrane filtration method was used to account of fecal coliform colonies. The experiment was conducted by mixing 0.25g of each Gly-Cl-Sil.@cotton, Gly-Cl-Sil.@cotton/ZnO, or Gly-Sil.@cotton only (control) with 50ml polluted sample. Each sample was loaded on a syringe with a Nitrocellulose membrane with pore size of 0.45 ?m. The flow rate was adjusted to 6min/10ml and then the membrane was transferred to agar plates to be incubated at 40–44°C for 24 hours. The inhibition rate (Y) in percentage was calculated by the equation:
Y = Wt-Qt × 100
Wt
Y = Wt-Qt × 100
Wt
where, Wt. is the number of bacterial colonies in the control sample; and Qt is the number of colonies of samples treated with Gly-Cl-Sil.@cotton, Gly-Cl-Sil.@cotton/ZnO, or Gly-Sil.@cotton only materials.
Oxidase test was used to check that some of the colonies which grown on MacConkey medium are Pseudomonas aeruginosa colonies. A random colony was picked up from the incubated MacConkey agar plate. Then, it placed on a filter paper disk infused with oxidative reagent (1% N, N, N, N-tetramethyl-p-phenylenediamine dihydrochloride). If blue color develops when checking the colony with oxidase tablets that means it is a Pseudomonas colony.
Results and Discussion
Preparation
The impregnation process for both cotton materials: the free cotton and coated cotton/ZnO includes the following steps (Figure 1):
The impregnation process for both cotton materials: the free cotton and coated cotton/ZnO includes the following steps (Figure 1):
- Functionalization of 3-(aminopropyl) triethoxysilane with ethylmonochloroacetate in a 1:1 molar ratio results of the formation of 3-glycinatepropyltriethoxysilane. That was evident from the FTIR spectrum (Figure 1) [35].
- Coating of cotton or cotton/ZnO-NPs with hydrolyzed 3- glycinatepropyltriethoxysilane using Cur-Pad-Dry method [20]. Therefore, polycondensation of biocidal functional silane groups with the hydroxyl groups of the surface of cotton fibers to form crosslinked siloxane coating Gly-sil.@cotton or Gly-sil.@cotton-ZnO -NPs materials [20] (Figure 1).
- The N-halamines glycinate coated cotton (Gly-Cl-sil.@cotton) or glycinate coated cotton/ZnO-NPs (G-Cl-sil.@cotton/ZnO-NPs) were obtained by treatment the glycinate siloxane coated cotton or coated cotton/ZnO-NPs with sodium hypochlorite solution at PH 7(Figure 1).
a) Synthesis Step
(EtO)3Si-(CH2)3-NH2 + Cl-CH2CO2C2H5 →
(EtO)3Si-(CH2)3-NH-CH2CO2C2H5+ HCl
(EtO)3Si-(CH2)3-NH-CH2CO2C2H5+ HCl
b) Cur-Pad-Dry method
(EtO)3Si-(CH2)3-NH-CH2CO2C2H5 + EtOH/H2O + cotton or cotton-ZnO-NPs
↓
↓
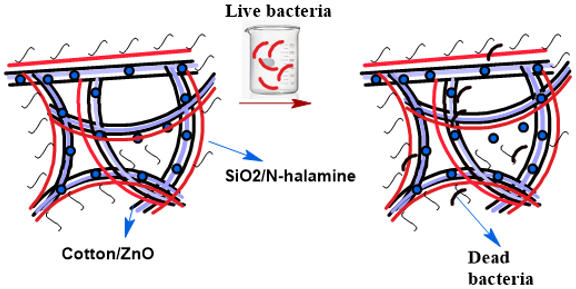
Figure 1: Coating and chlorination of functionalized glycinate siloxane coated cotton and cotton/ZnO-NPs.
FTIR Spectra
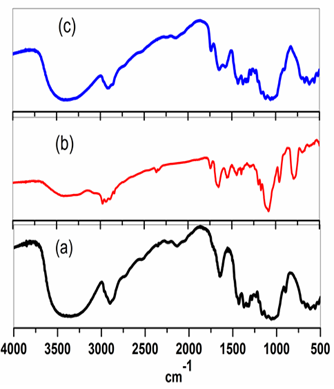
The FTIR spectra of free cotton, coated glycinate/cotton and coated glycinate/cotton/ZnO-NPs are depicted in Figure 2 (a-c). The IR spectra of these materials showed three regions of absorption. The first region is at 3500–3000 cm-1, the peaks in this region were assigned due to ?(O-H) and ?(N-H). The second region is at 1750–1600 cm-1, the peak at 1750 cm-1 is due to ?(CO), whereas the peak is due to 1670 cm-1 due to s(N-H), which is slightly shifted to lower frequency at ca. 1660 cm-1 upon chlorination with sodium hypochlorite, indicating the conversion of N-H to N-Cl [18-20,35-37]. The peak at 1620 cm-1 is assigned due to s(O-H). The third region of absorptions are assigned at 1200–800 cm-1 are due to ?(Si-O) symmetrical and asymmetrical vibrations stretching and bending vibrations, these assignments were based on published literature data of similar systems [35-37]. The shoulder at 960 cm-1 is due to single silanol vibration ?(Si-OH). Peaks around 1489 cm-1 and 2928 cm-1 ascribed to C-H was enhanced after modification of cotton with glycinate silane. FTIR spectra showed two bands at ca. 1750 cm-1 and 1670 cm-1 which assigned to the carbonyl and amine bands (C=O & N-H) of the methyl glycinate structure moieties.
The FTIR spectra of free cotton, coated glycinate/cotton and coated glycinate/cotton/ZnO-NPs are depicted in Figure 2 (a-c). The IR spectra of these materials showed three regions of absorption. The first region is at 3500–3000 cm-1, the peaks in this region were assigned due to ?(O-H) and ?(N-H). The second region is at 1750–1600 cm-1, the peak at 1750 cm-1 is due to ?(CO), whereas the peak is due to 1670 cm-1 due to s(N-H), which is slightly shifted to lower frequency at ca. 1660 cm-1 upon chlorination with sodium hypochlorite, indicating the conversion of N-H to N-Cl [18-20,35-37]. The peak at 1620 cm-1 is assigned due to s(O-H). The third region of absorptions are assigned at 1200–800 cm-1 are due to ?(Si-O) symmetrical and asymmetrical vibrations stretching and bending vibrations, these assignments were based on published literature data of similar systems [35-37]. The shoulder at 960 cm-1 is due to single silanol vibration ?(Si-OH). Peaks around 1489 cm-1 and 2928 cm-1 ascribed to C-H was enhanced after modification of cotton with glycinate silane. FTIR spectra showed two bands at ca. 1750 cm-1 and 1670 cm-1 which assigned to the carbonyl and amine bands (C=O & N-H) of the methyl glycinate structure moieties.
Thermal Analysis
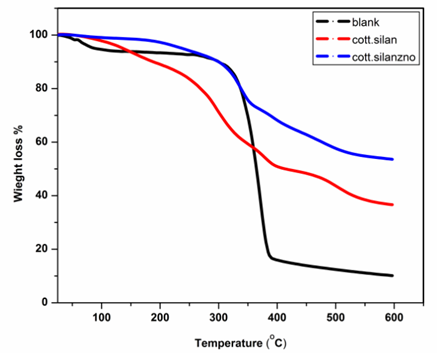
Thermogravimetric analysis (TGA) for blank cotton, glycinate coated cotton (Gly-Sil.@cotton) and glycinate coated cotton-ZnO (Gly-Sil.@cotton/ZnO-NPs) were examined under nitrogen atmosphere at 20–600ºC at a rate of 10ºC/minute. Thermogravimetric analysis (TGA) for blank cotton, Gly-Sil.@cotton and Gly-Sil.@cotton/ZnO-NPs after 5 washing cycles are presented in (Figure 3(a-c)). The total weight loss was found 89%, 62% and 45%, respectively. This reveals that around 27% of glycinate functional group was adsorbed onto cotton fabrics and that about 17% ZnO-NPs were coated onto the cotton fiber (Figure 3 (a-c)) [10,33,35].
Thermogravimetric analysis (TGA) for blank cotton, glycinate coated cotton (Gly-Sil.@cotton) and glycinate coated cotton-ZnO (Gly-Sil.@cotton/ZnO-NPs) were examined under nitrogen atmosphere at 20–600ºC at a rate of 10ºC/minute. Thermogravimetric analysis (TGA) for blank cotton, Gly-Sil.@cotton and Gly-Sil.@cotton/ZnO-NPs after 5 washing cycles are presented in (Figure 3(a-c)). The total weight loss was found 89%, 62% and 45%, respectively. This reveals that around 27% of glycinate functional group was adsorbed onto cotton fabrics and that about 17% ZnO-NPs were coated onto the cotton fiber (Figure 3 (a-c)) [10,33,35].
XRD Analysis
Figure 4 (a & b) displays XRD patterns for cotton/ZnO-NPs and Gly-Sil@cotton/ZnO-NPs, respectively. The diffraction patterns of ZnO-NPs in the two materials revealed that ZnO-NPs are present in crystalline form. The major peaks at 2? =31.3º, 35.1º, and 36.5º, 47.2º, 56.5º, and 62.8º are indexed with presence of diffraction planes (100), (002), (101), (102), (110) and (103), respectively. These diffraction planes are well indexed with PDF file (JCPDS 5-664) of hexagonal wurtzite structure [10,11]. The low intensity of diffraction pattern peaks in case of G-Sil/cotton/ZnO-NPs is probably due coating of cotton with G-Sil. These results were remarkably similar to those that had previously been published [10,11]. The diffraction peaks that were seen below 30o are typical of pristine cotton.
Figure 4 (a & b) displays XRD patterns for cotton/ZnO-NPs and Gly-Sil@cotton/ZnO-NPs, respectively. The diffraction patterns of ZnO-NPs in the two materials revealed that ZnO-NPs are present in crystalline form. The major peaks at 2? =31.3º, 35.1º, and 36.5º, 47.2º, 56.5º, and 62.8º are indexed with presence of diffraction planes (100), (002), (101), (102), (110) and (103), respectively. These diffraction planes are well indexed with PDF file (JCPDS 5-664) of hexagonal wurtzite structure [10,11]. The low intensity of diffraction pattern peaks in case of G-Sil/cotton/ZnO-NPs is probably due coating of cotton with G-Sil. These results were remarkably similar to those that had previously been published [10,11]. The diffraction peaks that were seen below 30o are typical of pristine cotton.
Scanning electron microscopic (SEM).
The SEM analysis of Gly-Sil.@cotton and Gly-Sil.@cotton/ZnO-NPs) is presented in Figure 5 (images a,b). SEM image of glycinate siloxane coated cotton (Figure 5, image a) showed that a layer of glycinate siloxane material coated blank cotton fibers. The SEM image of glycinate siloxane coated cotton/ZnO-NPs (Figure 5, image b) showed a layer of glycinate siloxane material coated cotton/ZnO-NPs composite to form Gly-sil.@cotton/ZnO-NPs, which is fully covered all cotton surface fibers, The agglomerates of glycinate/siloxane-ZnO-NPs were dense and compact.
The SEM analysis of Gly-Sil.@cotton and Gly-Sil.@cotton/ZnO-NPs) is presented in Figure 5 (images a,b). SEM image of glycinate siloxane coated cotton (Figure 5, image a) showed that a layer of glycinate siloxane material coated blank cotton fibers. The SEM image of glycinate siloxane coated cotton/ZnO-NPs (Figure 5, image b) showed a layer of glycinate siloxane material coated cotton/ZnO-NPs composite to form Gly-sil.@cotton/ZnO-NPs, which is fully covered all cotton surface fibers, The agglomerates of glycinate/siloxane-ZnO-NPs were dense and compact.
Chlorination Results
The Gly-Sil.-Cl@Cotton, and Gly-Sil.-Cl@cotton/ZnO materials were obtained by treatment the corresponding materials Gly-Sil.@cotton, and Gly-Sil.@cotton/ZnO with sodium hypochlorite solution (1.5% NaClO, pH = 10) at room temperature for 0.5h). The chlorinated materials were filtered and repeatedly washed with deionized water and dried at 50ºC for 1h. The loaded chlorine contents (mmol Cl/g) were measured by iodometric/thiosulfate titration [18-20]. The results are listed in Table 1. The chlorination results of Gly-Cl-Sil.@cotton is the same as that of the corresponding Gly-Cl-Sil.@cotton/ZnO.
The Gly-Sil.-Cl@Cotton, and Gly-Sil.-Cl@cotton/ZnO materials were obtained by treatment the corresponding materials Gly-Sil.@cotton, and Gly-Sil.@cotton/ZnO with sodium hypochlorite solution (1.5% NaClO, pH = 10) at room temperature for 0.5h). The chlorinated materials were filtered and repeatedly washed with deionized water and dried at 50ºC for 1h. The loaded chlorine contents (mmol Cl/g) were measured by iodometric/thiosulfate titration [18-20]. The results are listed in Table 1. The chlorination results of Gly-Cl-Sil.@cotton is the same as that of the corresponding Gly-Cl-Sil.@cotton/ZnO.
| N-halamines | mmol Cl /g |
| Gly-Cl-Sil. @cotton | 3.3 |
| Gly-Cl-Sil. @cotton/ZnO | 3.2 |
Table 1: Chlorine content (mmol Cl/g) of G-Cl-Sil. @cotton and G-Cl-Sil.@cotton/ZnO
Stability and Reproducibility
To ensure stability of the materials GlyCl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO after being used for inhibition, the used material was washed thoroughly with 50 mL distilled water for 5 min before chlorination. Despite that the process was repeated 5 times, same results of inhibition percentage (%) for Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO are almost unchanged Table 2. This indicates that Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials are stable after used for 5 times.
To ensure stability of the materials GlyCl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO after being used for inhibition, the used material was washed thoroughly with 50 mL distilled water for 5 min before chlorination. Despite that the process was repeated 5 times, same results of inhibition percentage (%) for Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO are almost unchanged Table 2. This indicates that Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials are stable after used for 5 times.
| Times of chlorination | The inhibition rate% for Gly-Cl-Sil.@cotton or Gly-Cl-Sil.@cotton/ZnO materials |
| 1st | 100 |
| 2nd | 100 |
| 3rd | 100 |
| 4th | 100 |
| 5th | 97 |
Table 2: The stability tests of Gly-Sil-Cl. @cotton or Gly-Sil.-Cl@Cotton/ZnO materials.
Antibacterial tests
The antibacterial tests of Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials for polluted water samples against fecal coliform, mostly E. coli, are given in Table 3 and Figure 6. The results showed approximately 100% removal of fecal coliformand E. coli from both water samples treated with Gly-Cl-Sil.@cotton (Figure 6a) and treated with Gly-Cl-Sil.@cotton/ZnO (Figure 6b). The control sample of parent material Gly-Sil.@cotton (Figure 6c) showed that growth of fecal coliform (blue colonies) and they were not affected by Gly-Sil.@cotton. The result indicates that both materials, Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO, have strong disinfection effect on fecal coliformand E. coli.
The antibacterial tests of Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials for polluted water samples against fecal coliform, mostly E. coli, are given in Table 3 and Figure 6. The results showed approximately 100% removal of fecal coliformand E. coli from both water samples treated with Gly-Cl-Sil.@cotton (Figure 6a) and treated with Gly-Cl-Sil.@cotton/ZnO (Figure 6b). The control sample of parent material Gly-Sil.@cotton (Figure 6c) showed that growth of fecal coliform (blue colonies) and they were not affected by Gly-Sil.@cotton. The result indicates that both materials, Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO, have strong disinfection effect on fecal coliformand E. coli.
The antimicrobial effect of Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials was also examinedon Pseudomonas aeruginosa by using McConkey agar (Figure 7). Samples treated by Gly-Cl-Sil.@cotton (Figure 7a) and treated with Gly-Cl-Sil.@cotton/ZnO (Figure 7b) were shown complete elimination of Pseudomonas aeruginosa. The sample in Figure 7c represents the control sample of contaminated water with only Gly-Sil.@cotton. The result shows growth of bacteria colonies on the control plate.
Oxidase test was conducted to validate the presence of Pseudomonasaeruginosa bacteria on the McConkey agar samples. The result shows a development of blue color when colonies were checked by oxidase test (Figure 8).
Synthesized Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials were shown the ability to inhibit growth of fecal coliform, mostly E. coli, and Pseudomonas aeruginosa and have potential to be used as antibacterial agents.
| N-halamine | The inhibition rate % |
| Gly-Cl-Sil. @cotton | 100 |
| Gly-Cl-Sil. @cotton/ZnO | 100 |
Table 1: Inhibition rate (%) of Gly-Cl-Sil.@cotton and Gly-Cl-Sil.@cotton/ZnO materials.
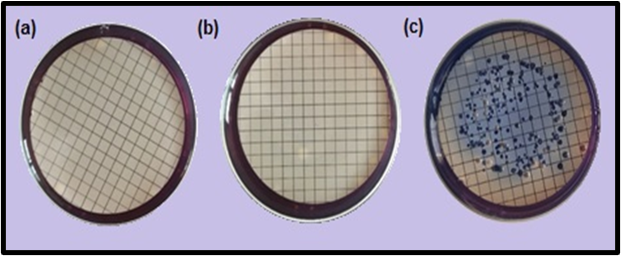
Figure 6: Antimicrobial tests of, (a) Gly-Cl-Sil.@cotton sample (b) Gly-Cl-Sil.@cotton/ZnO sample (c) control sample only Gly-Sil.@cotton.
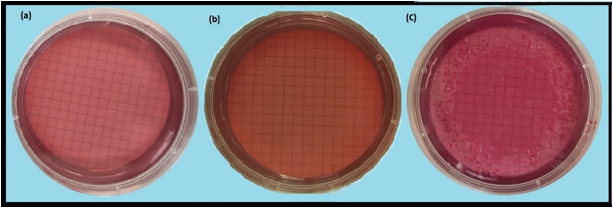
Figure 7: Antimicrobial test of (a) Gly-Cl-Sil.@cotton sample (b) Gly-Cl-Sil.@cotton/ZnO sample (c) Control sample only Gly-Sil.@cotton.
Conclusions
Two functionalities, the N-halamine glycinate and ZnO-NPs were successfully impregnated onto the surface of cotton fibers. The functionalized 3-glycinepropyltrimethoxysilane was prepared by reflux of 3-(aminopropyl) triethoxysilane (APTES) with ethylmonochloroacetate and subsequently coated onto cotton or cotton/ZnO -NPs fibers via Cur-Pad-Dry method. N-halamine glycinate coated cotton or coated cotton/ZnO-NPs were obtained by treatment 3-glycinate-functionalized silane coated cotton or cotton/ZnO-NPs with diluted sodium hypochlorite. TGA results functionalized materials compared with that of free cotton, reveals that 14% weight percent of glycinate and 11 % weight of ZnO-NPs were coated onto cotton fabrics. The antibacterial activity against fecal coliform, mostly E. coli, and Pseudomonas aeruginosa for both coated cotton and cotton/ZnO-NPs samples were showing promising result as disinfection materials.In this study, the cotton substrate was coated with two different antibacterial coatings on its surface, N-halamine-glycinate and ZnO-NPs cites. The presence of the two different centers could increasing antibacterial activity could helped to prevent the problem of antibacterial activity failure. This is based on the presence of a synergistic antibacterial strategy of using two antimicrobial agents.
Acknowledgement
The authors would like to express their sincere thanks for the Chemistry Department of Al Azhar University of Gaza for their support for this research.
The authors would like to express their sincere thanks for the Chemistry Department of Al Azhar University of Gaza for their support for this research.
References
- Zhang, M.; Wang, C.; Wang, H.; Jian, M.; Hao, X.; Zhang, Y. (2017). Carbonized Cotton Fabric for High-Performance Wearable Strain Sensors. Adv. Funct. Mater. 27, 1604795.
- Sandin, G.; Peters, G.M. (2018). Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 184, 353–365. Int.J. Mol. Sci. 21, 6531 13 of 14.
- Gao, Y.; Cranston, R. (2008). Recent advances in antimicrobial treatments of textiles. Text. Res. J. 78, 60–72.
- Yang, L.; Zhan, C.D.; Huang, X.Y.; Hong, L.Z.; Fang, L.M.; Wang,W.; Su, J.Y. (2020). Durable Antibacterial Cotton Fabrics Based on Natural Borneol-Derived Anti-MRSA Agents. Adv. Healthc. Mater. 9, e2000186.
- Liu, Y.; Li, J.; Cheng, X.L.; Ren, X.H.; Huang, T.S. (2015). Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J. Mater. Chem. B, 3, 1446–1454.
- Xu, Q. et al. (2018). Preparation of copper nanoparticles coated cotton fabrics with durable antibacterial properties. Fibers and Polymers 19, 1004–1013.
- Xu, Q., Wu, Y., Zhang, Y., Fu, F. & Liu, X. (2016). Durable antibacterial cotton modifed by silver nanoparticles and chitosan derivative binder. Fibers and Polymers 17, 1782–1789.
- Li, Z. et al. (2017). Te room temperature electron reduction for the preparation of silver nanoparticles on cotton with high antimicrobial activity. Carbohydrate Polymers 161, 270–276.
- Abramov, O. V. et al. (2009). Pilot scale sonochemical coating of nanoparticles onto textile to produce biocidal fabrics. Surf Coat Technol. 204, 718–722.
- El-Nahhal, I. M. et al. (2013). Nano-structured zinc oxide–cotton fibers: synthesis, characterization and applications. J. Mater. Sci.: Mater. 24, 3970–3975.
- El-Nahhal, I. M. et al. (2012). Nanostructured copper oxide-cotton fibers: synthesis, characterization, and applications. Int. Nano Lett. 2, 1–5.
- Perelshtein, I. et al. (2009). CuO-cotton nanoparticles: formation, morphology and antibacterial activity. Surf Coat Technol. 204, 54–57.
- Mikhailovskaya, A.P. (2018). Factors Determining the E_ectiveness of Dyeing Fiber Materials Using QuaternaryAmmonium Salts. Fibre Chem. 50, 188–192.
- Kang, J.K.; Lee, S.C.; Kim, S.B. (2019). Synthesis of quaternary ammonium functionalized silica gel through grafting of dimethyl dodecyl 3-(trimethoxysilyl) propyl ammonium chloride for nitrate removal in batch and column studies. J. Taiwan Inst. Chem. Eng. 102, 153–162.
- Musaev, Y.I.; Khashirova, S.Y.; Musaeva, E.B.; Kirzhinova, I.K. New Nanocomposites: Cotton-Cellulose-Ketimines Based on Guanidine and Aminoguanidine. Fibre Chem. 2018, 50, 60–63.
- Dong, A., et al. (2011). "Synthesis of N-halamine-functionalized silica–polymer core–shell nanoparticles and their enhanced antibacterial activity." Nanotechnology 22(29): 295602.
- Liu, C., et al. (2018). "Novel inorganic-based N-halamine nanofibrous membranes as highly effective antibacterial agent for water disinfection." ACS applied materials & interfaces 10(51): 44209-44215.
- Wang, Y., et al. (2020). "N-halamine modified mesoporous silica coated cotton as multipurpose protective fibrous matercials." Cellulose 27(17): 10461-10471.
- Chen, Y., Ma, Y., He, Q., Han, Q., Z hang, Q., Chen, Q. (2019). Construction of pyridinium/N-chloramine polysiloxane on cellulose for synergistic biocidal application. Cellulose 26: 5033-5049.
- Chen, Y., Wang, Y., Feng, C., He, Q., Chen, Q., Wang, Z., Han, Q. (2020). Novel quat/di-N-halamines silane unit with enhanced synergism polymerized on cellulose for development of superior biocidability. Int. J. Biol. Macromol. 154: 173-181.
- Cheng, X., Li, R., Du, J., Sheng, J., Ma, K., Ren, X., Huang, T.-S. (2015). Antimicrobial activity of hydrophobic cotton coated with N-halamine. Polym. Adv. Technol. 26: 99-103.
- Dong, A., Xue, M., Lan, S., Wang, Q., Zhao, Y., Wang, Y., Zhang, Y., Gao, G., Liu, F.,Harnoode, C. (2014). Bactericidal evaluation of N-halamine-functionalized silica nanoparticles based on barbituric acid. Colloids Surf., B 113: 450-457.
- Ren, X., Kou, L., Liang, J., Worley, S.D., Tzou, Y.-M., Huang, T. (2008). Antimicrobial efficacy and light stability of N-halamine siloxanes bound to cotton. Cellulose 15: 593-598.
- Kou, L., Liang, J., Ren, X., Kocer, H.B, Worley, S., Broughton, R., Huang, T. (2009). Novel N-halamine silanes. Colloids Surf., A 345: 88-94.
- Wu, L., et al., (2014). Synthesis of a novel multi N-halamines siloxane precursor and its antimicrobial activity on cotton. Applied Surface Science,. 314: p. 832-840.
- Chen, Y., X.-s. Zhong, and Q. Zhang, (2012). Synthesis of CO2-philic polysiloxane with N-halamine side groups for biocidal coating on cotton. Industrial & Engineering Chemistry Research,. 51(27): p. 9260-9265.
- Jie Z, Zhang B, Zhao L, Yan X, Liang J. (2014). Regenerable antimicrobial silica gel with quaternarized N-halamine. J Mater Sci 49(9): 3391–3399.
- Ates B, Cerkez I. (2017). Dual antibacterial functional regenerated cellulose fibers. J Appl Polym Sci134(21): 44872.
- Rahma H, Nickel R, Skoropata E, Wroczynsky Y, Rutley C, Manna PK, Hsiao CH, Ouyang H, van Lierop J, Liu S. (2016). Quaternized N-chloramine coated magnetic nanoparticles: a trifecta of superior antibacterial activity, minimal residual toxicity and rapid site removal. RSC Adv 6: 65837–65846.
- Li L, Pu T, Zhanel G, Zhao N, Ens W, Liu S. (2012). New biocide with both N-chloramine and quaternary ammonium moieties exerts enhanced bactericidal activity. Adv Healthcare Mater 1(5): 609–620.
- Liu Y, Liu Y, Ren X, Huang T. (2014). Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Appl Surf Sci 296: 231–236.
- Yong Chen1, Qi Chen, Zhendong Wang, Zengqi Yan, Qiang Zhang, and Qiuxia Han. (2022). Bactericidal silicone with one quaternary ammonium salt and two N-halamine sites in the repeating unit for improved biocidability on magnetic Submicrospheres. J Mater Sci 57: 2100–2114.
- I.M. El-Nahhal, J. K. Salem, R. Anbar, F.S. Kodeh, A. Elmanama, (2020). Preparationand antimicrobial activity of ZnO-NPs coated cotton starch and their functionalized ZnO-Ag/ cotton and Zn(II) curcumin/cotton materials. Scientific reports. 10: 5410.
- I.M. El-Nahhal, J. K. Salem, R. Anbar, F.S. Kodeh , A. Elmanama. (2022). CuO-NPs, CuO-Ag nanocomposite and Cu(II)-curcumin complex coated cotton/starched cotton antimicrobial material, Materials Chemistry and Physics, 285: 126099.
- El Nahhal I.; Aqad M.; Kodeh F; Safi Z.; Wazzan N. (2023). N-Halamine-modified mesoporous silica for water disinfection. Materials Chemistry and Physics. 293, P 126936.
- El-Nahhal, I.M., F.R. Zaggout, and N.M. El-Ashgar, (2000). Uptake of divalent metal ions (Cu2+, Zn2+ and Cd2+) by polysiloxane immobilized glycinate ligand system. Analytical letters,. 33(15): p. 3373-3395.
- Ahmed, M.A., et al., (2016). Synthesis and characterization of immobilized-polysiloxane monoamine-thiol triacetic acid and its diamine and triamine derivatives. Journal of Sol-Gel Science and Technology,. 78(3): p. 660-672.
Citation: Issa M. El Nahhal, Hayfa Habes Almutairi, Mustafa Al Aqad and Fawzi S Kodeh. (2023). Novel Glycinate N-halamine Siloxane coated Cotton and Cotton/ZnO-NPs Composites and their Antibacterial Activities. Journal of Pharmacy and Drug Development 5(1).
Copyright: © 2023 Issa M. El Nahhal. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.